Abstract
Tropical forests are being cleared at an accelerating rate, despite being one of the most important habitats for global biodiversity. Many remaining tropical forest tracts are now highly degraded and fragmented, which presents a major problem for sensitive and threatened forest-dwelling species that depend on this habitat for survival. In this study, we assessed the impacts of forest fragmentation, and its associated edge-effects, on tree species diversity, tree size, and structural diversity within the transitional forests of north west Madagascar. Using data collected from 9,619 trees within 200 vegetation plots, we calculated species diversity indices, a range of dendrometry measurements, and Shannon-Weaver diversity indices of structure, which we compared among core and edge areas of a continuous forest and a fragmented forest. We found that species diversity, tree size, and structural diversity was significantly reduced in fragmented forest, and within forest edge areas in comparison to core, continuous forest. We also observed species diversity and structural diversity to be strongly influenced by the total size, core area size, and shape of forest fragments. Whilst we found fragmentation and edge-effects to individually impact tree species diversity, size and structural diversity, fragmentation and edge-effects are strongly correlated and affect natural forest synergistically. Our results provide evidence that forest fragmentation seriously degrades habitat quality and integrity of transitional forests, which is of great concern for the threatened species that inhabit them. Urgent conservation efforts are needed to halt ongoing forest fragmentation throughout the tropics, and reforestation and restoration efforts are required to reconnect isolated forest patches and to reduce forest edge area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are arguably the most ecologically important habitat type in the world as they harbor more than 50% of the world’s terrestrial floral and faunal species (Wilson 1988; Duivenvoorden et al. 2002; Bonan 2008). Despite their importance for global biodiversity, tropical forests are being destroyed at a faster rate than any other ecosystem (Myers 1988) and it is now estimated that over half of the world’s tropical forests (~ nine million km²) have been cleared in the last century (Poorter et al. 2016; McFarland 2018). The primary driver of tropical deforestation has historically been human population growth (Godfray et al. 2010; Busch and Ferretti-Gallon 2017), as the increasing population has resulted in an increased global demand for food and other resources, resulting in large-scale agricultural expansion and resource extraction within tropical forest habitats (Myers 1993; Foley et al. 2005). Due to the ongoing deforestation, much of the remaining forest habitat in tropical regions is now extensively fragmented (Taubert et al. 2018), and areas of isolated forest fragments that are geographically separated by expanses of grassland now characterize landscapes that were once continuous forest tracts (Wade et al. 2003; Brinck et al. 2017). Further to this, many of the remaining forest fragments have high forest edge areas in comparison to continuous forests (Brinck et al. 2017), resulting in a high level of edge-effects which have an influence on the functioning of the fragment’s ecological processes (Fahrig 2003; Laurance et al. 2007). Many forest fragments are also degraded, of poor habitat quality and low ecological integrity (Coe et al. 2013; de Paula et al. 2015) and forest fragments vary greatly in their size and shape (Hill and Curran 2003; Taubert et al. 2018). Tropical forest fragmentation is therefore regarded as one of the biggest threats to animal and plant populations worldwide (Turner, 1996).
Many forest-dwelling species now survive only in fragmented tropical forest (Shelton 1985), and it is paramount to understand how the forest fragmentation process impacts on forest population composition and diversity (Zuidema et al. 1996). This is also important for the protection and restoration of forest habitat and the species-specific conservation of forest species, many of which are classified as threatened on the IUCN Red List (Pimm et al. 1995). The effects of fragmentation and forest area on species diversity are already well-studied (e.g., Laurance and Bierregaard 1997; Pimm 1998; Brosi et al. 2008; Dayananda et al. 2016), and the relationship between animal and plant species diversity and forest fragment size (e.g., Bierregaard et al. 1992; Benítez-Malvido and Martínez‐Ramos 2003; Pardini et al. 2005; Harvey et al. 2006; Tripathi et al. 2010; Santana et al. 2021) and fragmentation associated edge-effects (e.g., Laurance 1991; Laurance et al. 2007; Sundarapandian and Karoor 2013; Vetter et al. 2013; Razafindratsima et al. 2018; Santana et al. 2021) is particularly well documented. However, conservation of threatened species, and conservation of the forest ecosystem as a whole, also requires a specific understanding of how forest fragmentation effects the forest habitat itself (Arroyo-Rodríguez and Mandujano 2006; Echeverría et al. 2007). Some studies have already found that forest fragmentation impacts the floral species diversity (e.g., Scariot 1999; Paciencia and Prado 2005; Viña and Estévez-Varón 2019), structural diversity (e.g., Benitez-Malvido 1998; Laurance et al. 2001; Wassie et al. 2010) and the availability of potential food sources for animals (e.g., Hart et al. 2013; Hadley et al. 2014) within a forest. Most studies document forest fragmentation as having a negative effect on forest floral and structural diversity, and some investigations also report positive relationships between forest fragment size or shape and tree species diversity (e.g., Ranta et al. 1998; Hill and Curran 2003; Page et al. 2010) and differences between forest interior and edge (e.g., Oliveira-Filho et al. 1997; Oliveira et al. 2004). As forest fragmentation becomes ever more profound within tropical regions, efforts to understand the relationships between habitat quality and forest fragmentation, fragment size, shape, and edge-area must be intensified in order to maintain and manage what forest habitat remains, and to mitigate fragmentation drivers (Hill and Curran 2003).
The island of Madagascar has arguably experienced the highest deforestation and forest fragmentation rates of any country in the world (Goodman et al. 2018). It has been estimated that up to 80% of Madagascar’s original forest cover has been cleared in the last century alone (Harper et al. 2007; Vieilledent et al. 2018) and much of the little forest habitat that remains is now fragmented, degraded, and sporadically distributed within a matrix of grassland and savannah (Vieilledent et al. 2018). Madagascar is considered a biodiversity hotspot and the ongoing forest fragmentation has put enormous pressures on its biodiversity, of which 85% of its animals and 90% of its plants are endemic (Ganzhorn et al. 2001; Isambert et al. 2011), and the island is now one of the world’s primary conservation concerns (Schwitzer et al. 2013). Some studies of the effects of forest fragmentation on Madagascar’s forest floral diversity and structure have been conducted at various localities throughout the island, and forest fragmentation has been documented to have mixed effects on forest habitat between different studies undertaken at different locations (Cadotte et al. 2002; Irwin et al. 2010; Malcolm et al. 2017; Razafindratsima et al. 2018). In addition, floral species diversity and structural diversity have been observed to be variable between forests at different localities (Ratovonamana et al. 2011; Andriaharimalala et al. 2012), over degradation gradients (Ganzhorn 1987; Hending et al. 2020) and between edge and core areas (Lehman 2016; Malcolm et al. 2017), suggesting that the effects of fragmentation on forest habitat quality are variable and possibly site-specific. These past studies, coupled with Madagascar’s high bioclimatic zonation (Brown et al. 2016), advocate that an understanding of forest fragmentation induced changes in forest habitat is required on a localized scale in order to successfully manage and restore forest habitat within areas of high conservation priority. Further, this localized knowledge is also required to protect Madagascar’s threatened animal taxa, which are often confined to small areas and are thus specialized to the forests within their respective ranges (Wilmé et al. 2006).
We conducted this study within the Sahamalaza-Iles Radama National Park (hereafter referred to as SIRNP) of the Sambirano domain, north west Madagascar. Forest habitat structure has already been reported to vary throughout SIRNP and strong habitat and micro-habitat preferences have already been documented for resident animal species, particularly lemurs, that live within it (e.g., Seiler et al. 2013, 2014; Hending et al. 2017a, 2021a). However, there are little data on how fragmentation affects tree species diversity, dendrometry (physical structure and size of a tree), and structural diversity. Further, nothing is known of the effects of fragment spatial geometry on tree diversity, size, and structure within the National Park, and the wider Sambirano domain more generally. In this study, we investigated how both forest fragmentation and edge-effects impact tree species diversity, size, and structural diversity, and whether the effects of fragmentation and edge-effects are independent or synergistic. Tree species diversity, tree size, and forest structural diversity have been observed to vary between continuous and fragmented forest, between forest edge and core areas, and among forest fragments of varying area and shape in Madagascar and other areas of the tropics more-generally (e.g., Didham and Lawton 1999; Brown and Gurevitch 2004; Harper et al. 2005; Krishnadas et al. 2019; Albiero-Júnior et al. 2021). However, fragmentation and edge-effects are often correlated and not independent of each other (Ewers et al. 2007; Malcolm et al. 2017). We therefore tested the hypothesis that forest fragmentation and edge-effects impact tree species diversity, tree size, and structural diversity synergistically. Specifically, we predicted higher tree species diversity, higher mean tree size, and greater forest structural diversity in continuous forests, core areas, and larger forest fragments than in fragmented forests, edge areas, and smaller forest fragments respectively, and that fragmentation effects and edge-effects would be strongly correlated and non-independent (as in Hill and Curran 2003; Ewers et al. 2007; Krishnadas et al. 2019).
Methods
Study site
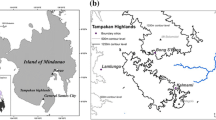
SIRNP is a 26,000 hectare protected area located primarily on the remote Sahamalaza Peninsula in Madagascar’s Sofia region, between the latitudes of 14°04’S − 14°37’S and longitudes of 47°52’E − 48°04’E (Fig. 1). In addition to its National Park status, SIRNP has also been designated a UNESCO Biosphere Reserve since 2007 (Rode et al. 2013). Despite this current protection, it has historically undergone severe deforestation and habitat fragmentation (Seiler et al. 2013, 2014). Much of SIRNP is now characterized by grassland and savannah, although there are some remaining isolated forest tracts and matrices of gallery and scrub forest (Volampeno et al. 2011). SIRNP is located in Madagascar’s Sambirano domain, an area of the north west of the island that is characterized by sub-humid transitional forests, and the vegetation of the National Park is comprised of a mixture of both deciduous and evergreen plant species, many of which are locally endemic (Koechlin 1972; Du Puy and Moat, 1996). SIRNP has a hot, sub-humid and seasonal climate, with a distinct hot, wet season (November - April) and a cooler dry season (May – October). The mean temperature range of SIRNP is 20.6–32.0 °C, with an extreme temperature range of 13.2–39.1 °C (Volampeno et al. 2011; Hending et al. 2017b) and an annual mean precipitation of ~ 1,600 mm (Schwitzer et al. 2007).
The Sahamalaza-Iles Radama National Park and the location of Ankarafa forest (A) and Anabohazo forest (B). Figure created in in ArcMap, with a scale of 1:7,000,000 for Madagascar and a scale of 1:350,000 for the zoomed panel. Red lines represent the protected area boundary (inner), and a three kilometre buffer (outer)
SIRNP is an ideal site at which to investigate the effect of forest fragmentation and its associated edge-effects on tree species diversity and forest structure. This is firstly because the National Park contains two remaining forests: the 1,169 ha Anabohazo forest, a continuous, undisturbed forest block that has undergone little disturbance (Randriatahina et al. 2014), and the 1,020 ha Ankarafa forest, a fragmented forest composed of ten fragments of varying size, shape, and with varying levels of forest edge-core ratios (Seiler et al. 2013). Secondly, the edge-core gradient of SIRNP’s forest has already been established from transects measuring the variation of microclimatic and abiotic variables (temperature, humidity, wind prevalence, and light intensity) from the forest edge into the forest core. This edge buffer has been reported as 165 m (Mandl 2018, unpublished thesis). The Anabohazo and Ankarafa forests are separated by only ~ 25 km of savannah, and this provides a rare opportunity to study the effects of forest fragmentation on a localized scale, where environmental covariates are more easily controlled, in comparison to other studies that have occurred over a larger area (e.g., Scariot 1999; Laurance et al. 2001).
Sampling of vegetation diversity and structure
Between 06/03/2019 and 15/05/2022, we conducted 200 vegetation plots, each measuring 20 × 20 m, to characterize tree species diversity, tree size, and forest structure within both the Anabohazo (N = 100) and Ankarafa (N = 100) forests. The 100 Ankarafa plots were located within ten separate forest fragments (10 plots per fragment) that varied in size and shape (Supplementary File 1). In Anabohazo forest, 50 of these plots were located within the forest core whilst 50 were within the forest edge area, defined as being within 165 m of the forest edge. In Ankarafa, 45 plots were located within the forest core whilst 55 were within the forest edge area (five plots in both edge and core for nine of the 10 Ankarafa fragments); this is because the Ankarafa II fragment had no core area (Supplementary File 1), and so all 10 plots for this fragment were located within a forest edge area. The vegetation plot locations were situated at ~ 50 m intervals along a system of established transects within each forest (Anabohazo N = 7, Ankarafa N = 10 (one per fragment). The plots themselves were established a minimum of 10 m from the transect line. Within each plot, we identified all trees with a circumference at breast height (CBH) of ≥ 16 cm (Armstrong et al. 2011) using the knowledge of local guides and we noted their colloquial (local) names. We then consulted the existing literature of the flora of Madagascar (Schatz, 2001) to identify the trees to species or genus level where possible and to determine whether they were an endemic native, a non-endemic native, or an introduced species to Madagascar. For each identified tree in the plot, we also measured the CBH with a measuring tape and we used a laser rangefinder (Excelvan, El Monte, California, USA) to measure the tree’s total height, bole height and crown diameter. We measured crown diameter by positioning two members of the field team under opposite sides of the widest point of the tree’s crown, and we measured the distance between the two researchers with the laser rangefinder. We converted our CBH values to diameter at breast height (DBH) values by dividing CBH by π. To measure tree density, we used the point-centre quarter method (Ganzhorn 2003), where we measured the distance from each identified tree to the nearest adjacent tree over two metres tall in four geographic directions using a measuring tape. We then calculated the mean of these four point-to-plant distances, squared it, and then calculated its inverse to obtain density values of the number of trees per square metre (N/m2) as measure of the microhabitat density around each tree.
Analysis of vegetation species diversity
To analyze how tree species diversity varied between the forests, and between edge and core areas, we first used the EstimateS software (Colwell 2013) to obtain values for the expected number of species (S) in the edge and core areas of each forest using rarefaction (Colwell and Coddington 1994). We formed subsets of data by pooling the plots for each forest and the separate edge and core areas of each forest, and we specified the number of knots for the rarefaction as the number (N) of trees included within each subset of data (Colwell et al. 2004). We specified for EstimateS to extrapolate the data up to a maximum of 5,000 knots for all data subsets, as the sampled number of trees was uneven between the two forests, and edge and core areas. We then constructed species-individual curves (with 95% confidence intervals) using the rarified and extrapolated S values so that tree species diversity could be compared between the Anabohazo and Ankarafa forests, and the edge and core areas. We did this by assessing the overlap between the 95% confidence intervals of the curves as a conservative means of establishing statistical significance (Colwell 2013).
We also used the EstimateS software to calculate Chao 1 species diversity indices (with 95% confidence intervals) for each forest’s core and edge areas to statistically compare species diversity among sampled areas. We chose this approach, rather than conducting analysis on standardized N species/N trees values, as these indices compensate for bias caused by undetected species and therefore represent lower bounds for the true species diversity of each sampled area (Chao 1984, 1987). For the species-individual curves, we compared Chao 1 species diversity between the two forests and their edge and core areas by assessing the overlap between 95% confidence intervals of the curves. Chao 1 indices cannot be extrapolated, and so we compared the indices at the point of maximum runs for the area with the fewest sampled individuals (Hending et al. 2023). We constructed all species-individual curves and Chao 1 index-individual curves in R Studio (2020) using the ‘ggplot2’ package (Wickham 2016).
Analysis of vegetation structure
Prior to analysis, we assigned our DBH, tree height, crown diameter, and tree density values into frequency bins. The frequency bins were as follows; DBH: 5.0-9.9 cm, 10.0-14.9 cm through to 45.0-49.9 and ≥ 50.0 cm; height: 1.50–2.99 m, 3.00–4.49 m through to 13.50–14.99 m and ≥ 15.00 m; crown diameter: < 1.99 m, 2.00–2.99 m, 3.00–3.99 m through to 9.00–9.99 m and ≥ 10.00 m; tree density: 0.00–0.49 n/m2, 0.50–0.99 n/m2 through to 4.00–4.49 n/m2 and ≥ 4.50 n/m2. We then used these frequency datasets to calculate Shannon-Weaver structural diversity indices (H) (Shannon and Weaver 1949) for each variable for each plot using the formula:
where Pi = the fraction of the entire population made up of species i, and S = total number of classification bins. This was so that we could then conduct analysis of structural diversity (following Ganzhorn 1987; Andersohn 2004).
Statistical analysis
We conducted all statistical analyses in R Studio using a 95% confidence interval. To check that all data fields were of normal distribution and homogenous variance, we performed Levene tests using the ‘car’ package (Fox and Weisberg 2019) on each data field that we wanted to compare. Whilst all Levene test results were not significant at the plot level, all Levene test results were significant at the individual tree level (Supplementary File 2). We therefore used Multivariate Generalized Linear Mixed Models (GLMMs) in the ‘lme4’ package (Bates et al. 2015) to compare tree species diversity, dendrometry, and structural diversity between (1) the two forests, and (2) between edge and core areas, and to test the relationship of these two fixed effects. Within all GLMMs, we assigned the tree diversity, dendrometry, or structural diversity metric that we were comparing as the response variable (one response variable per model), and we used two grouping variables (forest and edge/core). Although our study was localized within SIRNP to minimize random effects from environmental and climatic variables between the 200 plots, we included mean annual temperature and annual precipitation values as random factors to control for fine-scale environmental differences between plots. We obtained the values of mean temperature and precipitation for each vegetation plot from WorldClim (Fick and Hijmans 2017: 1 km resolution) using the ‘raster’ (Hijmans 2021) and ‘sp’ (Pebesma and Bivand 2005) R packages. We did not include elevation as an additional random factor, as elevation correlates with temperature and precipitation (Hending 2021a). To compare mean tree species per plot and structural diversity Shannon-Weaver indices between forest areas, we conducted our GLMMs at the plot level (N = 200), where all data points were vegetation plot means. To compare tree dendrometry measurements between forest areas, we conducted our GLMMs at the individual tree level (N = 9,619), where all data points corresponded to individual trees. Although individual trees can be regarded as independent data points, we ensured that any plot-based effects were removed from the subsequent GLMM results by including the vegetation plot ID, mean temperature and precipitation as random factors within the models. The full results of all GLMMs can be viewed in Supplementary File 3. In addition, we also performed GLMMs with one grouping variable with four categories (Anabohazo core, Anabohazo edge, Ankarafa core, and Ankarafa edge) to further explore how tree species diversity, tree size and tree structural diversity varied between the four surveyed forest areas. The results of these single fixed-effect GLMMs can be viewed in Supplementary File 4. To investigate the relationship of tree diversity, size, and structural diversity with fragment size, core area size, and fragment shape (Patton 1975), we performed linear models (LMs, full results in Supplementary File 5). As with the GLMMs, we included mean temperature and annual precipitation values for each forest fragment as random factors within our LMs.
Results
Tree species diversity
In total, we identified and measured a total of 9,619 trees from 225 different species (Anabohazo core N = 2,446, Anabohazo edge N = 2,095, Ankarafa core N = 2,250, Ankarafa edge N = 2,828). We confirmed the presence of 203 tree species within Anabohazo forest (core N = 189, edge N = 134), whilst we were able to confirm the presence of 118 tree species in Ankarafa forest (core N = 95, edge N = 107) (Supplementary File 6). We were able to identify 94 tree species to the species level, 120 species to the genus level, whilst we could not identify 11 tree species. The mean number of tree species per vegetation plot was significantly different among forest areas, with Anabohazo core plots having a higher mean number of tree species than Anabohazo edge and both areas of Ankarafa (Supplementary File 4). Anabohazo forest had a significantly higher expected number of tree species than Ankarafa forest when comparing the interpolated data (at the point where N = 2,095 individuals) and the extrapolated data (N = 5,000 individuals) (Fig. 2A). Anabohazo core also had a significantly higher expected number of tree species than Anabohazo edge. Both Anabohazo forest areas had a significantly higher Chao 1 species diversity index than both Ankarafa forest sites, although there was no difference between core and edge areas in both forests (Fig. 2B). Both forest fragmentation (Estimate = -7.568, df = 25.144, t = -4.488, P < 0.001) and edge-effects (Estimate = -5.939, df = 193.255, t = -5.066, P < 0.001) influenced tree species diversity, and there was a significant interaction between the two variables (Estimate = 3.921, df = 193.065, t = 2.364, P = 0.019, Correlation = 0.310) (Supplementary File 3).
A: Expected number of tree species (S) calculated by interpolation (solid lines) and extrapolated up to 5,000 runs (dashed line), and B: Chao 1 species diversity indices, for four different forest areas within the Sahamalaza-Iles Radama National Park of north west Madagascar. Dotted lines in A and B represent upper and lower 95% confidence intervals. Rarefaction, extrapolation, diversity indices and associated confidence intervals were computed in EstimateS (Colwell 2013)
Our linear models highlight that tree species diversity correlates positively with forest fragment size (R = 0.554), forest core area percentage (R = 0.452), and fragment shape index (R = 0.566) (Fig. 3). However, the results of these LMs were not statistically significant (Supplementary File 5).
The relationship between forest fragment total area (red), forest fragment core area percentage (blue), and forest fragment shape index (green) with mean tree species per vegetation plot for 10 forest fragments within Ankarafa forest of the Sahamalaza-Iles Radama National Park, north west Madagascar. Relationships investigated with linear models (full results in Supplementary File 5). Shaded areas represent 95% confidence intervals
Dendrometry
Tree DBH, height, crown diameter, and tree density were significantly different between the four compared forest areas (Table 1; Fig. 4, Supplementary File 4). Both forest fragmentation and edge-effects had significant impacts on mean DBH, height, crown diameter and tree density, (Table 1, Supplementary File 3). Interactions between fragmentation and edge-effects were also significant for all four dendrometry variables (Table 1, Supplementary File 3).
Summary of tree DBH (cm), height (m), crown diameter (m), and tree density (N/m2) for core and edge areas of Anabohazo and Ankarafa forest in the Sahamalaza-Iles Radama National Park of north west Madagascar. Boxplots display the median, interquartile ranges, and minimum and maximum value upon removal of all outliers (full ranges in Table 1). Boxplot notches represent 95% confidence intervals. Dots represent individual trees
Our LMs highlight that mean tree DBH and mean crown diameter had a strong positive correlation with forest fragment size (R = 0.919 and R = 0.773 respectively), forest core area percentage (R = 0.868 and R = 0.719 respectively), and fragment shape index (R = 0.779 and R = 0.304 respectively), whilst mean tree height and tree density had a strong negative correlation with fragment size (R = 0.887 and R = 0.754 respectively), core area percentage (R = 0.877 and R = 0.420 respectively), and shape (R = 0.841 and R = 0.538 respectively) (Fig. 5). Although almost all of these results indicated strong correlations (R > 0.700), the results of our LMs indicate that most of these correlations were not statistically significant (Supplementary File 5).
The relationship between forest fragment total area (red), forest fragment core area percentage (blue), and forest fragment shape index (green) with mean tree DBH (first row), mean tree height (second row), mean tree crown diameter (third row), and mean tree density (fourth row) per vegetation plot for 10 forest fragments within Ankarafa forest of the Sahamalaza-Iles Radama National Park, north west Madagascar. Relationships investigated with linear models (full results in Supplementary File 5). Shaded areas represent 95% confidence intervals
Tree structural diversity
DBH, height, crown diameter, and tree density Shannon-Weaver diversity were significantly different between the four compared forest areas (Table 2; Fig. 6, Supplementary File 4). Forest fragmentation had significant effects on DBH, crown diameter and tree density Shannon-Weaver diversity, whilst edge-effects significantly impacted DBH, tree height and crown diameter Shannon-Weaver diversity (Table 2, Supplementary File 3). Interactions between fragmentation and edge-effects were significant for DBH and tree height Shannon-Weaver diversity, but not for crown diameter or tree density (Table 2).
Summary of Shannon-Weaver structural diversity indices (SW) for DBH, height, crown diameter, and tree density for core and edge areas of Anabohazo and Ankarafa forest in the Sahamalaza-Iles Radama National Park of north west Madagascar. Boxplots display the median, interquartile ranges, and minimum and maximum values. Boxplot notches represent 95% confidence intervals. Dots represent individual data points (plot structural diversity SW values)
Our linear models highlight that tree DBH, height, and crown diameter structural diversity correlated positively with forest fragment size (all R > 0.700) and forest core area percentage (all R > 0.630). Whilst DBH structural diversity also correlated positively with fragment shape index (R = 0.717), height and crown diversity correlated negatively (R = 0.469 and R = 0.337 respectively). Tree density diversity indices correlated negatively with all three fragment-related variables (Fig. 7). Similar to the dendrometry variable results, structural diversity variables also had very strong correlations with fragment size, core area, and shape (R > 0.700), yet results of the LMs indicate that most results were not statistically significant (Supplementary File 5).
The relationship between forest fragment total area (red), forest fragment core area percentage (blue), and forest fragment shape index (green) with mean Shannon-Weaver structural diversity indices (SW) for tree DBH (first row), tree height (second row), tree crown diameter (third row), and tree density (fourth row) per vegetation plot for 10 forest fragments within Ankarafa forest of the Sahamalaza-Iles Radama National Park, north west Madagascar. Relationships investigated with linear models (full results in Supplementary File 5). Shaded areas represent 95% confidence intervals
Discussion
Tree species diversity
The results of our study demonstrate that forest fragmentation and forest edge-effects both individually have detrimental effects on tree species diversity within the transitional forests of north west Madagascar (Fig. 2). Further, there were significant positive interactions between the effects of forest fragmentation and edge-effects, and so both processes appear to impact tree species diversity synergistically. We observed fewer tree species in smaller fragments and those with lower core forest areas, suggesting that tree species diversity correlates to the magnitude and severity of the forest fragmentation (Fig. 3). These findings conform to our original hypothesis, and mirror the results from other studies that have assessed how vegetation diversity is impacted by forest fragmentation (Benítez-Malvido and Martínez‐Ramos 2003; Hill and Curran 2003; Aguirre-Jaimes et al. 2021; Santana et al. 2021). Whilst our study highlights a higher species diversity in continuous forest in comparison to fragmented forest, tree species diversity is likely to be even greater in continuous forest (Anabohazo) than we report, as our species-individual and Chao 1-individual curves had not yet plateaued at our point of statistical comparison (Fig. 2). This postulates that a high number of rare tree species are present within Anabohazo. Both curves had however plateaued for fragmented forest (Ankarafa), suggesting that forest fragmentation, and indeed fragment size (Fig. 3, Hill and Curran 2003), likely limits tree species diversity within a forest area (Almoussawi et al. 2020). The greater species diversity in continuous forest can be attributed to the presence of rare species (Fig. 2B), species sensitive to habitat degradation (Tabarelli et al. 2004) and shade tolerant species (Metzger 2000), which would otherwise be absent in fragmented forests. Fragment age is also likely to influence tree species diversity, as species diversity can be expected to be lower in older fragments (Ross et al. 2002; Goosem et al. 2016); as SIRNP forest fragment age data is not something that we were able to obtain either by historical GIS maps or discussions with local guides, this was something we could not investigate or control for in this study.
Interestingly, within fragmented forest, we observed a higher tree species diversity in forest edge area than in core area. This observation contrasts to core-edge comparisons from the continuous forest (Figs. 2 and 3). Tree species diversity is likely to be higher in core areas of continuous forest due to the presence of species sensitive to edge-effects and degraded habitat (Tabarelli et al. 2004). However, these species may be less frequent in fragmented forest, making them more difficult to detect, or absent entirely. The presence of tall, emergent trees within core areas of fragmented forests would also restrict the growth of edge-tolerant species (Budke et al. 2010), which would be more abundant at the forest edge; we observed shade-intolerant species such as Harungana madagascariensis and Strychnos spinosa almost exclusively within forest edge areas (Supplementary File 6). This, coupled with the presence of successional and exotic species (Waddell et al. 2020), may result in higher species diversity within edge areas of fragmented forests. Sensitive species will continue to decline as fragment size and core area decreases (Hill and Curran 2003; Laurance et al. 2018), and we observed diversity decline in correlation with decreasing fragment size here (Fig. 3). Whilst our results were not statistically significant despite high R values, this can be attributed to our small sample size (N = 10 fragments).
Tree size
Our results for tree size mirror those for species diversity; tree size and density was higher in continuous forest than in fragmented forest, and tree size was greater in core forest areas in comparison to edge areas (Table 1; Fig. 4). Similar to tree species diversity, fragmentation and edge-effects effect tree size individually, but there were strong positive relationships between both variables indicating that fragmentation effects drive edge-effects and vice-versa. These findings align with our hypothesis, and the findings of previous studies (Laurance et al. 2000, 2006; Magnago et al. 2015; Almeida et al. 2019). Whilst we found fragmented forest and edge areas to contain some large, fast-growing species (e.g., Canarium madagascariensis, Ceiba pentandra, Mangifera indica), these species were also present within continuous and core forest, along with specialised, old-growth, emergent canopy species, contributing to the significantly higher mean tree height in continuous and core areas. Large, specialised species are often absent in fragmented, degraded, and edge areas of forests, where they are replaced by successional, photophilic and generalist species (Laurance et al. 2006), often with lower biomass (Razafindratsima et al. 2018). This could be an explanatory factor for lower mean tree size in fragmented forests and edge areas. Tree size in forest edge areas is often limited by factors that cause extensive damage to vegetation, such as microclimate, wind exposure, soil desecration, fire, and browsing animals that are unable to access core forest areas (Laurance and Curran 2008; Magnago et al. 2015; Almeida et al. 2019). Such edge-effects would be much more prominent within fragments with lower core areas, resulting in altered tree size in fragmented forests. Further, additional disturbance and anthropogenic factors that affect tree size, such as cutting by humans, can often be observed many hundreds of metres into a forest patch (Laurance 1991). Tree size would therefore remain unaffected only in areas of continuous forest far from edge boundaries, which explains lower tree diversity in fragments of all sizes.
We found tree size and density to vary among our sampled fragments, an observation mirroring previous studies in other areas of the tropics (Tripathi et al. 2010; Almeida et al. 2019). Whilst tree DBH and crown diameter correlated strongly with fragment size, core area, and shape (Fig. 5), tree height and density had a weak negative correlation with these variables. Our results suggest that fragment size and shape affect tree dendrometry, yet our small sample size resulted in opposing trends between size variables and statistically insignificant results despite high R values (Supplementary File 5).
Tree structural diversity
Similar to tree species diversity and size, our results demonstrate that forest structural diversity is higher in continuous forest and forest core areas in comparison to fragmented forest and edge areas (Table 2; Fig. 6). Whilst fragmentation and edge-effects had individual impacts on three of the four structural diversity variables each (Table 2), fragmentation did not have an individual significant effect on tree height structural diversity, and edge-effects did not significantly affect tree density structural diversity on an individual level. However, structural diversity of DBH and height appears to be synergistically affected by both fragmentation and edge-effects, whilst crown diameter and tree density appear are only impacted by each variable individually (i.e., fragmentation does not drive edge-core differences in crown diameter and tree density structural diversity, and vice-versa). The findings for DBH and height conform to our original hypothesis, and our findings for all variables align with trends observed in other geographic areas of Madagascar and the tropics (Benitez-Malvido 1998; Laurance et al. 2001; Wassie et al. 2010; Muluneh et al. 2021). Forest structural diversity also appears to have a general positive correlation with fragment size and core area size (with the exception of tree density SW diversity), and negatively with shape index (with the exception of DBH SW diversity) (Fig. 7, Supplementary File 5). These results provide evidence to suggest that high structural diversity can only be retained within intact, undisturbed forest core areas, most likely due to higher species diversity, the retention of both smaller understorey species and large, old-growth species, and a greater range of tree forms (Memiaghe et al. 2016; Ali et al. 2020). Structural diversity loss also appears relative to the scale and degree of forest fragmentation and habitat degradation (e.g., Didham and Lawton 1999; Brown and Gurevitch 2004; Echeverría et al. 2007; Hending et al. 2020). However, we must restate that our fragment characteristic-related LMs were mostly not statistically significant, despite high R values.
In addition to lower tree species diversity, forest structural diversity within fragmented forest and edge areas is also likely to be caused by changes in microclimate, wind exposure, soil degradation, fire, and other factors that damage tree structure and limit growth (Laurance and Curran 2008; Magnago et al. 2015; Almeida et al. 2019). Whilst lower structural diversity is indicative of habitat degradation within forest ecosystems (Milodowski et al. 2021), reduced tree structural diversity is also correlated positively with species diversity of resident animals and non-woody plants (Tews et al. 2004; Lenière and Houle 2006; Mert and Yalçınkaya 2017; Hending et al. 2020). Lower structural diversity, coupled with reduced tree species diversity and density, diminishes the availability of travel strata, feeding trees, and sleeping sites for forest-dwelling animals (e.g., Seiler et al. 2013), many of which are threatened and dependent on forest habitat for survival. Forest fragmentation-induced structural diversity loss is therefore of great conservation concern, particularly for sensitive species that are specialised to continuous, pristine forest core habitat (Augusto et al. 2000). Fortunately, fragments and edge areas are valuable habitat for many generalist, gap-tolerant species, and many forest flora and faunal are capable of thriving within both pristine and degraded forest areas (Fahrig et al. 2019). This is definitely true for numerous species within our own study area, and Madagascar more-generally (e.g., Hending 2021b).
Conservation implications and conclusion
This study has demonstrated that forest fragmentation and edge-effects are highly detrimental to tree species diversity, tree size, and structural diversity, and the impacts of fragmentation and edge-effects are often correlated, and therefore synergistic. Even core areas within larger forest fragments exhibit significantly reduced tree species diversity and structural diversity, and such trends are linked with reduced overall floral and faunal biodiversity, and reduced ecosystem functionality (Tews et al. 2004; Hertzog et al. 2019). Our findings provide further evidence that continued forest fragmentation may drive forest ecosystem collapse, and only core areas of unfragmented, continuous forests are able to retain normal levels of tree species diversity and structural diversity. Tropical forests remain one of the most important habitat types for global biodiversity (Wilson 1988; Bonan 2008), and substantial habitat restoration efforts need to be made to halt fragmentation and limit the loss of intact core forest. In many areas of the tropics, including in our study area (Cotton 2021; Hending et al. 2021b), reforestation efforts are already underway to restore areas of primary forest lost due to unsustainable slash-and-burn agriculture, charcoal production, and mining (Chazdon 2008; Locatelli et al. 2015; Taubert et al. 2018). These efforts will be crucial to reconnect isolated forest patches, increase and regenerate core forest areas, and safeguard the future of core forest ecosystems (Chazdon 2008; Di Sacco et al. 2021).
Forests throughout Madagascar and the wider tropics continue to be fragmented at an alarming rate (Harper et al. 2007; Taubert et al. 2018; Vieilledent et al. 2018). The situation in Madagascar is of particular concern, as over 80% of the island’s original forest cover has already been cleared, and 46% of remaining forest areas are located within only 100 m of a forest edge (Vieilledent et al. 2018). Coupled with the ongoing threats posed by agricultural expansion, unsustainable natural resource extraction, and climate change (Myers 1993; Foley et al. 2005; Hending et al. 2022), continued forest fragmentation renders tropical forests as arguably the most threatened of all natural habitat types globally, despite being the most important for terrestrial biodiversity and carbon sequestration (Duivenvoorden et al. 2002; Bonan 2008; Keith et al. 2009). This study provides further evidence that urgent conservation actions, and new environmental policy and legislation mandates need to be implemented to protect, restore, and renew areas of forest throughout the tropics, and to ensure the future survival of the threatened taxa that inhabit these vitally important habitats.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguirre-Jaimes A, López-Acosta JC, Dirzo R (2021) Tropical rainforest fragmentation affects plant species richness, composition and abundance depending on plant-size class and life history. Bot Sci 99(1):92–103
Albiero-Júnior A, Venegas-González A, Camargo JLC, Roig FA, Tomazello-Filho M (2021) Amazon forest fragmentation and edge effects temporarily favored understory and midstory tree growth. Trees 35(6):2059–2068
Ali A, Mattsson E, Nissanka SP, Wang LQ (2020) Topmost trees and foremost species underlie tropical forest structure, diversity and biomass through opposing mechanisms. For Ecol Manag 473:118299
Almeida DR, Stark SC, Schietti J, Camargo JL, Amazonas NT, Gorgens EB, Rosa DM, Smith MN, Valbuena R, Saleska S, Andrade A, Mesquita R, Laurance SG, Laurance WF, Lovejoy TE, Broadbent EN, Shimabokoru YE, Parker GG, Lefsky M, Silva CA, Brancalion PHS (2019) Persistent effects of fragmentation on tropical rainforest canopy structure after 20 year of isolation. Ecol Appl 29(6):e01952
Almoussawi A, Lenoir J, Jamoneau A, Hattab T, Wasof S, Gallet-Moron E, Garzon‐Lopez CX, Spicher F, Kobaissi A, Decocq G (2020) Forest fragmentation shapes the alpha–gamma relationship in plant diversity. J Veg Sci 31(1):63–74
Andersohn C (2004) Does tree height determine epiphyte. diversity? Selbyana 25(1):101–117
Andriaharimalala T, Roger E, Rajeriarison C, Ganzhorn JU (2012) Phenology of different vegetation types in the dry forest of Andohahela National Park, southeastern Madagascar. Malagasy Nat 6:24–45
Armstrong AH, Shugart HH, Fatoyinbo TE (2011) Characterization of community composition and forest structure in a Madagascar lowland rainforest. Trop Conserv Sci 4:428–444
Arroyo-Rodríguez V, Mandujano S (2006) Forest fragmentation modifies habitat quality for Alouatta palliata. Int J Primatol 27(4):1079–1096
Augusto A, Tabanez J, Viana VM (2000) Patch structure within brazilian Atlantic forest fragments and implications for conservation. Biotropica 32(4b):925–933
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Benitez-Malvido J (1998) Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv Biol 12(2):380–389
Benítez-Malvido J, Martínez‐Ramos M (2003) Impact of forest fragmentation on understory plant species richness in Amazonia. Conserv Biol 17(2):389–400
Bierregaard RO, Lovejoy TE, Kapos V, dos Santos AA, Hutchings RW (1992) The biological dynamics of tropical rain forest fragments. Bioscience 42:859–866
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320(5882):1444–1449
Brinck K, Fischer R, Groeneveld J, Lehmann S, De Paula MD, Pütz S, Sexton JO, Song D, Huth A (2017) High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat Comm 8(1):1–6
Brosi BJ, Daily GC, Shih TM, Oviedo F, Durán G (2008) The effects of forest fragmentation on bee communities in tropical countryside. J Appl Ecol 45(3):773–783
Brown KA, Gurevitch J (2004) Long-term impacts of logging on forest diversity in Madagascar. Proc Natl Acad Sci 101(16):6045–6049
Brown JL, Sillero N, Glaw F, Bora P, Vieites DR, Vences M (2016) Spatial biodiversity patterns of Madagascar’s amphibians and reptiles. PLoS ONE 11(1):e0144076
Budke JC, Jarenkow JA, de Oliveira-Filho AT (2010) Intermediary disturbance increases tree diversity in riverine forest of southern Brazil. Biodivers Conserv 19(8):2371–2387
Busch J, Ferretti-Gallon K (2017) What drives deforestation and what stops it? A meta-analysis. Rev Env Econ Policy 11(1):3–23
Cadotte MW, Franck R, Reza L, Lovett-Doust J (2002) Tree and shrub diversity and abundance in fragmented littoral forest of southeastern Madagascar. Biodivers Conserv 11(8):1417–1436
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Chazdon RL (2008) Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320:1458–1460
Coe MT, Marthews TR, Costa MH, Galbraith DR, Greenglass NL, Imbuzeiro HM, Levine NM, Malhi Y, Moorcroft PR, Muza MN, Powell TL, Saleska SR, Solorzano LA, Wang J (2013) Deforestation and climate feedbacks threaten the ecological integrity of south–southeastern Amazonia. Phil Trans R Soc B 368(1619):20120155
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9. User’s guide and application published at http://purl.oclc.org/estimates
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Phil Trans R Soc B 345:101–118
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Cotton S (2021) Growing links for lemurs: towards an effective reforestation of Sahamalaza-Iles Radama National Park. Final Report, IUCN
Dayananda SK, Goodale E, Lee MB, Liu JJ, Mammides C, Pasion BO, Quan RC, Slik JF, Sreekar R, Tomlinson KW, Yasuda M (2016) Effects of forest fragmentation on nocturnal asian birds: a case study from Xishuangbanna, China. Zool Res 37(3):151–158
de Paula MD, Groeneveld J, Huth A (2015) Tropical forest degradation and recovery in fragmented landscapes—simulating changes in tree community, forest hydrology and carbon balance. Glob Ecol Conserv 3:664–677
Di Sacco A, Hardwick KA, Blakesley D, Brancalion PH, Breman E, Cecilio Rebola L, Chomba S, Dixon K, Elliott S, Ruyonga G, Shaw K, Smith P, Smith RJ, Antonelli A (2021) Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob Chang Biol 27(7):1328–1348
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31(1):17–30
Du Puy D, Moat J (1998) Vegetation mapping and classification in Madagascar (using GIS): implications and recommendations for the conservation of biodiversity. In: Huxley CR, Lock JM, Cutler DF (eds) Chorology, Taxonomy and Ecology of the Floras of Africa and Madagascar. Kew Royal Botanical Gardens, London, pp 97–117
Duivenvoorden JF, Svenning JC, Wright SJ (2002) Beta diversity in tropical forests. Science 295(5555):636–637
Echeverría C, Newton AC, Lara A, Benayas JMR, Coomes DA (2007) Impacts of forest fragmentation on species composition and forest structure in the temperate landscape of southern Chile. Glob Ecol Biogeog 16(4):426–439
Ewers RM, Thorpe S, Didham RK (2007) Synergistic interactions between edge and area effects in a heavily fragmented landscape. Ecolog 88(1):96–106
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annual Rev Ecol Evol Syst 34(1):487–515
Fahrig L, Arroyo-Rodríguez V, Bennett JR, Boucher-Lalonde V, Cazetta E, Currie DJ, Eigenbrod F, Ford AT, Harrison SP, Jaeger JA, Koper N, Martin AE, Martin JL, Metzger JP, Morrison P, Rhodes JR, Saunders DA, Simberloff D, Smith AC, Tischendorf L, Vellend M, Watling JI (2019) Is habitat fragmentation bad for biodiversity? Biol Conserv 230:179–186
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int J Clim 37(12):4302–4315
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309(5734):570–574
Fox J, Weisberg S (2019) An R companion to Applied regression [Third edition]. Sage, Thousand Oaks, CA
Ganzhorn JU (1987) A possible role of plantations for primate conservation in Madagascar. Amer J Primatol 12:205–215
Ganzhorn JU (2003) Habitat description & phenology. In: Setchell JM, Curtis DJ (eds) Field and Laboratory Methods in Primatology: a practical guide. University Press, Cambridge, UK, pp 40–56
Ganzhorn JU, Lowry PP, Schatz GE, Sommer S (2001) The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. Oryx 35(4):346–348
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967):812–818
Goodman SM, Raherilalao MJ, Wohlauser S (2018) The Terrestrial protected Areas of Madagascar: their history, description, and Biota. Association Vahatra, Antananarivo, Madagascar
Goosem M, Paz C, Fensham R, Preece N, Goosem S, Laurance SG (2016) Forest age and isolation affect the rate of recovery of plant species diversity and community composition in secondary rain forests in tropical Australia. J Veg Sci 27(3):504–514
Hadley AS, Frey SJ, Robinson WD, Kress WJ, Betts MG (2014) Tropical forest fragmentation limits pollination of a keystone understory herb. Ecology 95(8):2202–2212
Harper KA, Macdonald SE, Burton PJ, Chen J, Brosofske KD, Saunders SC, Euskirchen ES, Roberts DAR, Jaiteh MS, Esseen PA (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19(3):768–782
Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F (2007) Fifty years of deforestation and forest fragmentation in Madagascar. Env Conserv 34(4):325–333
Hart LA, Grieve GRH, Downs CT (2013) Fruiting phenology and implications of fruit availability in the fragmented Ngele Forest Complex, KwaZulu-Natal, South Africa. South Afr J Bot 88:296–305
Harvey CA, Medina A, Sánchez DM, Vílchez S, Hernández B, Saenz JC, Maes JM, Casanoves F, Sinclair FL (2006) Patterns of animal diversity in different forms of tree cover in agricultural landscapes. Ecol Appl 16(5):1986–1999
Hending D (2021a) Niche-separation and conservation biogeography of Madagascar’s fork-marked lemurs (Cheirogaleidae: Phaner): evidence of a new cryptic species? Glob Ecol Conserv 29:e01738
Hending D (2021b) Environmental drivers of Cheirogaleidae population density: remarkable resilience of Madagascar’s smallest lemurs to habitat degradation. Ecol Evol 11(11):5874–5891
Hending D, McCabe G, Holderied M (2017a) Sleeping and ranging Behavior of the Sambirano mouse lemur, Microcebus sambiranensis. Int J Primatol 38(6):1072–1089
Hending D, Holderied M, McCabe G (2017b) The use of vocalizations of the Sambirano mouse lemur (Microcebus sambiranensis) in an acoustic survey of habitat preference. Int J Primatol 38(4):732–750
Hending D, Andrianiaina A, Maxfield P, Rakotomalala Z, Cotton S (2020) Floral species richness, structural diversity and conservation value of vanilla agroecosystems in Madagascar. Afr J Ecol 58(1):100–111
Hending D, Drew H, Holderied MW (2021a) Habitat use of constant frequency echolocating bats in north-west Madagascar with acoustic evidence for a possible new species. Acta Chiropt 23(1):153–164
Hending D, Randrianarison H, Holderied M, McCabe G, Cotton S (2021b) The kapok tree (Ceiba pentandra (L.) Gaertn, Malvaceae) as a food source for native vertebrate species during times of resource scarcity and its potential for reforestation in Madagascar. Austral Ecol 46(8):1440–1444
Hending D, Holderied M, McCabe G, Cotton S (2022) Effects of future climate change on the forests of Madagascar. Ecosphere 13(4):e4017
Hending D, Andrianiaina A, Rakotomalala Z, Cotton S (2023) Conservation value of vanilla agroecosystems for vertebrate diversity in north-east Madagascar. Oryx 57(1):118–128
Hertzog LR, Boonyarittichaikij R, Dekeukeleire D, de Groote SR, van Schrojenstein Lantman IM, Sercu BK, Smith HK, de la Peña E, Vandegehuchte ML, Bonte D, Martel A, Verheyen K, Lens L, Baeten L (2019) Forest fragmentation modulates effects of tree species richness and composition on ecosystem multifunctionality. Ecology 100(4):e02653
Hijmans RJ (2021) raster: Geographic Data Analysis and Modeling. R package version 3.4–10
Hill JL, Curran PJ (2003) Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. J Biogeogr 30(9):1391–1403
Irwin MT, Wright PC, Birkinshaw C, Fisher B (2010) Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol Conserv 143:2351–2362
Isambert B, Bergsten J, Monaghan MT, Andriamizehy H, Ranarilalatiana T, Ratsimbazafy M, Andriniainimanana JR, Vogler AP (2011) Endemism and evolutionary history in conflict over Madagascar’s freshwater conservation priorities. Biol Conserv 144(6):1902–1909
IUCN (2020) The IUCN RedList of Threatened Species. https://www.iucnredlist.org/ [Accessed 09 June 2020]
Keith H, Mackey BG, Lindenmayer DB (2009) Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc Natl Acad Sci 106(28):11635–11640
Koechlin J (1972) Flora and vegetation of Madagascar. In: Battistini R, Richard-Vindard G (eds) Biogeography and Ecology in Madagascar. Springer, Dordrecht, pp 145–190
Krishnadas M, Kumar AN, Comita LS (2019) Edge effects reduce α-diversity but not β‐diversity during community assembly in a human‐modified tropical forest. Ecol Appl 29(8):e01996
Laurance WF (1991) Edge effects in tropical forest fragments: application of a model for the design of nature reserves. Biol Conserv 57(2):205–219
Laurance WF, Bierregaard RO (1997) Tropical forest remnants: ecology, management and conservation of fragmented communities. University Press, Chicago, IL
Laurance WF, Curran TJ (2008) Impacts of wind disturbance on fragmented tropical forests: a review and synthesis. Austral Ecol 33(4):399–408
Laurance WF, Delamônica P, Laurance SG, Vasconcelos HL, Lovejoy TE (2000) Rainforest fragmentation kills big trees. Nature 404(6780):836–836
Laurance WF, Pérez-Salicrup D, Delamônica P, Fearnside PM, D’Angelo S, Jerozolinski A, Pohl L, Lovejoy TE (2001) Rain forest fragmentation and the structure of amazonian liana communities. Ecol 82(1):105–116
Laurance WF, Nascimento HE, Laurance SG, Andrade A, Ribeiro JE, Giraldo JP, Lovejoy TE, Condit R, Chave J, Harms KE, D’Angelo S (2006) Rapid decay of tree-community composition in amazonian forest fragments. Proc Natl Acad Sci 103(50):19010–19014
Laurance WF, Nascimento HE, Laurance SG, Andrade A, Ewers RM, Harms KE, Luizao RC, Ribeiro JE (2007) Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2(10):e1017
Laurance WF, Camargo JL, Fearnside PM, Lovejoy TE, Williamson GB, Mesquita RC, Meyer CF, Bobrowiec PE, Laurance SG (2018) An amazonian rainforest and its fragments as a laboratory of global change. Biol Rev 93(1):223–247
Lehman SM (2016) Edge effects on tree dendromics, abiotic, and mouse lemur densities in western dry forests in Madagascar. In: Lehman S, Radespiel U, Zimmermann E (eds) The dwarf and mouse lemurs of Madagascar: Biology, Behavior and Conservation Biogeography of the Cheirogaleidae. University Press, Cambridge, pp 462–476
Lenière A, Houle G (2006) Response of herbaceous plant diversity to reduced structural diversity in maple-dominated (Acer saccharum Marsh.) Forests managed for sap extraction. For Ecol Manag 231(1–3):94–104
Locatelli B, Catterall CP, Imbach P, Kumar C, Lasco R, Marín-Spiotta E, Mercer B, Powers JS, Schwartz N, Uriarte M (2015) Tropical reforestation and climate change: beyond carbon. Restor Ecol 23(4):337–343
Magnago LFS, Rocha MF, Meyer L, Martins SV, Meira-Neto JAA (2015) Microclimatic conditions at forest edges have significant impacts on vegetation structure in large Atlantic forest fragments. Biodivers Conserv 24(9):2305–2318
Malcolm JR, Valenta K, Lehman SM (2017) Edge effects in tropical dry forests of Madagascar: additivity or synergy? Landsc Ecol 32(2):327–341
Mandl I (2018) Nightly encounters: the social, spatial and vocal ecology of the Sahamalaza sportive lemur, Lepilemur sahamalaza, in north-western Madagascar. Doctoral dissertation, University of Bristol
McFarland BJ (2018) The context of tropical rainforest deforestation and degradation. Conservation of Tropical Rainforests. McFarland BJ, Ed.), Palgrave Macmillan, Cham, pp 7–58
Memiaghe HR, Lutz JA, Korte L, Alonso A, Kenfack D (2016) Ecological importance of small-diameter trees to the structure, diversity and biomass of a tropical evergreen forest at Rabi, Gabon. PLoS ONE 11(5):e0154988
Mert A, Yalçınkaya B (2017) Relationship between some wild mammals and forest structural diversity parameters. J Env Biol 38(5):879–884
Metzger JP (2000) Tree functional group richness and landscape structure in a brazilian tropical fragmented landscape. Ecol Appl 10(4):1147–1161
Milodowski DT, Coomes DA, Swinfield T, Jucker T, Riutta T, Malhi Y, Svátek M, Kvasnica J, Burslem DF, Ewers RM, Teh YA (2021) The impact of logging on vertical canopy structure across a gradient of tropical forest degradation intensity in Borneo. J Appl Ecol 58(8):1764–1775
Muluneh MG, Feyissa MT, Wolde TM (2021) Effect of forest fragmentation and disturbance on diversity and structure of woody species in dry afromontane forests of northern Ethiopia. Biodivers Conserv 30(6):1753–1779
Myers N (1988) In: Biodiversity (Wilson EO, Peter FM (eds) Tropical forests and their species going, going? National Academy Press, Washington, DC, USA, pp 28–35
Myers N (1993) Tropical forests: the main deforestation fronts. Env Conserv 20(1):9–16
Oliveira MA, Grillo AS, Tabarelli M (2004) Forest edge in the brazilian Atlantic forest: drastic changes in tree species assemblages. Oryx 38(4):389–394
Oliveira-Filho AT, De Mello JM, Scolforo JRS (1997) Effects of past disturbance and edges on tree community structure and dynamics within a fragment of tropical semideciduous forest in south-eastern Brazil over a five-year period (1987–1992). Plant Ecol 131(1):45–66
Paciencia MLB, Prado J (2005) Effects of forest fragmentation on pteridophyte diversity in a tropical rain forest in Brazil. Plant Ecol 180(1):87–104
Page NV, Qureshi Q, Rawat GS, Kushalappa CG (2010) Plant diversity in sacred forest fragments of western ghats: a comparative study of four life forms. Plant Ecol 206(2):237–250
Pardini R, de Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124(2):253–266
Patton DR (1975) A diversity index for quantifying habitat edge. Wild Soc Bull 3(4):171–173
Pebesma EJ, Bivand RS (2005) Classes and methods for spatial data in R. R News. 5(2), https://cran.r-project.org/doc/Rnews/
Pimm SL (1998) Ecology – the forest fragment classic. Nature 393:23–24
Pimm SL, Russell GJ, Gittleman JL, Brooks TM (1995) The future of biodiversity. Science 269(5222):347–350
Poorter L, Bongers F, Aide TM, Zambrano AMA, Balvanera P, Becknell JM, Boukili V, Brancalion PHS, Broadbent EN, Chazdon RL, Craven D, de Almeida-Cortez JS, Cabral GL, de Jong BHJ, Denslow JS, Dent DH, DeWalt SJ, Dupuy JM, Durán SM, Espírito-Santo MM, Fandino MC, César RG, Hall JS, Hernandez-Stefanoni JL, Jakovac CC, Junqueira AB, Kennard D, Letcher SG, Licona JC, Lohbeck M, Marín-Spiotta E, Martínez-Ramos M, Massoca P, Meave JA, Mesquita R, Mora F, Muñoz R, Muscarella R, Nunes YRF, Ochoa-Gaona S, de Oliveira AA, Orihuela-Belmonte E, Peña-Claros M, Pérez-García EA, Piotto D, Powers JS, Rodríguez-Velázquez J, Romero-Pérez IE, Ruíz J, Saldarriaga JG, Sanchez-Azofeifa A, Schwartz NB, Steininger MK, Swenson NG, Toledo M, Uriarte M, van Breugel M, van der Wal H, Veloso MDF, Vester HFM, Vicentini A, Vieira ICG, Bentos TV, Williamson GB, Rozendaal DMA (2016) Biomass resilience of neotropical secondary forests. Nature 530(7589):211–214
R Studio Team (2020) RStudio: Integrated Development for R. RStudio. PBC, Boston, MA. http://www.rstudio.com/
Randriatahina G, Ratsoavina F, Volampeno S, Schwitzer C (2014) Rapid assessment of lemur species in Anabohazo forest, Sahamalaza-Iles Radama National Park. Lemur News 18:22–24
Ranta P, Blom TOM, Niemela JARI, Joensuu E, Siitonen M (1998) The fragmented Atlantic rain forest of Brazil: size, shape and distribution of forest fragments. Biodivers Conserv 7(3):385–403
Ratovonamana RY, Rajeriarison C, Roger E, Ganzhorn JU (2011) Phenology of different vegetation types in Tsimanampetsotsa National Park, southwestern Madagascar. Malagasy Nat 5:14–38
Razafindratsima OH, Brown KA, Carvalho F, Johnson SE, Wright PC, Dunham AE (2018) Edge effects on components of diversity and above-ground biomass in a tropical rainforest. J Appl Ecol 55(2):977–985
Rode EJ, Nekaris KAI, Markolf M, Schliehe-Diecks S, Seiler M, Radespiel U, Schwitzer C (2013) Social organisation of the northern giant mouse lemur Mirza zaza in Sahamalaza, north western Madagascar, inferred from nest group composition and genetic relatedness. Contrib Zool 82(2):71–83
Ross KA, Fox BJ, Fox MD (2002) Changes to plant species richness in forest fragments: fragment age, disturbance and fire history may be as important as area. J Biogeogr 29(5–6):749–765
Santana LD, Prado-Junior JA, Ribeiro JHC, Ribeiro MAS, Pereira KMG, Antune K, Carvalho FA, Van den Berg E (2021) Edge effects in forest patches surrounded by native grassland are also dependent on patch size and shape. For Ecol Manag 482:118842
Scariot A (1999) Forest fragmentation effects on palm diversity in central Amazonia. J Ecol 87(1):66–76
Schatz, GE. (2001) Generic Tree Flora of Madagascar. Missouri Botanical Garden, SaintLouis, USA & Royal Botanic Gardens, Kew, UK.
Schwitzer N, Randriatahina GH, Kaumanns W, Hoffmeister D, Schwitzer C (2007) Habitat utilization of blue-eyed black lemurs, Eulemur macaco flavifrons (Gray, 1867), in primary and altered forest fragments. Primat Conserv 22:79–87
Schwitzer C, Mittermeier RA, Davies N, Johnson S, Ratsimbazafy J, Razafindramanana J, Louis EE, Rajaobelina S (2013) Lemurs of Madagascar: a strategy for their conservation 2013–2016. IUCN SSC Primate Specialist Group, Bristol UK
Seiler M, Holderied M, Schwitzer C (2013) Effects of habitat degradation on sleeping site choice and use in Sahamalaza sportive lemurs (Lepilemur sahamalazensis). Int J Primatol 34(2):260–280
Seiler M, Holderied M, Schwitzer C (2014) Habitat selection and use in the critically endangered sahamalaza sportive lemur lepilemur sahamalazensis in altered habitat. Endanger Species Res 24(3):273–286
Shannon CE, Weaver W (1949) The Mathematical Theory of Communication. University Press, Urbana, Illinois
Shelton N (1985) Logging versus the natural habitat in the survival of tropical forests. Ambio 14(1):39–41
Sundarapandian S, Karoor PJ (2013) Edge effects on plant diversity in tropical forest ecosystems at Periyar Wildlife sanctuary in the western ghats of India. J For Res 24(3):403–418
Tabarelli M, Cardoso da Silva JM, Gascon C (2004) Forest fragmentation, synergisms and the impoverishment of neotropical forests. Biodivers Conserv 13(7):1419–1425
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS, Rödig E, Wiegand T, Huth A (2018) Global patterns of tropical forest fragmentation. Nature 554(7693):519–522
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31(1):79–92
Tripathi OP, Upadhaya K, Tripathi RS, Pandey HN (2010) Diversity, dominance and population structure of tree species along fragment-size gradient of a subtropical humid forest of northeast India. Res J Env Earth Sci 2(2):97–105
Turner IM, Chua KS, Ong JSY, Soong BC, Tan HTW (1996) A century of plant species loss from an isolated fragment of lowland tropical rain forest. Conserv Biol 10(4):1229–1244
Vetter D, Rücker G, Storch I (2013) A meta-analysis of tropical forest edge effects on bird nest predation risk: Edge effects in avian nest predation. Biol Conserv 159:382–395
Vieilledent G, Grinand C, Rakotomalala FA, Ranaivosoa R, Rakotoarijaona JR, Allnutt TF, Achard F (2018) Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biol Conserv 222:189–197
Viña A, Estévez-Varón JV (2019) Effects of fragmentation on tree species diversity in a lowland tropical forest area of the Andean foothills of colombia. Boletín Científico. Centro de Museos Museo de Historia Natural 23(2):109–132
Volampeno MSN, Masters JC, Downs CT (2011) Home range size in the blue-eyed black lemur (Eulemur flavifrons): a comparison between dry and wet seasons. Mamm Biol 76(2):157–164
Waddell EH, Banin LF, Fleiss S, Hill JK, Hughes M, Jelling A, Yeong KL, Ola BB, Sailim AB, Tangah J, Chapman DS (2020) Land-use change and propagule pressure promote plant invasions in tropical rainforest remnants. Landsc Ecol 35(9):1891–1906
Wade TG, Riitters KH, Wickham JD, Jones KB (2003) Distribution and causes of global forest fragmentation. Conserv Ecol 7(2):7
Wassie A, Sterck FJ, Bongers F (2010) Species and structural diversity of church forests in a fragmented Ethiopian Highland landscape. J Veg Sci 21(5):938–948
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York. https://ggplot2.tidyverse.org
Wilmé L, Goodman SM, Ganzhorn JU (2006) Biogeographic evolution of Madagascar’s microendemic biota. Science 312(5776):1063–1065
Wilson EO (1988) In: Wilson B, Peter EO FM, (eds) The current state of biological diversity. National Academy Press, Washington, DC, USA, pp 3–18
Zuidema PA, Sayer JA, Dijkman W (1996) Forest fragmentation and biodiversity: the case for intermediate-sized conservation areas. Env Conserv 23(4):290–297
Acknowledgements
We thank our field-guides Raumiald, Mahatsara, Avitsara, Hervé, Aristide and Jacquis, and our cooks Klariny, Klarice and Marceline for all their help and enthusiasm during our time together in the field. We also express our sincere gratitude to the people of Ambinda, Betsimpoaka, Marovato, Maropapango, Antanandava, Antanambao Manambaro, Antafiabe, Maromandia and Ambolobozo for their logistical assistance. We are grateful to MICET and MNP for their facilitation services and for kindly allowing us to conduct research within the Sahamalaza-Iles Radama National Park (permit numbers 245/19 and 124/22 - MEEF/SG/DGGE/DAPRNE/SCBE.Re). We thank National Geographic Society, Global Wildlife Conservation, Primate Conservation Inc., La Vallée des Singes, Idea Wild, Primate Society of Great Britain, AEECL, the University of Bristol, Bristol Zoological Society, and many private donors for kindly funding this fieldwork. Finally, we thank the Editor and two anonymous reviewers for providing helpful comments that enabled us to improve the quality of this manuscript.
Funding
This study was funded by National Geographic Society, Global Wildlife Conservation, Primate Conservation Inc., La Vallée des Singes, Idea Wild, Primate Society of Great Britain, AEECL, the University of Bristol, and Bristol Zoological Society.
Author information
Authors and Affiliations
Contributions
DH, MH, GM and SC conceived the ideas and designed methodology; DH, HR, NNMA, and CRH collected the data; DH analyzed the data; DH led the writing of the manuscript. All authors contributed to editing the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical note
All research complied with UK Home Office policies when working with animals and all research adhered to the legal requirements of Madagascar. Research in the Sahamalaza-Iles Radama National Park was permitted by Madagascar National Parks (Permit numbers 245/19 and 124/22 – MEEF/SG/DGGE/DAPRNE/SCBE.Re). We consulted the Code of Best Practices for Field Primatology when planning all methods undertaken in this study.
Additional information
Communicated by Arshad Ali.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hending, D., Randrianarison, H., Andriamavosoloarisoa, N.N.M. et al. Forest fragmentation and its associated edge-effects reduce tree species diversity, size, and structural diversity in Madagascar’s transitional forests. Biodivers Conserv 32, 3329–3353 (2023). https://doi.org/10.1007/s10531-023-02657-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02657-0