Abstract
Investigating the ecology of a species and the spatial requirements needed for their survival within an environment can improve and help develop conservation measures. In this study, we reviewed the literature describing the importance of fine-scale landscape characteristics on the distribution of penguin species. We then investigated little penguin nest-site use across eleven colonies in South Australia, with a focus on nest type, vegetation cover, nest entrance orientation, proximity to the nearest active nest, and side of the island. We showed that both abiotic and biotic variables were important for nest-site use in penguins and that the specific variables varied between species and populations. Little penguins in South Australia did not appear to use nest sites randomly, and active nests were mostly found on the northern side of the island and facing east or west. Our study highlights the importance of gaining a better understanding of penguin nest-site use, and their fitness consequences for populations, to ensure effective conservation outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the spatial requirements of a species and the factors that influence their distribution can be fundamental to develop appropriate conservation and management measures (e.g., Guderyahn et al. 2016; Jaworski et al. 2019; Louzao et al. 2006). Seabirds are considered bio-indicators of the marine environment (e.g., Croxall et al. 2002; Lascelles et al. 2012) and the most threatened group of birds (reviewed in Dias et al. 2019). Due to their status and environmental importance, their conservation is essential. Yet most landscape research to date on seabirds have primarily focused on their requirements at sea (such as food availability) with limited focus on how their land requirements affect their distribution (but see for example Eveillard-Buchoux et al. 2019; Paredes and Zavalaga 2001; Seddon and Davis 1989).
While seabird conservation depends on many factors (e.g., Dias et al. 2019; Trathan et al. 2015), the importance of land-based fine-scale landscape characteristics, such as nest sites, has been overlooked relative to marine habitat features. Fine-scale landscape characteristics (also referred to as small natural features) can have a disproportionate ecological influence on species by providing resources that otherwise limit population size or distribution, similar to keystone species in ecology (Hunter Jr 2017; Hunter et al. Jr 2017). Nest sites are particularly important for breeding success in seabirds (e.g., Colombelli-Négrel 2019; Frere et al. 1992; Gandini et al. 1999; Kim and Monaghan 2005b; Michielsen et al. 2019; Paredes and Zavalaga 2001). For example, nest sites with high vegetation cover can offer more protection from conspecifics, climate, and/or predators than less covered nests, thereby increasing breeding success (Bukacińska and Bukaciński 1993; Burger 1977; Frere et al. 1992; Gandini et al. 1999; Kim and Monaghan 2005a, b). Different nest types also offer differing microclimate, which in turn influence hatching success and chick survival (Bull 2000; Colombelli-Négrel 2019; Renner and Davis 2001) as well as thermoregulation (Colombelli-Négrel 2019), and thus can have long-term impacts on individual fitness. Therefore, identifying nest-site characteristics that may limit species distribution and/or promote higher breeding success should constitute a key objective in seabirds’ conservation (Eveillard-Buchoux et al. 2019; Rönkä et al. 2008).
Little penguins (Eudyptula minor) are a seabird species endemic to the southern coast of Australia and parts of New Zealand (Marchant and Higgins 1990). South Australia host approx. 100 little penguin colonies, with 20+ colonies suspected to have declined in the last two decades for reasons not well understood (Colombelli-Négrel 2015, 2020; DEWNR 2016; Wiebkin 2011). The Granite Island colony, for example, experienced one of the strongest declines as the population went from > 1500 adult little penguins in 2001 to < 30 adults since 2012 (Colombelli-Négrel 2020). Very little is known regarding the importance of fine-scale habitat and landscape characteristics on the distribution of penguins and their breeding habitat. For little penguins, Marker (2016) showed in Tasmania that the proximity of their foraging site to their breeding site was important for their distribution at the regional level. More locally, their breeding distribution was influenced by the potential for human disturbances (Marker 2016), as well as the distance between neighbouring nests (Tasmania; Marker 2016), the amount and type of vegetation near their nests (Western Australia; Clitheroe 2021), the aspect of the nests (Western Australia; Clitheroe 2021), and the proximity of landing sites (Victoria and Western Australia; Clitheroe 2021; Weerheim et al. 2003).
In this study, we first reviewed the literature describing the importance of fine-scale landscape characteristics on the distribution of penguin species. We then asked which habitat characteristics predicted nest-site use (active/inactive) in little penguins. Based on the literature, we specifically focused on nest type, orientation, and location, as well as vegetation cover and proximity to other active nests. To determine if we were correct in our hypotheses, we measured nest-site characteristics at active and inactive nests during population surveys of 11 penguin colonies on islands off the South Australia coastline. We used colonies that varied in population trends (see methods) to also determine whether differences in activity, rather than topography or vegetation, influenced nest-site use. We predicted (1) that active nests will be clustered together, and thus distance between neighbouring nests would be an important predictor for the presence of other active nests (Marker 2016), and (2) that active little penguin nests will be found sheltered from the elements, either facing away from the prevailing winds (north and south winds in South Australia) or with high vegetation cover to reduce high temperatures inside the nest, because little penguins breeding success can be influenced by nest type and local environmental conditions, such as temperature and wind (Colombelli-Négrel 2019; Johnson and Colombelli-Négrel 2021).
Materials and methods
Literature search
We searched for studies that specifically investigated whether fine-scale habitat and landscape characteristics explained penguin habitat use or for studies that mentioned such characteristics in their results. Using Google Scholar, we conducted a literature search of all studies published until 2021 with the following terms: ‘penguin’, ‘distribution’, ‘nest’, (including ‘nest site’) and ‘habitat’. We focused on papers published in refereed journals, but also included book chapters, reports and thesis that were available online and relevant (see Fig. 1).
Summary of the 39 studies used in this review. The figure presents for each region (Antarctica, South America, Southern Africa, and Australasia) the number of (i) peer-reviewed studies, (ii) reports and theses, (iii) studies that focused on selection at the nest site, (iv) studies that focused on selection at the scale of general breeding habitat, and (v) studies that examined breeding success and its association with habitat characteristics
We clustered the results of our literature review into four regions: (1) South America – Emperor penguins (Aptenodytes forsteri), King penguins (A. patagonicus), Adélie penguins (Pygoscelis adeliae), Chinstrap penguins (P. antarctica) and Gentoo penguins (P. papua); (2) Australasia – Yellow-eyed penguins (Megadyptes antipodes), Australian little penguins, (Eudyptula novaehollandiae), Little blue penguins (E. minor), White-flippered penguins (E. minor albosignata), Snares penguin (Eudyptes robustus), Fiordland crested penguin (E. pachyrhynchus) and Royal penguin (E. schlegeli); (3) Antarctica (including island located in the Southern Ocean) – King penguins, Gentoo penguins, Magellanic penguins (Spheniscus magellanicus), Humboldt penguins (S. humboldti), and Galapagos penguins (S. mendiculus); and (4) southern African – African penguin (Spheniscus demersus) (see Fig. 1). We found no study matching our criteria for the Erect-crested penguin (E. sclateri), the Macaroni penguin (E. chrysolophus), and the two Rockhopper penguins (E. chrysocome and E. moseleyi).
Spatial distribution of little penguins in South Australia
Study sites
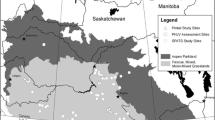
Between May 2016 and October 2018, we surveyed 11 little penguin colonies in South Australia. GPS coordinates, years studied and population trends for each colony are presented in Table 1. In this study, we used colonies that varied in their population trends and were either: (i) well-studied (i.e., information regarding population sizes and population trends was available across several consecutive years or even decades; Troubridge Island, Granite Island, and Emu Bay on Kangaroo Island), (ii) little studied (i.e., information regarding population sizes was available for some years but was patchy; English Island, Hareby Island, Spilsby Island) or (iii) data deficient (i.e., no or little information regarding population sizes was available Wardang Island, Smith Island, Rabbit Island, Goose Island, Louth Island) (see also DEWNR 2016). The locations of the colonies are presented in Fig. 2.
Distribution of the eleven islands surveyed for the presence of little penguin nests in South Australia. Islands marked with black triangles represent well-studied little penguin colonies, those with dark grey triangles represent the little-studied little penguin colonies, and those with light grey triangles are data deficient
Study species
Little penguins in Australia are known to breed both on the mainland and on offshore islands (Dann 1991; Marchant and Higgins 1990; Stevenson and Woehler 2007). During their breeding season, they become central-place foragers, often foraging within 20–30 km of their breeding site (Collins et al. 1999; Hoskins et al. 2008). Little penguins are monogamous and asynchronous breeders; breeding occurs between May and February (Colombelli-Négrel 2015, 2018; Johannesen et al. 2003; Reilly and Cullen 1981), with each breeding pair producing up to two clutches per breeding season (Colombelli-Négrel 2015; Johannesen et al. 2003; Kemp and Dann 2001; Reilly and Cullen 1981). During incubation and the first two weeks of the ‘chick-rearing’ period, parents take turns sitting on the eggs or chicks (Miyazaki and Waas 2003). Incubation lasts 33–44 days and chicks fledge 8–9 weeks after hatching (Chiaradia and Nisbet 2006; Colombelli-Négrel 2015; Kemp and Dann 2001; Numata et al. 2004).
Population surveys
Population surveys were used to determine the location of active vs. inactive nests. We surveyed each colony during the second peak of the breeding season, either by inspecting the entire colony, or, in the case of large islands, by using transects or 30 × 30 m quadrats in areas where little penguins were present (see below). Such areas were determined using a combination of (1) random searches, (2) local knowledge from park rangers (and habitants for habited islands), (3) knowledge from previous surveys, and (4) helicopter surveys (when possible) to determine potential penguin habitat within an island. The peaks of the breeding season for each colony were determined using local knowledge from park rangers (and habitants for habited islands) and knowledge from previous surveys (see Table 1). Except on Troubridge, Goose and Rabbit islands, where nests were located all over the island, nests were only found along the coastline no further than 120 m from the sea (see also Weerheim et al. 2003). Therefore, transects were positioned along the coast following the coastline, and all transects were marked with a Garmin GPS 62 or 64 s.
Granite Island (2016–2018), English Island (2018) and Goose Island (2017–2018) were surveyed entirely each year. Troubridge Island (2016–2018) and Emu Bay (2016–2017) were surveyed entirely in 2016 and using quadrats in 2017 (both colonies) and 2018 (Troubridge Island). Data extrapolated from the quadrat surveys on Troubridge Island were comparable to surveys conducted over the entire island (Colombelli-Négrel 2017, 2018). All the other colonies were surveyed using transects. All the islands in the lower Spencer Gulf (English Island, Hareby Island, Spilsby Island, Smith Island, Rabbit Island, and Louth Island) were surveyed in 2018.
Nests were searched within ~ 1 m of our transects on the land side and all the way to the water on the water side. The number and length of transects varied between colonies due to the size and shape of the islands and the vegetation present (number of transects ranging from 2 to 9 per colony, 3.5 on average; length of transects ranging from 400 m to 1 km, 700 m on average). For each colony, once a nest was found, we noted its status noted as active or inactive. Active nests were identified if they contained egg, chick or adult penguins or if they had clear evidence of recent (i.e., in the current year) penguin presence, such as fresh excrement, strong penguin smell, fresh digging or penguin feathers (Colombelli-Négrel 2015; Schumann et al. 2013). Deep nests were checked using a burrow-scope (Faunatech Austbat, Australia). A burrow was recorded as inactive if none of the above criteria was found or if it had cobwebs or overgrown vegetation at the entrance indicating that no large animal was or had been regularly entering/exiting the burrow. While little penguins are asynchronous breeders, they have two peaks during their breeding season in South Australia (Colombelli-Négrel, pers. obs.). As our surveys occurred during the second peak of breeding, all birds would have either bred in the first peak or started breeding in the second peak and, hence, we are confident that inactive nests remained inactive after our surveys. All nests were marked with a Garmin GPS 62 or 64 s.
Nest-site characteristics
While surveying each colony, the following nest-site characteristics were noted for each nest: (1) nest-site use (active or inactive as describe above), (2) nest type (categorized as either: surface (scrapes under an open bush), sand (nests dug in soft sand), bush (scrapes deep under a thick bush), rock (burrows under boulder or in rock crevices) or artificial (plastic boxes with rocks, metal drums or concrete structure) (Colombelli-Négrel 2019; see also Marker 2016), (3) vegetation cover (percentage of vegetation immediately above the nest (up to 0.5 m) estimated visually when standing 1 m away; 0-100% following Colombelli-Négrel 2019); (4) nest entrance orientation [recorded as a score between 1 (north facing) and − 1 (south facing)]; (5) proximity to the nearest active nest (in meters), and (6) location within the colony/side of the island (recorded in degrees as a value between 1 and 360 degrees in relation to north = 360 degrees; i.e., a north-east side would be recorded as 45 degrees and a southern side would be recorded as 180 degrees). Nest-site characteristics were collected once per nest site. To ensure independence of the data, when colonies were visited more than once, we only collected nest-site characteristics during the last survey.
Statistical analysis
All statistical analyses were performed in SPSS version 25.0 for Windows (SPSS Inc., Chicago, IL, USA). Prior to analysis, we assessed collinearity between the predictor variables using the variance inflation factors (VIF) analysis; all VIF values were well < 2, confirming no collinearity (Zuur et al. 2009). We then modelled penguin nest-site use (active vs. inactive) using a General Linear Model with a binary distribution and six fixed factors (colony, nest type, vegetation cover, nest entrance orientation, proximity to the nearest active nest, and side of the island). We investigated differences in nest-site characteristics between colonies using MANOVAs and Bonferroni post hoc pairwise comparisons.
Results
Literature review
The type (biotic and abiotic) and number (between one and nine) of habitat variables measured varied between studies (Fig. 1; Table 2). Most studies focused on habitat use at the nest site (i.e., presence of active nests; 71% − 27 studies out of 38), while 8% (3/38) focused on habitat use at the scale of general breeding habitat (i.e., colony size) and 21% (8/38) focused on breeding success; only one study (3%) focused on both presence of active nests and colony size and four studies (11%) investigating habitat use at the nest site also examined breeding success and its association with habitat characteristics (Fig. 1; Table 2). Only 60% of the studies (23 out of 38 studies) statistically evaluated the influence of habitat characteristics on habitat use in penguins and only two studies (5%) compared nest-site characteristics between active and inactive nests (Table 2). The remaining 40% simply described penguin habitat use or mentioned nest-site characteristics in their results but did not statistically evaluate their importance for habitat or nest-site use.
Habitat variables influencing nesting site use or colony size varied between studies, regions, and species (Fig. 3; Table 2). Penguins in Antarctica were commonly reported nesting on snow-free or ice-free surfaces as well as stable sea ice platforms, up to several kilometres from the sea. Penguins in South America and Southern Africa nested in sheltered areas, often under shrubs or bushes, up to 1 km from the sea. Penguins in Australasia nested in rock crevices, burrows (man-made or artificial) or under thick vegetation, less than 500 m from the sea. In addition, habitat variables important for nest use and breeding success differed between cavity vs. surface nesting species. For surface nesting species, abiotic and topographical variables as well as the proximity of conspecifics were important factors determining nest use, while both abiotic (slope, aspect, location within the colony) and biotic (vegetation cover) variables were important for breeding success (Fig. 3; Table 2). For burrow nesting species, both abiotic (location within the colony, distance to shore and neighbours) and biotic (vegetation cover, habitat type) variables were important for both nest use and breeding success (Fig. 3; Table 2).
Little penguin population surveys and nest distribution
A total of 394 nests were found across nine islands, of which 238 were active. For 78% of the surveyed islands (excluding English and Smith Islands where no penguins were present), active nests were found at higher densities on the northern side of the island. For the remaining 22%, nests were found towards the southern side of the island. The best predictors for the presence of active little penguin nests were the side of the island and nest entrance orientation (GLM; Table 3): active nests were mostly found on the northern side of the island, including northeast and northwest sides, and facing east or west. Hareby Island significantly had more inactive nests than the other colonies (GLM; Table 3). All colonies significantly differed in their nest-site characteristics: nest type (MANOVA: F8, 393 = 41.10; P < 0.0001), vegetation cover (F8, 393 = 14.87; P < 0.0001), nest entrance orientation (F8, 393 = 9.70; P < 0.0001), proximity to the nearest active nest (F8, 393 = 8.20; P < 0.0001), and side of the island (F8, 393 = 70.17; P < 0.0001; Table S1). We present in Table S2 our sample sizes and a summary of the nest-site characteristics measured in this study in relation to active and inactive nests.
Discussion
Nest-site in birds is critical for courtship, pairing, and reproductive success (e.g., Colombelli-Négrel and Kleindorfer 2009; Mainwaring et al. 2014; Stokes and Boersma 1998). Several factors may influence nest-site selection and use, especially those that offer protection from predators and environmental extremes and thus increase breeding success and survival of nesting birds (Colombelli-Négrel 2019; Colombelli-Négrel and Kleindorfer 2009; Frere et al. 1992; Michielsen et al. 2019; Stokes and Boersma 1998). Our literature review demonstrated that both abiotic and biotic variables were important for nest-site use in penguins, but that the specific variables varied between studies, regions, and species (Table 2), maybe due to adaption to local environment. Our study on South Australian little penguins further supports these finding and showed that little penguins in this region did not appear to use nest sites randomly, but instead likely based their nest-site use on abiotic factors such as the side of the island and the orientation of the nest entrance. Our study highlights that a clearer understanding of nest-site use in penguins is required to better manage their breeding habitat (see also Eveillard-Buchoux et al. 2019; Hunter Jr et al. 2017).
Similar to other studies in penguins (Bried and Jouventin 2001; Challies and Burleigh 2004; Long and Litchwark 2017; Morandini et al. 2021; Ratz and Murphy 1999), the location of a nest within a colony was an important factor for nest activity in South Australian little penguins. However, in our study such location was not defined as centre vs. edge, as found in most studies, but rather as a northerly side. Indeed, we found that active little penguin nests were more likely to be found on the northern side of the island. A study on Tasmanian little penguins also found that active nests were more likely to be found on north-facing slopes (Marker 2016), suggesting that a northerly side may be important for the establishment of little penguin nests within a colony/island. This may be due to the potential impact that the winter prevailing winds (when little penguins start breeding in South Australia) could have on the nest micro-climate and/or on the coastal waters, which remains to be tested.
Additionally, we found that active little penguin nests were more likely to face an eastern or western aspect, similar to what was observed in Western Australia by Klomp et al. (1991) and Clitheroe (2021). Again, this may be due to the prevailing winds, and how winds influence nest microclimate (i.e., temperature and humidity) (Ropert-Coudert et al. 2004), which would suggest that nest microclimate (and not orientation per se) may be driving nest use in little penguins. Numerous studies in birds have highlighted the importance of the nest microclimate for habitat use (e.g., Colombelli-Négrel 2019; Lei et al. 2014; Rhodes et al. 2009), even sometimes suggesting that it may play a larger role than vegetation or topography. In penguins, nest temperature is important for thermoregulatory behaviours and chick development (Frere et al. 1992; Frost et al. 1976; Lei et al. 2014), while nest humidity is important for egg shell integrity and hatching success (Colombelli-Négrel 2019). Additional studies separating the effects of microclimate, winds, and orientation on nest use may help answer this question.
Surprisingly, and contrary to other studies on little penguins (Clitheroe 2021; Dann 1994; Marker 2016), vegetation cover was not an important factor explaining the presence of active nests in our study. Vegetation cover (either adjacent to the nest or directly above) is an important variable for both surface and burrow nesting penguin species (Fig. 3; Table 2), likely to minimise predation risk (Frere et al. 1992; Stokes and Boersma 1998) and/or exposure to extremes environments (Kim and Monaghan 2005a). It should be noted however, that most colonies in this study did not have terrestrial predators, which may have impacted our results. The impact of vegetation may also have been mitigated by interaction with other variables (not measured in this study), such as substrate. Indeed, substrate composition can influence vegetation growth and structure (see Borboroglu et al. 2002) as well as help maintain nests’ microclimate and stability (Stokes and Boersma 1991). It should also be noted that most previous studies investigated the importance of vegetation cover in relation to the presence vs. absence of penguin nests (Clitheroe 2021; Dann 1994; Marker 2016) rather than nest-site use (this study), which may also explain the observed difference in results.
Social interactions or the close presence of conspecifics may also influence selection/use of nest-sites in penguins (Ainley et al. 1995; Marker 2016; Santora et al. 2020; Volkman and Trivelpiece 1981). However, in South Australian little penguins, we found no correlation between the presence of active nests and the distance to the next active nest. Similarly, we found that only Hareby Island significantly differed in the percentage of active vs. inactive nests from the other colonies. It is possible that little penguins experience reduced breeding success when nesting in high density – as found in African (Sherley et al. 2014) and Magellanic (Stokes and Boersma 2000) penguins (see also Colombelli-Négrel 2015, in little penguins) – due to competition for food, and thus could be avoiding nesting too close to conspecifics. Alternatively, it may simply be because little penguins are highly philopatric, returning to the same part of their colony (and often re-using the same nest) year after year (Reilly and Cullen 1981), regardless of the presence of conspecifics. This idea is supported by our nest survey on Rabbit Island, where a single nest was found in the middle of the island.
Nest type has been shown to be important for breeding success in several penguin species (Frere et al. 1992; Paredes and Zavalaga 2001; Seddon and Van Heezik 1991; Sherley et al. 2012), likely due to its impact on the thermodynamic characteristics of the nest (Colombelli-Négrel 2019; Frost et al. 1976; Lei et al. 2014; Seddon and Davis 1989). However, in little penguins, the importance of nest type for breeding success varied between studies, with some studies finding a positive influence (Bull 2000; Colombelli-Négrel 2019; Renner and Davis 2001) and others not (Boyer 2010; Braidwood et al. 2011; Geurts 2006). In this study, we found that nest type did not influence the likelihood of finding an active nest vs. an inactive nest. Nest type use may be influenced by other factors, such as proximity to prey supply, predator presence and/or frequency of extreme weather events. The most advantageous combination of landscape characteristics, frequency and intensity of adverse factors, and beneficial factors that allows for the survival of individuals and population, is thus expected be unique for different species and populations (see also Table 2). This reinforces the importance of site-specific investigations for each species to ensure that more effective conservation measure are put in place.
To assess the fitness benefits of habitat selection, studies should also demonstrate increased fitness in used habitats (Jones 2001; reviewed in Chalfoun and Schmidt 2012). Indeed, breeding success in penguins can be influenced by both abiotic and biotic variables, such as slope, aspect, location within the colony, vegetation cover or habitat type (Bried and Jouventin 2001; Colombelli-Négrel 2019; McKay et al. 1999; Morandini et al. 2021; Schmidt et al. 2021; Stokes and Boersma 1998; Trivelpiece and Fraser 1996). Yet very few studies in our review investigated nest attributes for both nest-use and breeding success (Fig. 1; Table 2). In addition, the variables that correlated with nest-site use sometimes differed to those that correlated with improved breeding success (Fig. 3). Studies in other seabirds have suggested that habitat characteristics may be less important for breeding success than other factors, such as parental condition or experience (e.g., Pugesek and Diem 1983; Velando and Freire 2001), which may explain these contradictory results. Additionally, most studies assessing nest-site use in animals simply assume that nests or sites are voluntarily chosen without considering that they may be in fact selected randomly (see Goodenough et al. 2009). Additional studies experimentally testing nest selection in penguins are clearly needed.
Conclusion
Climate change is predicted to reduce the suitability or availability of breeding habitats for penguin species by altering the coastal environment (i.e., by melting nesting ice-surfaces or increasing sea-levels and thereby reducing sizes of low-lying islands for example; see Chambers et al. 2011; Colombelli-Négrel 2017; Dann and Chambers 2009; Schumann et al. 2013),. While some species may be able to adjust their distribution, the persistence of other species or populations may depend on well-planned habitat management such as timely prioritisation of conservation measures for species that will be most affected by a reduction in the suitability or availability of their breeding habitats (see Grémillet and Boulinier 2009). Our study and literature review emphasise that factors important for nest-site use in penguins varied between species and even populations; thereby highlighting the importance of gaining a better understanding of penguin habitat use, and their fitness consequences for populations, to ensure effective habitat management in the light of climate change (see also Eveillard-Buchoux et al. 2019; Hunter Jr et al. 2017).
Data availability
Data are available on the Flinders University data repository at https://doi.org/10.25451/flinders.22151966.
References
Ainley DG, Nur N, Woehler EJ (1995) Factors affecting the distribution and size of pygoscelid penguin colonies in the Antarctic. Auk 112:171–182
Bool NM, Wiebkin AS (2013) Census of little penguin population Troubridge Island. Report to the Department for Environment and Heritage, Adelaide
Borboroglu PG, Yorio P, Boersma PD, Valle HD, Bertellotti M (2002) Habitat use and breeding distribution of magellanic penguins in northern San Jorge Gulf, Patagonia Argentina. Auk 119:233–239
Boyer A-S (2010) Microbial infection of avian eggs: a threat to all synchronously incubating species? Case study of New Zealand’s little blue penguin (Eudyptula minor). Masters Thesis. Massey University, Auckland
Braidwood J, Kunz J, Wilson KJ (2011) Effect of habitat features on the breeding success of the blue penguin (Eudyptula minor) on the West Coast of New Zealand. NZ J Ecol 38:131–141
Bried J, Jouventin P (2001) The King Penguin Aptenodytes patagonicus, a non-nesting bird which selects its breeding habitat. Ibis 143:670–673
Brothers N (1983) Seabird Islands: Sterile Island, Tasmania. Corella 137:91–92
Bukacińska M, Bukaciński D (1993) The effect of habitat structure and density of nests on territory size and territorial behaviour in the black-headed gull (Larus ridibundus L). Ethology 94:306–316
Bull LS (2000) Factors influencing little penguin Eudyptula minor egg success on Matiu-Somes Island, New Zealand. Emu 100:199–204
Burger J (1977) Role of visibility in nesting behavior of Larus gulls. J Comp Physiol Psychol 91:1347
Chalfoun AD, Schmidt KA (2012) Adaptive breeding-habitat selection: Is it for the birds? Auk 129:589–599
Challies CN, Burleigh RR (2004) Abundance and breeding distribution of the white-flippered penguin (Eudyptula minor albosignata) on Banks Peninsula, New Zealand. Notornis 51:1–6
Chambers LE, Devney CA, Congdon BC, Dunlop N, Woehler EJ, Dann P (2011) Observed and predicted effects of climate on australian seabirds. Emu 111:235–251
Chiaradia A, Nisbet ICT (2006) Plasticity in parental provisioning and chick growth in little Penguins Eudyptula minor in years of high and low breeding success. Ardea 94:257–270
Clitheroe E (2021) Can artificial habitat mitigate impacts of climate change? Quantifying nesting habitat microclimate and use by little penguins (Eudyptula minor). PhD Thesis, Murdoch University, Perth
Collins M, Cullen JM, Dann P (1999) Seasonal and annual foraging movements of little penguins from Phillip Island, Victoria. Wildl Res 26:705–721
Colombelli-Négrel D (2015) Low survival rather than breeding success explains little penguin population decline on Granite Island. Mar Freshw Res 66:1057–1065
Colombelli-Négrel D (2016) Penguin monitoring and conservation. Report to the Adelaide and Mt Lofty Natural Resources Management Board, Adelaide, activities in Gulf St Vincent (July 2015 - June 2016
Colombelli-Négrel D (2017) Penguin monitoring and conservation activities. Report to the Adelaide and Mt Lofty Natural Resources Management Board, Adelaide, in the Gulf St Vincent (July 2016 - June 2017
Colombelli-Négrel D (2018) Penguin monitoring and conservation activities. Report to the Adelaide and Mt Lofty Natural Resources Management Board, Adelaide, in the Gulf St Vincent (July 2017 - June 2018
Colombelli-Négrel D (2019) Benefits, costs and trade-offs of nesting habitat selection in little penguins. J Ornithol 160:515–527
Colombelli-Négrel D (2020) Penguin monitoring and conservation activities in the Gulf St Vincent (June 2019 - May 2020). Report to the Adelaide and Mt Lofty. Natural Resources Management Board, Adelaide
Colombelli-Négrel D, Kleindorfer S (2009) Nest height, nest concealment, and predator type predict nest predation in superb fairy-wrens (Malurus cyaneus). Ecol Res 24:921–928
Copley P (1996) The status of seabirds in South Australia. In: Ross G, Weaver K, Greig J (eds) The Status of Australia’s Seabirds, pp. 139–180
Croxall JP, Trathan P, Murphy E (2002) Environmental change and Antarctic seabird populations. Science 297:1510–1514
Dann P (1991) Distribution, population trends and factors influencing the population size of little penguins Eudyptula minor on Phillip Island, Victoria. Emu 91:263–272
Dann P (1994) The abundance, breeding distribution and nest sites of blue penguins in Otago. New Z Notornis 41:157–166
Dann P, Chambers L (2009) Climate change and little penguins. Report for Western Port Greenhouse Alliance, Melbourne
Department of Environment, Water and Natural Resources (DEWNR) (2016) Conservation risk assessment report for little penguins in South Australia. DEWNR Technical Report 2016/33, Government of South Australia, Department of Environment, Water and Natural Resources, Adelaide
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537
Eveillard-Buchoux M, Beninger PG, Chadenas C, Sellier D (2019) Small-scale natural landscape features and seabird nesting sites: the importance of geodiversity for conservation. Landsc Ecol 34:2295–2306
Frere E, Gandini P, Boersma D (1992) Effects of nest type and location on reproductive success of the Magellanic Penguin Spheniscus magellanicus. Mar Ornithol 20:1–6
Fretwell PT, Trathan PN, Wienecke B, Kooyman GL (2014) Emperor penguins breeding on iceshelves. PLoS ONE 9:e85285
Frost P, Siegfried W, Burger A (1976) Behavioural adaptations of the Jackass Penguin, Spheniscus demersus to a hot, arid environment. J Zool 179:165–187
Gandini P, Frere E, Boersma D (1999) Nest concealment and its relationship to predation and reproductive success in the Magellanic Penguin at its southern-most continental colony. Ornitol Neotrop 10:145–150
Geurts JL (2006) The feeding and breeding ecology of little blue penguins (Eudyptula minor) from Tiritiri Matangi Island, New Zealand
Goodenough AE, Elliot S, Hart AG (2009) Are nest sites actively chosen? Testing a common assumption for three non-resource limited birds. Acta Oecol 35:598–602
Grémillet D, Boulinier T (2009) Spatial ecology and conservation of seabirds facing global climate change: a review. Mar Ecol Prog Ser 391:121–137
Guderyahn LB, Smithers AP, Mims MC (2016) Assessing habitat requirements of pond-breeding amphibians in a highly urbanized landscape: implications for management. Urban Ecosyst 19:1801–1821
Hoskins AJ, Dann P, Ropert-Coudert Y, Kato A, Chiaradia A, Costa DP, Arnould JPY (2008) Foraging behaviour and habitat selection of the little penguin Eudyptula minor during early chick rearing in Bass Strait, Australia. Mar Ecol Prog Ser 366:293–303
Hunter ML Jr (2017) Conserving small natural features with large ecological roles: an introduction and definition. Biol Conserv 211:1–2
Hunter ML Jr, Acuña V, Bauer DM, Bell KP, Calhoun AJ, Felipe-Lucia MR, Fitzsimons JA, González E, Kinnison M, Lindenmayer D (2017) Conserving small natural features with large ecological roles: a synthetic overview. Biol Conserv 211:88–95
Jabłoński B (1984) Distribution and numbers of penguins in the region of King George Island (South Shetland Islands) in the breeding season1980/1981. Pol Polar Res, pp. 17–30
Jaworski T, Plewa R, Tarwacki G, Sućko K, Hilszczański J, Horák J (2019) Ecologically similar saproxylic beetles depend on diversified deadwood resources: from habitat requirements to management implications. For Ecol Manage 449:117462
Johannesen E, Houston D, Russell J (2003) Increased survival and breeding performance of double breeders in little penguins Eudyptula minor, New Zealand: evidence for individual bird quality? J Avian Biol 34:198–210
Johnson B, Colombelli-Négrel D (2021) Breeding success in Southern Australian little penguins is negatively correlated with high wind speeds and sea surface temperatures. Condor 123:duaa062
Jones J (2001) Habitat selection studies in avian ecology: a critical review. Auk 118:557–562
Kemp A, Dann P (2001) Egg size, incubation periods and hatching success of little penguins, Eudyptula minor. Emu 101:249–253
Kim S-Y, Monaghan P (2005a) Effects of vegetation on nest microclimate and breeding performance of lesser black-backed gulls (Larus fuscus). J Ornithol 146:176–183
Kim S-Y, Monaghan P (2005b) Interacting effects of nest shelter and breeder quality on behaviour and breeding performance of herring gulls. Anim Behav 69:301–306
Klomp N, Meathrel C, Wienecke B, Wooller R (1991) Surface nesting by little penguins on Penguin Island, Western Australia. Emu 91:190–193
LaRue MA, Ainley DG, Swanson M, Dugger KM, Phil O, Lyver B, Barton K, Ballard G (2013) Climate change winners: receding ice fields facilitate colony expansion and altered dynamics in an Adélie penguin metapopulation. PLoS ONE 8:e60568
Lascelles BG, Langham GM, Ronconi RA, Reid JB (2012) From hotspots to site protection: identifying Marine protected areas for seabirds around the globe. Biol Conserv 156:5–14
Lawley E, Lawley J, Page B (2005) Effects of African boxthorn removal on native vegetation and burrowing of short-tailed shearwaters on Althorpe Island, South Australia. Trans R Soc S Aust 129:111–115
Lei BR, Green JA, Pichegru L (2014) Extreme microclimate conditions in artificial nests for endangered african penguins. Bird Conserv Int 24:201–213
Long R, Litchwark S (2017) A survey of Fiordland crested penguins/tawaki (Eudyptes pachyrhynchus) from Cascade River to Martins Bay, South Westland, New Zealand, 2014. Notornis 64:206–210
Louzao M, Hyrenbach KD, Arcos JM, Abelló P, de Sola LG, Oro D (2006) Oceanographic habitat of an endangered Mediterranean procellariiform: implications for marine protected areas. Ecol Appl 16:1683–1695
Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC (2014) The design and function of birds’ nests. Ecol Evol 4:3909–3928
Marchant S, Higgins PJ (1990) Handbook of australian, New Zealand and Antarctic birds; volume 1. Oxford University Press, Melbourne
Marker PF (2016) Spatial scale and nest distribution of little penguins (Eudyptula minor). PhD Thesis. University of Tasmania, Hobart
McKay R, Lalas C, Mckay D, McConkey S (1999) Nest-site selection by yellow-eyed Penguins Megadyptes antipodes on grazed farmland. Mar Ornithol 27:29–35
Michielsen RJ, Ausems AN, Jakubas D, Pętlicki M, Plenzler J, Shamoun-Baranes J, Wojczulanis-Jakubas K (2019) Nest characteristics determine nest microclimate and affect breeding output in an Antarctic seabird, the Wilson’s storm-petrel. PLoS ONE 14:e0217708
Miyazaki M, Waas JR (2003) Correlations between body size, defensive behaviour and reproductive success in male little Blue Penguins Eudyptula minor: implications for female choice. Ibis 145:98–105
Morandini V, Dugger KM, Lescroël A, Schmidt AE, Ballard G (2021) Maintenance of nest quality in Adélie penguins Pygoscelis adeliae: an additional benefit to life in the center. Polar Biol 44:1553–1562
Numata M, Davis LS, Renner M (2004) Growth and survival of chicks in relation to nest attendance patterns of little penguins (Eudyptula minor) at Oamaru and Motuara Island, New Zealand. NZ J Ecol 31:263–269
Paredes R, Zavalaga CB (2001) Nesting sites and nest types as important factors for the conservation of Humboldt Penguins (Sphensicus humboldti). Biol Conserv 100:199–205
Pugesek BH, Diem KL (1983) A multivariate study of the relationship of parental age to reproductive success in California gulls. Ecology 64:829–839
Quintana R (2001) Nest-site characteristics of a Gentoo Penguin Pygoscelis papua colony at Cierva point, Antarctic Peninsula. Mar Ornithol 29:109–112
Quintana R, Cirelli V, Orgeira J (2000) Abundance and spatial distribution of bird populations at Cierva Point, Antarct Peninsula. Mar Ornithol 28:21–27
Ralph MS (2008) Aspects of the breeding biology of the African Penguin on Bird Island, Algoa Bay. Masters Thesis. Nelson Mandela Metropolitan University, Gqeberha
Ratz H, Murphy B (1999) Effects of habitat and introduced mammalian predators on the breeding success of yellow-eyed Penguins Megadyptes antipodes, South Island, New Zealand. Pac Conserv Biol 5:16–27
Reilly P, Cullen J (1981) The little penguin Eudyptula minor in Victoria, II: Breed. Emu 81:1–19
Renner M, Davis LS, Island (2001) Survival analysis of Little Penguin Eudyptula minor chicks on Motuara Island, New Zealand. Ibis 143:369–379
Rhodes B, O’donnell C, Jamieson I (2009) Microclimate of natural cavity nests and its implications for a threatened secondary-cavity-nesting passerine of New Zealand, the South Island Saddleback. Condor 111:462–469
Robinson T, Canty P, Mooney T, Rudduck P (1996) South Australia’s Offshore Islands., Australian Heritage Commission: Canberra: Australian Government Publishing Service
Rönkä M, Tolvanen H, Lehikoinen E, von Numers M, Rautkari M (2008) Breeding habitat preferences of 15 bird species on south-western finnish archipelago coast: applicability of digital spatial data archives to habitat assessment. Biol Conserv 141:402–416
Ropert-Coudert Y, Cannell B, Kato A (2004) Temperature inside nest boxes of little penguins. Wildl Soc Bull 32:177–182
Salton M, Kliska K, Carmichael N, Alderman R (2019) Population status of the endemic royal penguin (Eudyptes schlegeli) at Macquarie Island. Polar Biol 42:771–781
Santora JA, LaRue MA, Ainley DG (2020) Geographic structuring of Antarctic penguin populations. Glob Ecol Biogeogr 29:1716–1728
Schmidt AE, Ballard G, Lescroël A, Dugger KM, Jongsomjit D, Elrod ML, Ainley DG (2021) The influence of subcolony-scale nesting habitat on the reproductive success of Adélie penguins. Sci Rep 11:1–15
Schumann N, Dann P, Arnould JP (2013) Use of terrestrial habitats by burrow-nesting seabirds in south-eastern Australia. Emu 113:135–144
Seddon PJ, Davis LS (1989) Nest-site selection by yellow-eyed penguins. Condor 91:653–659
Seddon PJ, Van Heezik Y (1991) Effects of hatching order, sibling asymmetries, and nest site on survival analysis of Jackass Penguin chicks. Auk 108:548–555
Sherley RB, Barham BJ, Barham PJ, Leshoro TM, Underhill LG (2012) Artificial nests enhance the breeding productivity of African Penguins (Spheniscus demersus) on Robben Island, South Africa. Emu 112:97–106
Sherley RB, Barham PJ, Barham BJ, Crawford RJ, Dyer BM, Leshoro TM, Makhado AB, Upfold L, Underhill LG (2014) Growth and decline of a penguin colony and the influence on nesting density and reproductive success. Popul Ecol 56:119–128
Steinfurth A (2007) Marine ecology and conservation of the Galápagos penguin, Spheniscus mendiculus PhD thesis. Christian-Albrechts-Universität, Ziel
Stevenson C, Woehler EJ (2007) Population decreases in little Penguins Eudyptula minor in southeastern Tasmania, Australia, over the past 45 years. Mar Ornithol 35:61–66
Stokes DL, Boersma PD (1991) Effects of substrate on the distribution of Magellanic Penguin (Spheniscus magellanicus) burrows. Auk 108:923–933
Stokes DL, Boersma PD (1998) Nest-site characteristics and reproductive success in magellanic penguins (Spheniscus magellanicus). Auk 115:34–49
Stokes DL, Boersma PD (2000) Nesting density and reproductive success in a colonial seabird, the magellanic penguin. Ecology 81:2878–2891
Trathan P, Croxall J, Murphy E (1996) Dynamics of Antarctic penguin populations in relation to inter-annual variability in sea ice distribution. Polar Biol 16:321–330
Trathan PN, García-Borboroglu P, Boersma D, Bost CA, Crawford RJ, Crossin GT, Cuthbert RJ, Dann P, Davis LS, De La Puente S (2015) Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conserv Biol 29:31–41
Trivelpiece WZ, Fraser WR (1996) The breeding biology and distribution of Adélie penguins: adaptations to environmental variability. Found Ecol Res West Antarct Penins 70:273–285
Velando A, Freire J (2001) How general is the central-periphery distribution among seabird colonies? Nest spatial pattern in the european shag. Condor 103:544–554
Volkman NJ, Trivelpiece W (1981) Nest-site selection among adélie, chinstrap and gentoo penguins in mixed species rookeries.Wilson Bull, pp. 243–8
Waluda CM, Dunn MJ, Curtis ML, Fretwell PT (2014) Assessing penguin colony size and distribution using digital mapping and satellite remote sensing. Polar Biol 37:1849–1855
Warham J (1974) The breeding biology and behaviour of the Snares crested penguin. J R Soc N Z 4:63–108
Weerheim MS, Klomp NI, Brunsting AMH, Komdeur J (2003) Population size, breeding habitat and nest site distribution of little penguins (Eudyptula minor) on Montague Island, New South Wales. Wildl Res 30:151–157
Whitehead MD, Johnstone GW (1990) The distribution and estimated abundance of Adélie Penguins breeding in Prydz Bay. Antarct Polar Biol 3:91–98
Wiebkin AS (2011) Conservation management priorities for little penguin populations in Gulf St Vincent. Report to Adelaide and Mount Lofty Ranges Natural Resources Management Board, Adelaide. SARDI Publication No. F2011/000188-1
Wiebkin AS (2012) Feeding and breeding ecology of little penguins (Eudyptula minor) in the Eastern Great Australian Bight. PhD Thesis, The University of Adelaide
Winter SJ (2000) Number and distribution of blue penguin (Eudyptula minor) nests in the Mount Maunganui area, Bay of Plenty. Notornis 47:160–162
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
Thanks to all the volunteers who helped collect the data, in particularly Sarah-Lena Reinhold for her contribution for the islands in the Spencer Gulf and Bianca Johnson for her help on Troubridge, Wardang and Goose Islands. Thanks to Barossa Helicopters and Rhyan Baffett for transport to the islands in the Spencer Gulf. Thanks to the local rangers on Wardang and Goose Islands (in particular Jasmine Swales and Max Barr) for their help with the surveys. Thanks to Chris and Judy Johnson for access to Troubridge Island. Thanks to Ingrid Stirnemann for advice on the manuscript and statistics.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding was provided by the Nature Foundation SA, Birds SA, Australian Geographic Society, The Alongside Wildlife Foundation, Flinders University, and the Adelaide & Mount Lofty Ranges Natural Resources Management Board.
Author information
Authors and Affiliations
Contributions
DCN designed the study and wrote the first draft of the manuscript. DCN and LI collected the data and analysed the data. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
This project was approved by the Flinders University Ethics Committee (E388-E449) and supported by a scientific permit to conduct the research (Y26040).
Consent to participate
Not applicable.
Consent for publication
All authors gave final approval of this manuscript for publication.
Additional information
Communicated by Stephen Garnett.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colombelli-Négrel, D., Iasiello, L. The importance of fine-scale landscape characteristics for habitat selection in penguins. Biodivers Conserv 32, 1369–1401 (2023). https://doi.org/10.1007/s10531-023-02557-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02557-3