Abstract
Context
Most tropical forest landscapes are highly fragmented, have habitat patches varying in size and shape, and display different degrees of perturbation, but with high conservation values. Therefore, a major goal of landscape ecology is to discover the actual spatial scale at which landscape composition and structure affect biological processes and biodiversity.
Objective
This study aimed to determine the landscape scale of effect governing the α and β diversities of woody species in a highly fragmented, semideciduous tropical forest.
Methods
We recorded the diversity of woody species in 19 plots scattered across a highly fragmented, semideciduous tropical forest landscape. Then, we used CART algorithms to evaluate the effects of landscape attributes on the α and β diversities of such species across 100 scales (10–1000 m) and tested continuous effects with generalized additive models.
Results
The shape and size of habitat patches in the range of 250–470 m determined α diversity. As for β diversity, nestedness was affected by the shape of forest patches at 510 m, whereas landscape heterogeneity affected species turnover within 100 m buffers.
Conclusion
While a previous study in a similar habitat reported effects at 800 m, the number, size, and shape of habitat patches in the current study accounted for the diversity of the focal plots within 100–510 m. Furthermore, CART effectively screened 100 scales, revealing which landscape attributes correlated the most with the diversity of woody plants. The findings provide valuable guidelines for conservation, restoration efforts, and public policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The loss and fragmentation of tropical forests have drastically modified the configuration of natural landscapes and the functions of ecosystems worldwide (Fischer and Lindenmayer 2007; Taubert et al. 2018). At present, the majority of tropical landscapes are characterized by habitat patches of varied sizes and shapes at different levels of disturbance and connectivity (Gardner et al. 2009; Pozo et al. 2011). Nonetheless, most tropical landscapes harbor a considerable portion of the original biodiversity of plants and some animal groups (Laurance 2008; Wheatley and Johnson 2009). The capacity of the taxa to move through the transformed habitat correlates with several factors, including their functional traits, the preservation of biotic interactions, and the spatial array of habitat patches in the landscape (Ricketts 2001). Therefore, the scale at which landscape properties (e.g., forest cover, the number and distribution of habitat patches, and mean patch size and shape) affect biodiversity also varies among taxa or functional groups (Tishendorf et al. 2003; Falcucci et al. 2007; Fletcher and Fortin 2018; Miguet et al. 2016).
The number of forest patches (a simple estimate of fragmentation) and their shape are landscape attributes that are most frequently associated with the diversity of biological communities. In particular, the shape of habitat patches informs their geometric complexity (Patton 1975), along with the environmental influence of the transformed matrix on the habitat, as edge effects reduce the quality-habitat areas of such patches (Laurance 1991). Edge effects are more significant in patches of complex shapes, such as forest patches along streams, than in forest patches of simpler geometries (e.g., circles). Therefore, the number of forest patches and their shape serve as surrogates of quality-habitat areas and have been shown to have a positive relationship with the α diversity of different taxa (Saunders et al. 1991; Ewers and Didham 2007; Fischer and Lindenmayer 2007; Keppel et al. 2010). Furthermore, landscape heterogeneity and habitat patch connectivity correlate positively with α diversity because highly heterogeneous landscapes tend to harbor species-rich communities rather than homogenous landscapes (Morelli et al. 2013; Perović et al. 2015). In addition, more connected habitat patches facilitate movement (gene flow) across the transformed matrix (McCluskey et al. 2022). Regarding β diversity, recent evidence has shown the significant positive effects of habitat fragmentation and the shape of habitat patches (Nicasio-Arzeta et al. 2021). Such effects can be attributed to different patches harboring different elements of biodiversity and the fact that forest patches of simple geometry are likelier to host both habitat specialists and rare species (Jamoneau et al. 2012).
Among the myriad landscape metrics believed to influence biological processes, a pivotal question in landscape ecology concerns the scale at which landscape properties exert their impact on biological processes and biodiversity—referred to as the “landscape scale of effect.” Historically, most studies have yielded inconclusive evidence regarding the reported scale of effect, primarily due to predefined scales often failing to encompass the actual scale of effect, and because a limited number of scales have been examined so far (Jackson and Fahrig 2015).
In the current study, we investigated the landscape scale of effect in the α and β diversities of woody species of highly fragmented, semideciduous tropical vegetation in the central Gulf of Mexico Lowlands. We tested 100 scales encompassing two orders of magnitude and four landscape metrics of up to seven land use types. We aimed to answer the following questions: (1) What spatial scales and landscape attributes govern the α diversity (species richness, Shannon, and Simpson true diversity estimates) of woody plants in forest remnants within a highly fragmented semideciduous tropical forest? (2) What spatial scales and landscape attributes govern the β diversity components (species turnover and nestedness) of woody plants in forest remnants within a highly fragmented semideciduous tropical forest? and (3) Are β diversity components (species turnover and nestedness) affected at the same spatial scales and by the same landscape attributes?
Theoretically, the scale at which landscape properties affect biological processes and biodiversity is intimately linked to the dispersal limitations of the taxa involved. Natural communities of tropical trees are known to be structures, partly due to the dispersal limitations to gene flow (movement of seeds and pollen), as discussed by Hubbell and Foster (1986) and Chave et al. (2002). Bats and birds are among the main disperser agents of woody species in the tropics (Howe and Smallwood 1982; Fleming and Kress 2011), whereas medium- and small-bodied mammals serve as secondary dispersers (Seidler and Plotkin 2006). Therefore, considering the mobility of birds (Cueto 2006; Jordano et al. 2007; Boscolo and Metzger 2009; Sica et al. 2020) and bats (Ripperger et al. 2015), along with previous reports on the landscape scale of effect in the diversity of tropical plant communities (Nicazio-Arzeta et al. 2021), we expect that landscape attributes will have significant effects on the diversity of woody plants at scales between 100 and 800 m. Specifically, because the area of habitat patches correlates positively with species richness (Cayuela 2006; Wies et al. 2021), we predict that α diversity would increase with the number and area of forest patches, as well as in landscapes with forest patches with simple geometries (e.g., circles). These specific patches have a smaller perimeter-to-area ratio than geometrically complex patches, thus maximizing the quality habitat at the core of the patches (Ewers and Didham 2007). We also predict that the nestedness component of the β diversity of focal plots is likely to increase with landscape heterogeneity (Nicazio-Arzeta et al. 2021). This is because heterogeneous landscapes tend to harbor subsets of the community of woody plants in remnant forest patches (Cook et al. 2005; Jamoneau et al. 2012). In addition, focal plots located within landscapes featuring predominantly geometrically simple forest patches (e.g., square-like) are likely to host habitat specialist taxa and potentially rare species, thus making significant contributions to β diversity through species turnover (Jamoneau et al. 2012). Due to the reduced likelihood of habitat-specialist species moving through the transformed matrix, we also expect that the landscape scale of effect on the turnover component of β diversity will occur at shorter spatial scales than the nestedness component, wherein habitat-generalist species are the primary contributors.
Methods
Study site
The study site was in the coastal plains of the Gulf of Mexico within the municipality of Tlalixcoyan in Veracruz State (extent, 14Q; longitude = 770,354.4 and 818,914.4; latitude = 2,058,615 and 2,097,975, Fig. 1). The municipality of Tlalixcoyan has an area of 974.71 km2 and an average elevation of 10 m above sea level. The weather is classified as Aw (i.e., tropical with summer rains) (Kottek et al. 2006), with an average mean temperature of 25.9 °C. The accumulated annual precipitation averages 1,400 mm. The amount of rainfall peaks between June and October, while the dry season generally occurs from November to May (García 1988). The soil in the municipality is vertisol, which is a type of fertile soil with a highly expandable clay content, resulting in cracks that open and close due to changes in humidity (FAO 2014).
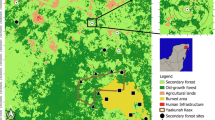
A Location of the study area in the municipality of Tlalixcoyan, Veracruz. B Classified image of the study site showing the location of 20 plots and 1000 m radii buffers. C Example of a focal plot with 10 buffers spaced every 100 m for illustration purposes; the study considered 100 buffers spaced every 10 m
Given that most natural vegetation in the municipality has been transformed, it is not easy to establish the original type of vegetation in the area (López and Dirzo 2007). However, some vegetation remnants and old secondary growth in abandoned lands reveal the structure of evergreen and semideciduous tropical forests, with canopy trees as high as 10–25 m. The following are representative canopy species in the area: Ficus spp., Tapirira mexicana, Cordia stellifera, Sabal mexicana, and Cedrela odorata. In the lower strata, Coccoloba barbadensis, Pithecellobium dulce, and some species of Vachellia and Randia were common (Juárez-Fragoso et al. 2017). According to the Instituto Nacional de Estadística y Geografia (INEGI 2016), land use in the municipality includes pasture (62%), agriculture (34%), secondary tropical forest (2.5%), water bodies (0.8%), urban centers (0.6%), and other tropical vegetation forms, such as savanna (0.1%).
Study design
This study was designed according to a classification of land-use types in the municipality of Tlalixcoyan (Fig. 1). The supervised classification was based on the maximum likelihood method applied over a Sentinel 2B satellite image collected by the sentinel mission of the European Space Agency with a 10 m resolution, less than 10% cloudiness, dated July 2020 (ESA 2015). The classified image shows seven land use types: (1) semideciduous tropical forest, (2) water bodies, (3) crops, (4) pastures, (5) pastures with small forest patches and isolated trees, (6) savannas, and (7) urban areas. The overall classification accuracy was 76% (Juárez-Fragoso et al. 2023). For this study, we defined 19 sampling sites across the classified image. All sites were immersed in forest vegetation, although the sizes of the forest fragments containing the focal plot varied.
The mean (± standard deviation, SD) distance between the focal plots was 19,561 ± 9,490 m, and the nearest pair of focal plots was 1,337 m apart. We generated 100 buffers around each focal plot from a radius of 10–1,000 m (i.e., every 10 m). We used 10 m increments in the radius of buffers because the classified image resolution was 10 × 10 m, and the study site had a long history of transformation characterized by ca. 15% of forest patches with an area less than 1000 m2 (Juárez-Fragoso et al. 2023). Thus, setting the 10 m radius increments allowed the gradual inclusion of tiny forest patches, which in many other studies were often excluded because of the selected grain size and the focus on medium to large forest patches (Zhang et al. 2020; Weis et al. 2021). In our study, buffers around a pair of focal plots overlapped when the radius was above 660 m. The maximum overlap (1000 m radius) was 21% of the buffer area, and the overlap at the upper limit of the predicted scale of effect (800 m) was less than 8% (Fig. S1). However, this fact is a minor concern because the overlap does not affect the response variable (e.g., plant diversity estimated in the focal plots), and computer simulation studies have shown that overlapping buffers do not affect the autocorrelation patterns of landscape metrics (Zuckerberg et al. 2012).
For each buffer size (10–1000 m radius), we evaluated one metric at the landscape level, as well as (1) landscape heterogeneity (the Shannon Index) and three metrics at the class level; (2) the number of habitat patches; (3) the mean area of habitat patches; and (4) the overall shape of the habitat patches (see Table S1 for the detailed calculation). The numerical expression of the shape of habitat patches was based on the ratio between the perimeter of a circle and the perimeter of a habitat patch of equal areas. In this way, values close to 1 indicate simple geometries, while values close to zero indicate complex geometries (i.e., highly irregular patches). The selected landscape metrics have been described in the literature as determiners of seed and seedling diversity (Hernández-Ruedas et al. 2018; San-José et al. 2020; Nicasio-Arzeta et al. 2021) and include animal populations, such as birds (Villard et al. 1999), bats (Ethier and Fahrig 2011), and terrestrial mammals (Thornton et al. 2011). The maximum buffer size analyzed in this study complied with a previous study (Jackson and Fahring 2012), which proposed that the scale of the effect of ecological processes should be investigated in buffers no smaller than 0.3–0.5 times the expected dispersion distance.
For each buffer size, the landscape units were defined according to the rule of four neighbors (Rook’s case contiguity) (Lloyd 2010). All metrics were calculated by using the spatial analysis packages Raster 2.9–5 (Hijmans and Elith 2019) and Terra 1.5–17 (Hijmans 2023), as well as following the formulas in the R Landscapes package (Hesselbarth et al. 2019). We calculated all metrics using the programming R language environment.
Field data collection
In the field, we marked a 10 × 10 m plot in each site and recorded all woody individuals with a diameter at breast height (DBH) equal to or greater than 2.5 cm. The size of the plots allowed us to minimize the so-called edge effects (30–50 m from the border) (Benitez-Malvido et al. 2018), particularly for plots in small forest patches. Based on our research group’s previous experience in the study site, the 10 × 10 m plots are sufficient to record the local composition of woody species with DBH ≥ 2.5 (c.f., López and Dirzo 2007; Juárez-Fragoso et al. 2017). All registered woody individuals were identified in the field to the species level, taking as references a floristic list (Juárez-Fragoso et al. 2017) and the ecological works carried out on the study site by our research group (Hernández-Hernández 2010; López and Dirzo 2007).
Data analysis
Diversity
To assess the completeness of the recorded sample of woody species, we used the sample coverage estimate proposed by Chao and Shen (2010). Sample coverage represents the proportion of individuals in a community belonging to any species included in the sample. The closer the sample coverage is to one, the more significant the fraction of the actual community represented in the model (Chao and Jost 2012).
For each 10 × 10 m plot, we calculated the species richness (0D), and the effective number of equivalent species 1D (eH, Shannon), and 2D (the inverse of Simpson, 1/Simpson) representing the effective number of species (Jost 2006). We also included the Shannon evenness index, as suggested by Cao and Hawkins (2019). Next, we calculated β diversity by partitioning the contribution of species turnover (i.e., changes in species composition with balanced abundances) and nestedness (i.e., abundance gradients of shared species) (Baselga 2010). Then, based on the distance matrix, we averaged the β diversity of each focal plot compared with all the other plots. To obtain the α diversity (Shannon and Simpson) and β diversity (Sørensen similarity) estimates, we used the R packages Vegan (Oksanen et al. 2020) and betapart (Baselga et al. 2021), respectively. All analyses were performed in R 4.1.1 (R Core Team 2021).
Scale of effect
We used machine learning classification and regression trees (CART) algorithms to determine the scale at which the landscape attributes showed the highest correlation with the diversity metrics (α and β). CART creates a recursive partition of a single response variable (e.g., α diversity) based on explanatory variables (i.e., landscape attributes estimated in buffers from 10 to 1,000 m in radii scaled up every 10 m). Although the procedure is computationally exhaustive, each partition obtained is the best possible partition based on the set or subset of data (De’Áth and Fabricius 2000). For each diversity metric, the response variable, we used a CART model that tested the whole set of predictor variables (three metrics of up to seven and-use types and one at buffer level, landscape diversity (Shannon), and all metrics estimated in 100 scales). The actual number of predictor variables was 769. CARTs are nonparametric models with no prior assumptions regarding data distribution and are robust enough to handle continuous linear/nonlinear relationships and threshold responses (Kallimanis et al. 2007). In addition, there is no penalization or limitation of predictor variables because the procedure is computationally exhaustive, and every predictor variable is dealt with individually in each partition. The sample size in our study (N = 19) limited the number of potential partitions, as no partition can have less than five observations (Breiman et al. 1984; Loh 2014). Then, we used a Monte Carlo test (Roff 2006) to establish whether the division of the CART model was statistically significant. The response variable was randomly shuffled and fitted to the model (random CART: rCART) using only those variables selected by the initial CART model. We repeated this procedure 1,000 times and evaluated whether the divisions in each of the rCART models reduced the residual deviance in a greater or an equal amount as in the initial model. This was done with the function tree in the R package of the same name (Therneau and Atkinson 2022) in R 4.1.1 (R Core Team 2021).
Next, we tested whether the diversity metrics responded in a linear/nonlinear way to the landscape metrics at the scales defined by the CART models. To this end, we used general additive models (GAM) to describe the potentially linear and nonlinear relationships (De’Ath and Fabricius 2000) of the diversity metrics with the landscape metrics at the scales defined by the CART models. In all cases, the GAM was fitted with a thin plate spline smoother (k = 3), which allowed linear and simple nonlinear responses, such as growth reaching a plateau. The GAM were fitted in the R package mgcv (Wood 2011) in R 4.1.1 (R Core Team 2021).
Results
Floristic richness
In the 19 plots, we registered 427 individuals, 67 species, and 60 genera of woody species with DBH ≥ 2.5 cm throughout Tlalixcoyan (Table S2). The most representative families were Leguminosae (15 species, 22%), Rubiaceae (six species, 9%), and Moraceae (five species, 7%), while the most representative genera were Ficus and Randia, with four species each. In addition, the most abundant species were Guazuma ulmifolia, with 52 individuals; Coccoloba barbadensis, with 48 individuals; and Parmentiera aculeata, with 32 individuals. The sample coverage estimate was 0.72 (i.e., the sampled species represented 72% of the woody stem, with DAP ≥ 2.5 at the study site). In the plots, the average mean species richness was 8.16 species, a standard deviation of 2.97 species, and a range between 3 and 14 species of woody plants with DAP ≥ 2.5 cm.
Species richness
The CART model accounted for over 75% (P = 0.007) of the total deviance in the first division (Fig. 2A, B). On average (± standard error), the species richness of focal plots (10.86 ± 0.91 species, n = 7) was 42.3% higher than the mean species of plots in landscapes with more irregular forest patches (7.6 ± 0.91 species, n = 12) at a scale of 470 m. The second division of the CART model occurred in plots with fewer regular forest patches (a reduction of 13% of the total deviance), but this was not significant (P = 0.058). The GAM accounted for 40.1% of the observed deviance, indicating that species richness was positively correlated with the shape of forest patches (F = 7.04, d.f. = 2, P = 0.004) on a scale of 470 m (Fig. 2C).
The landscape scale of effect on the species richness of the woody species of a highly fragmented subcaducifolious tropical forest. A Classification and regression tree (CART) showing the main divisions of the dataset caused by the shape of forest patches in 470 m radii buffers and a secondary division caused by the number of forest patches within 340 m. B Summarized species richness, mean ± standard error in each branch of the CART model (vertical correspondence); n = number of observations in each branch. In A and B, black-colored lines and symbols correspond to significant branches of the CART model, while those in gray color correspond to branches that likely occurred by chance. C The continuous effect of the mean shape of forest patches in 470 m radii buffers on species richness based on the general additive model (GAM)
Diversity and evenness
The CART models showed similar responses to the exponentials of Shannon’s and Simpson’s inverse diversity indices (Figs. 3A, B and S2A, B, respectively). The first division of the models accounted for 80.3% (P = 0.004) and 86.9% (P = 0.005) of the total deviance of the eH and 1/D, respectively. At a scale with a radius of 320 m, the respective diversities of the focal plots (eH = 8.14 ± 0.95; 1/D = 6.9 ± 0.86; n = 8) were 70 and 85% higher when the landscape included no fewer than three forest patches than when the landscape had fewer forest patches (eH = 4.81 ± 0.81; 1/D = 3.74 ± 0.59; n = 11). The second division of the CART models accounted for an additional 10.3% in both models, but the division was likely to occur by chance (P = 0.091 and P = 0.073 for eH and 1/D, respectively).
The landscape scale of effect on the diversity (eH, Shannon) and evenness of the woody species of a highly fragmented subcaducifolious tropical forest. A and D CART model showing the main divisions of the dataset caused by the number of patches of subcaducifolious tropical forest in 320 m radii buffers and the shape of forest patches in 600 m radii buffers for eH and evenness, respectively. The diagram also shows secondary divisions caused by the shape of forest patches and the extent of agricultural land for eH and evenness, respectively. B and E Summarized diversity and evenness, mean ± standard error in each branch of the CART models (vertical correspondence); n = number of observations in each branch. In A, B, D, and E, black-colored lines and symbols correspond to significant branches of the CART models, while those in gray color correspond to branches that likely occurred by chance. C and F The continuous effect of the number of forest patches in 320 m radii buffers and the mean shape of forest patches in 600 m radii buffers on diversity and evenness, respectively, based on the GAM
Regarding the exponentials of Shannon’s and Simpson’s reciprocal indexes, the GAM accounted for 55.6 and 56.2% of the total deviance, respectively. Furthermore, the diversity increased along with an increase in the number of forest patches in the landscape (F = 3.55, d.f. = 2, P = 0.029 for the Shannon index and F = 4.15, d.f. = 2, P = 0.026 for the Simpson index) (Figs. 3C and S2C).
For evenness, the CART model reduced total deviance by 84.7% in the first division (P = 0.01) (Fig. 3D, E). The community of woody species was more evenly composed (0.91 ± 0.02; n = 8) in landscapes (660 m) with forest patches of regular shape (shape = 0.66) than in those communities (0.81 ± 0.03) of plots in landscapes with less regular forest patches (shape = 0.6). Meanwhile, the second division of the CART model reduced deviance by an additional 10.3%, but the division likely occurred by chance (P = 0.064). For evenness, the GAM accounted for 30.6% of the total deviance, revealing that evenness increased as forest patches on the landscape had simpler geometric forms (Fig. 3F).
Beta diversity
Total β diversity ranged from 0.71 to 0.93, with an average of 0.8. The first division of the CART model on the nestedness component of the β diversity (unidirectional abundance gradients, βgra) reduced deviance by 87%. Plots in landscapes (buffer 510 m) with irregularly shaped forest patches of (shape < 0.51) had the highest values of nestedness (βgra = 0.084 ± 0.008, Fig. 4A, B), while nestedness was 55.6% lower (βgra = 0.035 ± 0.006) in plots where the landscape included regularly shaped forest patches (shape > 0.65). Although the second division of the CART model reduced deviance by an additional 21.8%, this division likely occurred by chance (P = 0.173). Meanwhile, the GAM accounted for 36.3% of the total deviance and showed that βgra correlated negatively with the shape of forest patches (F = 4.45, d.f. = 2, P = 0.0054) (Fig. 4C).
The landscape scale of effect on β diversity components (nestedness and turnover) of the woody species of a highly fragmented subcaducifolious tropical forest. A and D CART model showing the main divisions of the dataset caused by the mean shape of forest patches in 510 m radii buffers and the heterogeneity of the landscape (Shannon diversity) in 100 radii buffers for nestedness and species turnover, respectively. The diagram also shows the secondary divisions caused by the area of forest patches for both components of the β diversity. B and E Corresponding summarized nestedness and species turnover (mean ± standard error) in each branch of the CART models (vertical correspondence); n = number of observations in each branch. In A, B, D, and E, black-colored lines and symbols correspond to significant branches of the CART models, while those in gray color correspond to branches that likely occurred by chance. C and F The continuous effect of the mean shape of forest patches in 510 m radii buffers and the diversity of the landscape (Shannon) in 100 m radii buffers on nestedness and species turnover, respectively, based on the GAM
Regarding species turnover (balanced variation in abundance, βbal) (Fig. 4D, E), the first division of the CART model was statistically significant (P < 0.001), accounted for 93% of the total deviance, and revealed that plots in heterogeneous landscapes (i.e., the Shannon index at the scale of 100 m, H′ = 1.8) had higher species turnover (βbal = 0.85 ± 0.018) than those plots (βbal = 0.70 ± 0.023) in less variegated landscapes (H′ = 1.2). The GAM also showed that βto correlated positively with landscape heterogeneity, accounting for 27.7% of the total deviance (F = 2.77, d.f. = 2, p = 0.019) (Fig. 4F).
Discussion
In this study, we assessed the corresponding relationships between landscape attributes and the α and β diversities of woody species in a highly modified habitat covered by patches of semideciduous tropical forest and other tropical vegetation variants (López and Dirzo 2007; Juárez-Fragoso et al. 2017). In general, the scale at which the landscape configuration defined the diversity of woody species in the sampling plots varied from 100 to 660 m, confirming our prediction that significant effects of the landscape attributes would be observed at scales between 300 and 800 m. In addition, the landscape scale of effect for species richness, Shannon and Simpson diversities, and evenness occurred between 320 and 660 m. In accordance with our predictions, the scale of effect for the turnover component of the β diversity (100 m) was over five times shorter than the observed scale of effect on the nestedness component (510 m).
Overall, the observed scale of effect was smaller than previously reported for woody plants in other tropical forests, which was approximately 800 m (Nicasio-Arzeta et al. 2021). Several factors may have contributed to this discrepancy in the scale of effect observed between the current study and other studies done in the tropical forests of Mexico. First, regarding our methodology, we assessed the scale of effect across two orders of magnitude, buffers from 10 and up to 1,000 m in radius with 10 m increments. In contrast, Nicasio-Arzeta et al. (2021) analyzed buffers that were 13 scales across one order of magnitude (from 300 and up to 1500 m with 100 m increments). The ten-fold buffer size increment (100) compared with our study (10 m) limited the number of scales (300–600 or 700) within the range of scales of effects observed in this study and restricted the opportunity to find effects in the same range. Nonetheless, the reported scale of effect of 800 m is only 21% higher than the uppermost scale of effect observed in our study (660 m for evenness). On the other hand, some effects were observed at 100 m (βbal), a scale not considered by Nicasio-Arzeta et al. (2021).
Second, our study site harbors remnants of a semideciduous tropical forest, with over 80% of the vegetation cover transformed into agricultural and cattle ranching practices (Juarez-Fragoso et al. 2023) across an area with a size of 970 km2. In contrast, Nicasio-Arzeta et al. (2021) analyzed a tropical rainforest landscape with up to 60% of land cover transformed into farming and cattle ranching (Carabias et al. 2015) in an area less than 50 km2. Thus, the observed differences in the scale of effect may depend on the type of landscape. Nonetheless, as Jackson and Fahrig (2015) pointed out, a low number of scales leads to imprecise results, while there are no conceptual limitations to evaluating a large set of scales. Therefore, our analysis of 100 scales across two orders of magnitude may have produced more accurate results than previous studies. As such, we highlight the need to replicate our research in other tropical forest landscapes to disentangle methodological issues, landscape history and transformation, and vegetation cover types. To this end, the R code in the supplementary material will significantly benefit those willing to test 100 scales (or more).
Third, due to the extent of transformation of the vegetation in our study site, most of the forest remnants are of secondary semideciduous tropical forest, with few well-conserved original vegetation cover, which contrasts with other studies wherein the landscape still harbors significant patches of relatively well-conserved tropical forests (San-José et al. 2019, 2020; Nicasio-Arzeta et al. 2021). These characteristics may have narrowed the scale of effect compared with those observed in studies performed in landscapes with well-conserved forest patches, mainly because the vegetation at our study site had gradually regenerated by germplasm from the few neighboring forest patches and isolated trees and palms (Villicaña-Hernández et al. 2020). The observed scale of effect (100–660 m) may indicate dispersal limitations (van Breugel et al. 2019), which is congruent with copious evidence of landscape ecology indicating that habitat transformation and fragmentation limit the movement of individuals between forest patches. These affect, aside from ecological interactions (Debinski and Holt 2000), the gene flow among populations (Kwak et al. 1998; Sork et al. 1999; Coulon et al. 2004; Wang et al. 2011; Jackson and Fahrig 2014) that, in the case of plants, occurs in the form of seed dispersal and pollen movement. However, some studies have shown that the impacts of fragmentation on gene flow (mobility of individuals and gametes) are low or even null in highly mobile organisms (McCulloch et al. 2013; Jackson and Fahrig 2014).
Fourth, our study considered seven land-use categories (i.e., semideciduous tropical forests, pastures with scattered wooden vegetation, pure pastures, savanna, agricultural land, water bodies, and urban centers). Three metrics were calculated for each type of land use, and one metric characterized the landscape (i.e., landscape heterogeneity). All metrics were assessed every 10 m from the 10 to 1,000 m radius buffers. Thus, we tested over 500 predictor variables (landscape attributes on different scales) in the CART models. Consistent with previous studies, the relevant metrics used to account for the diversity of woody species in the focal plots included shape, size, the number of forest patches in the landscape, and overall landscape heterogeneity (the Shannon index) at scales ranging from 100 to 660 m. Overall, our findings align with those of Hu et al. (2011), who observed that large and symmetrical islands in Thousand Island Lake in southeast China harbored a high diversity of plants (Wilson et al. 2020). In addition, our findings are in line with those of other studies that analyzed the effects of landscape configuration on the α and β diversities of plants (Arroyo-Rodríguez et al. 2009; Nicazio-Arzeta et al. 2021), birds (Villard et al. 1999), and mammals (Arroyo-Rodríguez and Días 2010; Saldívar-Burrola et al. 2022).
A positive relationship between the shape of forest patches and species richness has been observed in tropical forests (Stanton et al. 2013). Meanwhile, forest edges are considered challenging environments for mature forest species (Hill and Curran 2003). For example, temperature and light availability increase toward forest edges, whereas air and soil humidity decrease (Murcia 1995). Therefore, tree mortality is usually higher on the edges than on the interiors of forest patches (Laurence et al. 2000). According to a simple geometric principle, in symmetrical patches (e.g., circles), the ratio of the perimeter over the squared root of the area equals a constant (2π). The percentage increases rapidly as irregularity increases (Feder 1988). Therefore, regularly shaped forest patches are likelier to harbor species-rich assembles than irregular forest patches, where environmental filters may operate along extensive borders, thus increasing overall mortality while favoring the survival of some light-demanding species (Poorter et al. 2008).
In addition, our results showed that α diversity increased along with the increase in the number and area of forest patches, which was also observed in previous studies (Fahrig 2003; Watson et al. 2005; Laurance 2008). In line with our results, Nicasio-Arzeta et al. (2021) also found that the species richness of seedlings in tropical rainforests increased when the landscape included large and highly aggregated forest patches. They concluded that connectivity was one of the leading landscape elements that promoted high species richness. In contrast, we observed no effect of overall landscape fragmentation, which complemented connectivity.
Similar to α diversity, the nestedness component of β diversity was influenced by the shape and size of the forest patches in the landscape. Nestedness was higher when irregularly shaped patches predominated in the landscape at a scale of 510 m. In contrast, regarding species turnover, the scale of effect occurred at 100 m and was determined by the heterogeneity of the landscape. Thus, species turnover increased as landscape heterogeneity increased. This finding supports the idea that substantial dispersal limitations exist in highly anthropized landscapes, such as those investigated in our study (Jacquemyn et al. 2001). In such landscapes, germplasm sources are primarily found on nearby vegetation remnants, and a variegated neighbor can potentially support different species (Arroyo‐Rodríguez et al. 2017). This finding also emphasizes the importance of secondary patches of tropical forests as reservoirs of biodiversity (Arroyo‐Rodríguez et al. 2017). In particular, we detected 67 species in 60 genera in our focal plots. Despite the predominance of species characteristics of early successional stages, such as Guazuma ulmifolia, Vachellia cornigera, Piscidia piscipula, Sabal mexicana, and Coccoloba barbadensis, we observed some species that are also typical in mature forests, such as Spondias mombin, Machaerium lindenianum, Cedrella odorata, and Malpighia glabra.
The role of small patches of secondary forests as reservoirs of woody species diversity was also observed in Hernández-Ruedas et al. (2014), who reported that a set of managed forest patches harbored as much plant diversity as mature forest plots in southern México. Thus, our findings demonstrated that secondary forests can also be significant reservoirs of plant communities (Laurence et al. 2000). Moreover, our results align with those of Chazdon et al. (2009) and Martinez-Ramos et al. (2016), who proposed that, in some regions, the conservation of small and managed forest patches could be more effective in preserving the diversity of vegetation and, potentially, some groups of fauna. As described above, very little primary vegetation was identified at the study site. Therefore, future conservation efforts in the region must focus on small secondary forest patches, as well as restoration, to increase landscape connectivity (Juárez-Fragoso et al. 2023).
Conclusion
The results of this study showed the spatial distributions of semideciduous tropical forest patches in terms of the shape, size, and number of forest patches. These results helped us understand the spatial distributions of the α and β diversities of woody species in a highly anthropized region. We analyzed 100 scales (10–1,000) with 10 m increments to determine the scale of effect, which was a significant improvement over previous studies that used only a few broadly spaced scales with little biological justification. However, our study relied on the traditional approach of arbitrarily defining a maximum buffer size and searching for the scale of effect within this boundary. Nonetheless, our methodology enabled us to detect significant scale effects halfway through the tested scales, thereby suggesting that our results were not limited or biased by the a priori definition of minimum and maximum buffer sizes. However, it remains to be determined whether the hierarchy of effects detected in this study (between 200 and 660 m) could be applied to other systems where remnants of conserved forests occur. Finally, although secondary forest patches predominantly characterized our study site, our findings provide valuable guidelines for conservation and restoration in other study sites and the formulation of public policies to encourage landowners to practice conservation.
References
Arroyo-Rodríguez V, Dias PAD (2010) Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am J Primatol 72:1–16
Arroyo-Rodríguez V, Pineda E, Escobar F (2009) Value of small patches in conserving plant-species diversity in highly fragmented rainforests. Conserv Biol 23:729–739
Arroyo-Rodríguez V, Melo FP, Martínez-Ramos M, Bongers F, Chazdon RL, Meave JA, Natalia Norden N, Santos BA, Leal IR, Tabarelli M (2017) Multiple successional pathways in human-modified tropical landscapes: new insights from forest succession, forest fragmentation and landscape ecology research. Biol Rev 92:326–340
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19:134–143
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F, Logez M (2021) betapart: partitioning beta diversity into turnover and nestedness components. R package version 1.5. 4. https://cran.r-project.org/package=betapart
Benitez-Malvido J, Lázaro A, Ferraz IDK (2018) Effect of distance to edge and edge interaction on seedling regeneration and biotic damage in tropical rainforest fragments: a long-term experiment. J Ecol 106:2204–2217
Boscolo D, Metzger JP (2009) Is bird incidence in Atlantic forest fragments influenced by landscape patterns at multiple scales? Landscape Ecol 24:907–918
Breiman L, Friedman J, Stone CJ, Olshen RA (1984) Classification and regression trees. Routledge, New York, p 368
Cao Y, Hawkins CP (2019) Weighting effective number of species measures by abundance weakens detection of diversity responses. J Appl Ecol 56:1200–1209
Carabias J, De la Maza J, Cadena R (2015) Conservación y desarrollo sustentable en la Selva Lacandona: 25 años de actividades y experiencia. Natura y Ecosistemas Mexicanos, Mexico City, p 694
Cayuela L (2006) Deforestación y fragmentación de bosques tropicales montanos en los Altos de Chiapas, México. Efectos sobre la diversidad de árboles. Ecosistemas 15:182–189
Chao A, Shen TJ (2010) User’s guide for program SPADE (Species prediction and diversity estimation). http://chao.stat.nthu.edu.tw/wordpress/wp-content/uploads/software/SPADE_UserGuide(20160621).pdf. Accessed Oct 2022
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547
Chave J, Muller-Landau HC, Levin SA (2002) Comparing classical community models: theoretical consequences for patterns of diversity. Am Nat 159:1–23
Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, Lamb D, Stork NE, Miller SE (2009) The potential for species conservation in tropical secondary forests. Conserv Biol 23:1406–1417
Cook WM, Yao J, Foster BL, Holt RD, Patrick LB (2005) Secondary succession in an experimentally fragmented landscape: community patterns across space and time. Ecology 86:1267–1279
Coulon A, Cosson JF, Angibault JM, Cargnelutti B, Galan M, Morellet N, Petit E, Aulagnier S, Hewison AJM (2004) Landscape connectivity influences gene flow in a roe deer population inhabiting a fragmented landscape: an individual–based approach. Mol Ecol 13:2841–2850
Cueto VR (2006) Escalas en ecología: su importancia para el estudio de la selección de hábitat en aves. El Hornero 21:1–13
De’Ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355
ESA (European Space Agency) (2015) www.esa.int/ (Accessed July 2020)
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landscape Ecol 26:865–876
Ewers RM, Didham RK (2007) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv Biol 21:926–936
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Ecol S 34:487–515
Falcucci A, Maiorano L, Boitani L (2007) Changes in land-use/land–cover patterns in Italy and their implications for biodiversity conservation. Landscape Ecol 22:617–631
FAO (Food and Agricultural Organization of the United Nations) (2014) World reference base for soil resources. International soil classification system or naming soils and creating legends for soil maps. FAO, Rome, p 192
Feder J (1988) The perimeter-area relation. In: Feder J (ed) Fractals: physics of solids and liquids. Springer, Boston, pp 200–211
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Global Ecol Biogeogr 16:265–280
Fleming TH, Kress WJ (2011) A brief history of fruits and frugivores. Acta Oecol 37:521–530
Fletcher R, Fortin M (2018) Spatial ecology and conservation modeling. Springer, Cham, p 541
García E (1988) Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana). Instituto de Geografía–UNAM, México
Gardner TA, Barlow J, Chazdon R, Ewers RM, Harvey CA, Peres CA (2009) Prospects for tropical forest biodiversity in a human-modified world. Ecol Lett 12:561–582
Hernández-Hernández D (2010) Caracterización del potencial de regeneración de la vegetación del palmar de Sabal mexicana y su correlación con algunos factores edáficos. Master’s Thesis. Instituto de Ecología. Mexico.
Hernández-Ruedas MA, Arroyo-Rodríguez V, Meave JA, Martínez-Ramos M, Ibarra-Manríquez G, Martínez E, Jamangapé G, Melo PFL, Santos BA (2014) Conserving tropical tree diversity and forest structure: the value of small rainforest patches in moderately–managed landscapes. PLoS ONE 9:e98931
Hernández-Ruedas MA, Arroyo-Rodríguez V, Morante-Filho JC, Meave JA, Martínez-Ramos M (2018) Fragmentation and matrix contrast favor understory plants through negative cascading effects on a strong competitor palm. Ecol Appl 28:1546–1553
Hesselbarth MHK, Sciaini M, With KA, Wiegand K, Nowosad J (2019) Landscape metrics: an open–source R tool to calculate landscape metrics. Ecography 42:1648–1657
Hijmans RJ, Elith J (2019) Spatial distribution models. https://rspatial.org/sdm/. Accessed Dec 2022
Hijmans R (2023) Terra: Spatial data analysis. R package version 1.7–39. https://CRAN.R-project.org/package=terra
Hill JL, Curran PJ (2003) Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. J Biogeogr 30:1391–1403
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Hu G, Feeley KJ, Wu J, Xu G, Yu M (2011) Determinants of plant species richness and patterns of nestedness in fragmented landscapes: evidence from land-bridge islands. Landscape Ecol 26:1405–1417
Hubbell SP, Foster RB (1986) Biology, chance, and history and the structure of tropical rain forest tree communities. In: Cody ML, Diamond J (eds) Community ecology. Harper & Row, New York, pp 314–329
INEGI (Instituto Nacional de Estadística y Geografía) (2016) Uso del suelo y vegetación, escala 1: 250,000, serie VI
Jackson HB, Fahrig L (2012) What size is a biologically relevant landscape? Landscape Ecol 27:929–941
Jackson ND, Fahrig L (2014) Landscape context affects genetic diversity at a much larger spatial extent than population abundance. Ecology 95:871–881
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Global Ecol Biogeogr 24:52–63
Jacquemyn H, Butaye J, Hermy M (2001) Forest plant species richness in small, fragmented mixed deciduous forest patches: the role of area, time and dispersal limitation. J Biogeogr 28:801–812
Jamoneau A, Chabrerie C-K, Decocq W (2012) Fragmentation alters beta-diversity patterns of habitat specialists within forest metacommunities. Ecography 35:124–133
Jordano P, Garcia C, Godoy JA, Garcia-Castano JL (2007) Differential contribution of frugivores to complex seed dispersal patterns. Proc Natl Acad Sci USA 104:3278–3282
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Juárez-Fragoso MA, López-Acosta JC, Velázquez-Rosas N (2017) Contribución al conocimiento ecológico y florístico de un palmar dominado por Sabal mexicana Mart. al sur del estado de Veracruz. México Polibotan 44:51–66
Juárez-Fragoso MA, Perroni-Ventura Y, Wesley D, Gómez-Díaz JA, Hernández-Gómez IU, Guevara R (2023) Identificando zonas potenciales de conservación en el trópico mexicano usando descriptores del paisaje. Madera y Bosques 29:e2922507
Kallimanis AS, Ragia V, Sgardelis SP, Pantis JD (2007) Using regression trees to predict alpha diversity based upon geographical and habitat characteristics. Biodivers Conserv 16:3863–3876
Keppel G, Buckley YM, Possingham HP (2010) Drivers of lowland rain forest community assembly, species diversity and forest structure on islands in the tropical South Pacific. J Ecol 98:87–95
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen–Geiger climate classification updated. Meteorol Z 15:259–263
Kwak MM, Velterop O, van Andel J (1998) Pollen and gene flow in fragmented habitats. Appl Veg Sci 1:37–54
Laurance WF (1991) Edge effects in tropical forest fragments: application of a model for the design of nature reserves. Biol Conserv 57:205–219
Laurance WF (2008) Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biol Conserv 141:1731–1744
Laurance WF, Delamônica P, Laurance S, Vasconcellos HL, Lovejoy TE (2000) Rainforest fragmentation kills big trees. Nature 404:836
Lloyd C (2010) Spatial data analysis: an introduction for GIS users. Oxford University Press, Oxford, p 224
Loh WY (2014) Fifty years of classification and regression trees. Int Stat Rev 82:329–348
López JC, Dirzo R (2007) Floristic diversity of Sabal palmetto woodland: an endemic and endangered vegetation type from Mexico. Biodivers Conserv 16:807–826
Martínez-Ramos M, Ortiz-Rodríguez IA, Piñero D, Dirzo R, Sarukhán J (2016) Anthropogenic disturbances jeopardize biodiversity conservation within tropical rainforest reserves. Proc Nat Acad Sci USA 113:5323–5328
McCluskey EM, Lulla V, Peterman WE, Stryszowska-Hill KM, Denton RD, Fries AC, Langen TA, Johnson G, Mockford SW, Gonser RA (2022) Linking genetic structure, landscape genetics, and species distribution modeling for regional conservation of a threatened freshwater turtle. Landscape Ecol 37:1017–1034
McCulloch ES, Tello S, Whitehead J, Rolón-Mendoza CMJ, Maldonado-Rodríguez RMCD, Stevens D (2013) Fragmentation of atlantic forest has not affected gene flow of a widespread seed–dispersing bat. Mol Ecol 22:4619–4633
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landscape Ecol 31:1177–1194
Morelli F, Pruscini F, Santolini R, Perna P, Benedetti Y, Sisti D (2013) Landscape heterogeneity metrics as indicators of bird diversity: determining the optimal spatial scales in different landscapes. Ecol Indic 34:372–379
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Nicasio-Arzeta S, Zermeño-Hernández IE, Maza-Villalobos S, Benitez-Malvido J (2021) Landscape structure shapes the diversity of tree seedlings at multiple spatial scales in a fragmented tropical rainforest. PLoS ONE 16:e0253284
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2020) Vegan: community ecology package. R Package Version 2:5–7
Patton DR (1975) A diversity index for quantifying habitat “edge.” Wildlife Soc B 3:171–173
Perović D, Gámez-Virués S, Börschig C, Klein AM, Krauss J, Steckel J, Rothenwöhrer C, Erasmi S, Tscharntke T, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríquez G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, Muller-Landau HC, Peña-Claros M, Webb CO, Wright IJ (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89:1908–1920
Pozo-Montuy G, Serio-Silva JC, Bonilla-Sánchez YM (2011) Influence of the landscape matrix on the abundance of arboreal primates in fragmented landscapes. Primates 52:139–147
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ricketts T (2001) The matrix matters: effective isolation in fragmented landscapes. Amer Nat 158:87–99
Ripperger SP, Kalko EK, Rodríguez-Herrera B, Mayer F, Tschapka M (2015) Frugivorous bats maintain functional habitat connectivity in agricultural landscapes but rely strongly on natural forest fragments. PLoS ONE 10:e0120535
Roff DA (2006) Randomization and Monte Carlo methods. In: Roff DA (ed) Introduction to computer-intensive methods of data analysis in biology. Cambridge University Press, Cambridge, pp 102–156
Saldívar-Burrola LL, Martínez-Ruíz M, Arroyo-Rodríguez V, Villalobos F, Duarte-Dias PA, López-Barrera F, Arasa-Gisbert R (2022) Can secondary forests mitigate the negative effect of old–growth forest loss on biodiversity? A landscape–scale assessment of two endangered primates. Landscape Ecol 37:3233–3238
San-José M, Arroyo-Rodríguez V, Jordano P, Meave JA, Martínez-Ramos M (2019) The scale of landscape effect on seed dispersal depends on both response variables and landscape predictor. Landscape Ecol 34:1069–1080
San-José M, Arroyo-Rodríguez V, Meave JA (2020) Regional context and dispersal mode drive the impact of landscape structure on seed dispersal. Ecol Appl 30:e02033
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Seidler TG, Plotkin JB (2006) Seed dispersion and spatial pattern in tropical trees. PLoS Biol 4:e344
Sica YV, Quintana RD, Bernardos JN, Calamari NC, Gavier-Pizarro GI (2020) Wetland bird response to habitat composition and configuration at multiple spatial scales. Wetlands 40:2513–2525
Sork VL, Nason J, Campbell DR, Fernandez JF (1999) Landscape approaches to historical and contemporary gene flow in plants. Trends Ecol Evol 14:219–224
Stanton DE, Negret BS, Armesto JJ, Hedin LO (2013) Forest patch symmetry depends on the direction of limiting resource delivery. Ecosphere 4:1–12
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS, Rödig E, Wiegand T, Huth A (2018) Global patterns of tropical forest fragmentation. Nature 554:519–522
Therneau T, Atkinson B (2022). _rpart: Recursive partitioning and regression trees_. R package version 4.1.19, <https://CRAN.R-project.org/package=rpart>
Thornton D, Branch L, Sunquist M (2011) Passive sampling effects and landscape location alter associations between species traits and response to fragmentation. Ecol Appl 21:817–829
Tishendorf L, Bender DJ, Fahrig L (2003) Evaluation of patch isolation metrics in mosaic landscapes for specialist vs. generalist dispersers. Landscape Ecol 18:41–50
van Breugel M, Craven D, Lai HR, Baillon M, Turner BL, Hall JS (2019) Soil nutrients and dispersal limitation shape compositional variation in secondary tropical forests across multiple scales. J Ecol 107:566–581
Villard MA, Trzcinski MK, Merriam G (1999) Fragmentation effects on forest birds: relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol 13:774–783
Villicaña-Hernández GJ, Martínez-Natarén DA, Álvarez-Espino RX, Munguía-Rosas MA (2020) Seed rain in a tropical dry forest and adjacent home gardens in the Yucatan. Trop Conserv Sci 13:1940082920974599
Wang R, Compton SG, Chen XY (2011) Fragmentation can increase spatial genetic structure without decreasing pollen–mediated gene flow in a wind–pollinated tree. Mol Ecol 20:4421–4432
Watson JEM, Whittaker RJ, Freudenberger D (2005) Bird community responses to habitat fragmentation: how consistent are they across landscapes? J Biogeogr 32:1353–1370
Wheatley M, Johnson C (2009) Factors limiting our understanding of ecological scale. Ecol Complex 6:150–159
Wies G, Arzeta SN, Ramos MM (2021) Critical ecological thresholds for conservation of tropical rainforest in human modified landscapes. Biol Conserv 255:109023
Wilson MC, Hu G, Jiang L, Liu J, Liu J, Jin Y, Yu M, Wu J (2020) Assessing habitat fragmentation’s hierarchical effects on species diversity at multiple scales: the case of Thousand Island Lake, China. Landscape Ecol 35:501–512
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royl Stat Soc B 73:3–36
Zhang JQ, Mammides C, Corlett RT (2020) Reasons for the survival of tropical forest fragments in Xishuangbanna, Southwest China. Forests 11:159
Zuckerberg B, Desrochers A, Hochachka WM, Fink D, Koening WD, Dickinson JL (2012) Overlapping landscapes: a persistent, but misdirected concern when collecting and analyzing ecological data. J Wildl Manag 76:1072–1080
Acknowledgements
MAJF would like to thank Consejo Nacional de Humanidades, Ciencia y Tecnología Mexico (CONAΗCYT) for his doctoral scholarship (465860). The researchers would also like to thank the Instituto de Ecología A.C. for funding the project and the local authorities of Tlalixcoyan, who backed the project and helped us contact various stakeholders to gain access to their lands throughout the municipality.
Funding
This project was supported by CONAHCYT, which provided the scholarship (465860) on behalf of MAJF.
Author information
Authors and Affiliations
Contributions
MAJF and RG conceived and designed the project. MAJF, YPV, JAGD, and RG conducted the fieldwork. MAJF and RG analyzed the data. MAJF, WD, and RG interpreted and discussed the results. All the authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
None of the authors have relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juárez–Fragoso, M.A., Perroni, Y., Dáttilo, W. et al. The landscape scale of effect on the alpha and beta diversities of woody species in a semideciduous tropical forest. Landsc Ecol 39, 33 (2024). https://doi.org/10.1007/s10980-024-01809-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01809-z