Abstract
Mounting evidence suggests declines in the abundance and diversity of wild bees. Increasing habitat that provides forage and nesting sites could boost struggling populations, particularly in urban, suburban and agricultural landscapes. The millions of acres beneath aerial electric transmission lines, sometimes referred to as easements or rights-of-way, must be kept free of tall-growing vegetation and hence have the potential to provide suitable habitat for many native species. Prior work has demonstrated that bee communities in easements managed using alternatives to episodic mowing were more diverse than in nearby open areas, however true control sites within the easements were unavailable. In order to compare vegetation management protocols, we conducted a two-year study which enabled us to directly compare transmission line easements in three locations currently undergoing Integrated Vegetation Management—a dynamic form of management involving spot removal and herbicide treatment of unwanted species (treatment) with nearby sites undergoing standard management protocols of yearly or biyearly mowing (control). Results show that treatment sites had significantly higher abundance and species richness than controls. Seasonal differences were pronounced, with the spring fauna most affected by differences in vegetation management. In addition, the older treatment sites house more social bees, more parasitic species and a more even distribution of bees across nesting guilds. Finally, we established that treatment sites had distinct bee communities, further increasing their value as sources for native bee populations in the landscape. Overall, the data clearly show the value of implementing alternative active vegetation management in the land under powerlines to achieve an increase in the abundance and diversity of wild bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Public interest in the health of wild bee communities has primarily stemmed from concerns surrounding the provision of pollination services for agriculture, exemplified by President Obama’s Presidential Memorandum back in 2014 mandating a federal effort to promote the health of pollinators in the United States. The focus on pollination services makes sense considering that the economic value of these services is estimated to be between 100 and 200 billion dollars per year worldwide (Gallai et al. 2009; Klein et al. 2007). Although not all these animal pollinators are bees (Rader et al. 2016), bees have been shown to be the most important animal pollinators of agricultural crops worldwide (Williams et al. 2001). Focus has traditionally been on managed honeybees (Apis mellifera), but recent analyses have shown that wild bees as a group contribute roughly the same amount to crop pollination (Kleijn et al. 2015). Despite their significance as a group, it is also true that not all bee species are equally adept at or needed for sufficient pollination of crops (Kleijn et al. 2015) and although a greater diversity of bees has been implicated in increased pollination services (Fontaine et al. 2005; Perfectti et al. 2009) and functional redundancy in pollinator networks leading to more stable pollination services (Kaiser-Bunbury et al. 2017), it would be misguided to focus only on agricultural economics. Pollination is not only a requirement for crop species, but unmanaged vegetation as well. Ollerton et al. (2011) estimates that 74% of all plant species in temperate biomes and 94% in tropical areas are serviced by animal pollinators, a majority of which are bees (Williams et al. 2001), therefore healthy native plant communities require healthy bee communities.
It would be tough to argue that bees are not an ecologically and economically important group, but are they in need of support? Such increasing interest in pollination services to both wild and managed plants has unearthed concerning data on the declining status of many members of this Superfamily (Apoidea), leading, for example, to the first official listing as Endangered of a bee species (Bombus affinis, the rusty-patched bumblebee) by the United States Fish and Wildlife Service in 2017. Honeybees have also been in a well documented decline due to pesticide use and disease (e.g., Simon-Delso et al. 2014; Chensheng et al. 2014; Vidau et al. 2011; Henry et al. 2012; Cresswell 2011). The implicated pesticides have been demonstrated to have both lethal and detrimental sub-lethal effects on multiple species of native bees (Mayer and Lunden 1997; Scott-Dupree et al. 2009; Stark et al. 1995) and more recently, neonicotinoids have been shown to produce long-term population changes in wild bees in England (Woodcock et al. 2016) and be correlated with decreased yields in bee pollinated crops in Finland (Hokkanen et al. 2017). In addition to pesticides and disease, non-domesticated bees must also contend with declining, degraded and fragmented habitat. Recent research indicates broad global pollinator declines that further jeopardize pollination services to agriculture and natural habitats (Potts et al. 2010; Vanbergen and Insect Pollinators Initiative 2013).
How do we reverse these trends? Bees collect the floral resources pollen and nectar to sustain themselves and to provision for their offspring, and therefore require access to flowering plants for the duration of their adult lifespans. For bees that specialize on a limited group of plants, resource requirements are inflexible. The majority of bee species are pollen generalists that forage from a broader group and are flexible in their diet, but even these bees have ‘favorite’ plants (Roulston and Goodell 2011). Bees also require a place to build a nest to house their offspring. The specifics of the nesting requirements differ widely between families and include many distinct types of soil, existing cavities and even solid wood (Mitchell 1960, 1962). The proximity of floral resources to the nesting site is important as well because a bee’s foraging range is limited (by size and other species-specific factors). Bees are very efficient and show preference for floral resources that are closest to their nesting site (Westrich 1996; Gathmann and Tscharntke 2002; Williams and Kremen 2007).
Based on these requirements, the habitats that are most populated with wild bees are early to mid successional habitats that are dominated first by grasses and herbaceous plants, then later perennials, shrubs and young trees. These provide floral resources over much of the year (as opposed to forested areas where blooms are limited to early spring by the shade of leaf cover), as well as a diversity of nesting substrates (King and Schlossberg 2014; Tonietto et al. 2016). In reality, pollinators of all sorts (e.g., butterflies, moths, beetles, flies) as well as many bird species and small mammals rely on these “open” habitats. Unfortunately, such areas are becoming increasingly patchy and ephemeral, particularly in the landscape of the Eastern US as farmland is either developed or allowed to grow into forest (Litvaitis et al. 1999; Askins 2001; DeGraaf and Yamasaki 2003).
Many grassroots initiatives organized by non-profit advocacy groups have begun to promote pollinator and bee friendly habitats across the landscape, often focusing on pollinator-attracting seed mixes and/or detailed instructions for meadow installations on private lands or offering certification to companies and farms (e.g., Bee Better certified™ Xerces Society). All of these seek to engage either the public or private industry in efforts to defray the costs of the vegetation management necessary to keep habitats in an open, early to mid-successional state, something many habitat reserves and parks often do not have the resources to do. Electric power transmission companies, however, have funding for this management written into their business plan, as it is the responsibility of the transmission companies to keep vegetation from interfering with power flow, and hence must be kept far below line-level. Of land that is actively managed in the US, transmission line easements rank near the top in terms of total area, covering over four million hectares, not including smaller, low-voltage lines (Goodrich-Mahony 2017). Although not nationally quantified, a study from New York State (Confer and Pascoe 2003) demonstrated that utilities manage nearly eight times the amount of shrubland than other agencies within the state. Companies meet their responsibility in a number of ways, but historically the most common technique is episodic mowing, or large-scale herbicide application for inaccessible areas. In periods between treatments, this land provides early successional habitat that is used by birds (Askins et al. 2012; Marshall and VanDruff 2002; King and Byers 2002; Marshall et al. 2002; Confer 2002; Knight and Kawashima 1993), butterflies and moths (Berg et al. 2011, 2013; Komonen et al. 2013; Schweitzer et al. 2010; Wagner 2007), small mammals (Litvaitis 2001; Macreadie et al. 1998; Johnson et al. 1979), plants (Wagner et al. 2014a, b) and wild bees (Wagner et al. 2014a, b; Russell et al. 2005). Although a growing body of literature is demonstrating the general importance of this habitat (e.g., Hill and Bartomeus 2016; Sydenham et al. 2016), Wojcik and Buchmann in their review (2012) conclude that more detailed research on pollinators is needed to further quantify these benefits. Getting utility companies on board with modifying their vegetation management to better accommodate wildlife, especially wild bee communities, has the potential to substantially supplement and possibly swamp other small-scale efforts.
We know that open habitats are good, but how can management in powerline corridors be evaluated and improved to better accommodate wild bee communities? What should our criteria be? If the goal is to promote pollination services, then we need both an abundance of bees and a diversity of species. Large population sizes are usually linked to lowered risk of extinction, and diversity of pollinators has been found to increase pollination services, likely through redundancy (Steffan-Dewenter and Westphal 2008; Winfree et al. 2008). Many would argue, however, that richness and abundance are not the only (or even the most critical) gauge of healthy bee communities and functional group analysis should be included in any assessment (e.g., Sheffield et al. 2013a). Analyzing the impact of vegetation management on the representation of particular groups/guilds based on body size, sociality, trophic level, nest site preferences, foraging specificity, etc., can tell us more about community health than richness measures. Again, what should our criteria for success be based on these functional categories?
As it has been documented that body size is related to foraging distance (Greenleaf et al. 2007), we expect larger bees to forage further from their nests, whereas small bees are more likely to be residents where they are collected. If our goal is to create source populations that are able to provide services (and colonizers) to surrounding areas, then we would hope that our vegetation management protocol would result in more resident bees (practically measured based on size) by providing adequate floral and nesting resources. The creation of a diversity of nesting resources (woody debris, standing dead, variation in stem size classes, bare ground, etc.) should result in the presence of bees with a diversity of nesting preferences present in our managed site. Similarly, as we know that social bees also can only exist in places that have the resources to support some minimum number of colony members (which must be above the resource threshold required for a solitary bee) and these resources must be available consistently throughout the season, quality habitats should have a higher representation of social bees. Further, as social bees have been shown to be more severely impacted by isolation from natural habitat and patch size (Jauker et al. 2013; Williams et al. 2010, Winfree et al. 2009; Ricketts et al. 2008; Klein et al. 2002), we would expect that the increased presence of social species would be an indication of successful management. The presence of parasitic bee species (cleptoparasites of other bee species) are also relevant, as they add a trophic level and are thought to only persist where populations of their hosts reach a stable threshold, likely due to a sufficient resource base (see Sheffield et al. 2013a). Therefore, we should look for a management protocol that increases the number of parasites. Finally, if our goal is to support populations of floral specialists, management should increase the dominance of native forbs and shrubs.
In summary, what is needed is a management protocol that promotes habitat heterogeneity and stability of the floral and nesting resources that wild bees require (Steffan-Deweneter and Westphal 2008; Winfree et al. 2008; Greenleaf et al. 2007; Kremen et al. 2002). It seems unlikely that current standard practices are the best. Large-scale applications of general herbicides, or mowing (without regard to flowering season), produce dramatic (albeit temporary) decreases in floral resources. When repeated regularly, these management techniques cause overall diminishment of the variety of floral resources, and of the complexity of habitat required for the accumulation of nesting substrates (Potts et al. 2010; Dixon 2009). In addition, episodic mowing on transmission line easements favors invasive plants, which can sometimes be problematic for native wildlife (Drake et al. 2016; Freeman et al. 2014; Bezemer et al. 2014). One management strategy currently being explored by transmission companies throughout the US could potentially mitigate both problems. Integrated Vegetation Management (IVM) eschews mowing in favor of selective topping and/or selective herbicide treatment of tall growing species and other undesirables. In its ideal form, this technique results in a stable mosaic of meadow and scrub habitat and has the added benefit of working with the native seed bank, thereby increasing the predominance of native forbs and shrubs (Johnstone and Haggie 2014). Historically, this method was used only when transmission lines passed through habitat reserves or other protected areas (see Russell et al. 2005). But now, through a combination of motivations including improving public relations and reducing long-term costs, many companies are considering wider implementation of IVM. Establishing whether or not this management style creates healthy source populations for wild bees will have far reaching ramifications throughout the industry, as environmental departments within these utility companies are currently awaiting evidence that these expenditures are worth their time. In addition, IVM is not something that can only be adopted in powerline corridors, but rather could serve as a template for management of land in other areas where working with the native seed bank is preferable than bringing in seeds or plantings.
In order to test the following hypotheses, we will compare wild bee richness, abundance and community composition across management styles on powerline corridors by conducting concurrent surveys across the season over a two-year period along with quadrat sampling to measure habitat variables associated with nest site availability.
Hypothesis 1
Sites managed with Integrated Vegetation Management will have a higher richness and abundance of wild bees compared with sites subjected to periodic mowing.
Hypothesis 2
Sites managed with Integrated Vegetation Management will have bee communities with a more even distribution of nesting preferences than mowed sites, reflecting the greater abundance of cavity and stem nesting options.
Hypothesis 3
Sites managed with Integrated Vegetation Management will have a higher proportion of small bodied species compared with mowed sites, because bee body sizes correlate with foraging range.
Hypothesis 4
Sites managed with Integrated Vegetation Management will have more social species due to the increased resource base (nesting and floral) as compared with mowed sites.
Hypothesis 5
Sites managed with Integrated Vegetation Management will have more rare species, including parasitic species (due to the increased density of their hosts) and floral specialists (due to increase floral diversity).
Hypothesis 6
Any differences between IVM and other treatments (see hypotheses 1–5 above) will rapidly increase after the beginning of implementation, as the IVM sites are undergoing rapid ecological succession.
Materials and methods
Quantifying the benefits of this management style in situ is problematic from a design perspective, as utility company land managers implement management plans linearly along continuous strips of land, so pseudoreplication is an issue. Also, if we want to evaluate the potential of these managed lands to house stable wild bee communities (by providing both nesting habitat and forage), we would not expect to be able to do so before multiple years of alternate management had passed. Considering these constraints, we were able to find a system that allows some replication across multiple ROW lines and which had been established 2 years prior to the start of our study, giving the bee communities time to respond. We were given access to two new IVM trials, established in 2009, in the same geographic region as the previous studied long-term IVM sites on the Patuxent Wildlife Research Refuge in Maryland, USA, thus enabling us to make comparisons between newly established IVM sites, long-term IVM sites and sites managed with standard protocols of episodic mowing all within the same geographic region.
Three study regions were identified in Maryland (See Supplemental Materials: Appendix 1), corresponding to implementation of Integrated Vegetation Management (IVM) by a public utility company. Overall, we selected nine sites undergoing the standard management practice of episodic mowing (Control) and 29 sites managed with IVM (Table 1). Of those 29, eight were recent implementations of the IVM management protocol (IVM_new) and 21 were long-established IVM (IVM_old).
Site descriptions
Ann Arundel County, Patuxent Wildlife Research Center
PWRC has two transmission line easements running through it, one belonging to Baltimore Gas & Electric (BGE) and one belonging to Potomac Electric Power Company (PEPCO). Both have been managed using IVM for the past 40–50 years, whereby tall growing and undesirable species are treated with selective herbicides and other species growing higher than 3 m are topped every 4–5 years. Twenty-one sections of this habitat were identified and collection transects place within (IVM_old).
Ann Arundel County, Davidsonville and Howard County, Columbia
Two IVM trials were established in adjacent Counties by BGE in 2009, one in the town of Davidsonville and the other in Columbia. The section in Columbia is roughly 2 km long, 78 m wide and in a very suburban area with a walking path and public park directly underneath the transmission lines. The section in Davidsonville is roughly 8 km long and 122 m wide and traverses a rural to suburban gradient. Three sections were chosen for study in Columbia and five in Davidsonville for a total of eight IVM treatment sites (IVM_new). In addition, nine sites undergoing standard BGE management (annual/biennial mowing) were identified in the vicinity (Control). Controls were selected based on proximity to the treatment sites to reduce the effects of landscape context. Note that four control and three treatment sites were added after the first collection periods in May and an additional control site was added in 2012.
Data collection
Bee surveys
To compare the bee communities collected from sections of the transmission line easements that were managed using the standard practice of periodic mowing (Controls) with those that were managed using integrated vegetation management (IVM_new, IVM_old), bees were surveyed using modified pan traps (see Westphal et al. 2008). These traps consist of small white, 100 ml plastic cups that have either been painted with fluorescent blue or yellow paint (Guerra Paint and Pigment Corp, Fluorescent Blue FLB00002, Fluorescent Yellow FLY00002) or left white. These bowls are filled with water, with just enough dish soap to break the surface tension. One transect consisting of 15 bee bowls (five of each color, alternating) spaced in 5 m intervals was placed directly on the ground in each site. If possible, bowls were placed in areas of low vegetation (along paths) to increase visibility, however in some cases where this was not possible, the vegetation was cleared in the immediate vicinity of the bowl. The bowls were cleared of bees once a day for 3 days, yielding three 24-hour sampling periods. All traps were cleared on the same days within a 2–3 h period in order to reduce the impact of weather, to which bees are notoriously sensitive. Bees were sampled in May 2011 and 2012, late June/early July 2011 and 2012 and August 2011 and 2012. Oertli and colleagues (2015) found that seasonal turnover in bee species was pronounced and occurred in three clusters—spring (April/May), early summer and late summer and our sampling regime follows that same pattern in an attempt to give the greatest breadth to our community analysis. In addition, it is likely that vegetation management may have different impacts on these seasonal communities. Collections across years are vital as some of the management protocols being evaluated are recent implementations and therefore the plant communities will be a dynamic state as they undergo ecological succession.
Bees collected were then processed and labeled in the laboratory. All individuals, where possible, were identified to species with the help of online and print keys (Ascher and Pickering 2017; Gibbs 2011) as well as consultation with relevant specialists and reference specimens provided by Sam Droege. Individuals that could not be confidently given a species name were either assigned to a morphospecies within a genus (e.g., Lasioglossum species A; see Oliver and Beattie 1996) if intact or kept at the genus level if identifying characteristics were missing. All specimens from Davidsonville and Columbia were identified by Kimberly Russell and all specimens collected from Patuxent Wildlife Research Center were identified by Sam Droege. To avoid richness inflation, morphospecies were eliminated from analyses if present in both Russell’s and Droege’s collections (within the same genus) as Droege’s specimens were not kept so comparison was not possible. In addition, effort was made to accommodate taxonomic changes over the course of the study, resulting in some species groups being combined for analysis. See Supplemental Materials: Appendix 2 for a detailed accounting of specimen exclusions and grouping notes for analyses.
Nesting habitat
Inventorying solitary bee nests within a habitat is difficult, if not impossible (Jerry Rosen, personal communication). As a surrogate, we hypothesize that if a greater diversity of nesting habitat exists in a particular habitat, in this case, sites managed with IVM, we should be able to detect this by looking at the identity of bee species found there and their associated nesting preferences. Based on prior work (Russell et al. 2005), we expect mowed areas to be dominated by ground nesting bees, whereas IVM sites should show a more even distribution of individuals across nesting type.
In order to quantify how vegetation management might affect nesting habitat diversity (thereby driving differences in resultant bee communities), we conducted quadrat surveys in a subset of our study sites. Few protocols exist for measuring nest site availability, due to the difficulty of locating bee nests in situ. However, one can measure vegetation characteristics that are likely correlates of nest site diversity (e.g., Potts et al. 2005; Grundel et al. 2010). We randomly chose eleven sites for the quadrat surveys, spread across our three study regions including four of our Control sites, four of our IVM_new sites and three of our IVM_old sites. Within each site, we created a ~ 35 m transect perpendicular to the bee bowl transect, centered on bowl eight. Along this transect we evenly spaced out three 1 m by 1 m quadrats. Within each quadrat, we measured coverage of bare ground, litter, dead woody vegetation, live woody vegetation, grass, herbaceous vegetation and overall vegetation, using the following intervals: 0–5, 5–10, 10–15, 15–25, 25–35, 35–50, 50–75, 75–90, and 90–100%. We also recorded the number of live stems > 3 mm and number of dead stems > 3 mm. Note that areas covering a fine mix of substrates were counted in all relevant categories, so the percentages for a quadrat can total more than 100%.
Data analysis
Hypothesis 1
Sites managed with Integrated Vegetation Management will have a higher richness and abundance of wild bees. The basic, replicated unit of data is the transect, with response variables being the richness and abundance of bees in a transect. ‘Noise’ is between-transect variation stemming from a combination of real, fine-spatial-scale differences in local vegetation and other factors, and sampling variation. Both richnesses and abundances are counts, but in field samples these are typically overdispersed (more high and low values compared to a random distribution of items into sample ‘bins’). Analyses of variance therefore used generalized linear modeling (GLM) with a quasi-Poisson distribution and F-tests for model comparison. The model fit results confirmed the over-dispersion, which was mild for richnesses and strong for abundances (dispersion factors between 1 and 2 and between 5 and 20 respectively—exact values for each analysis are given in Supplemental Materials: Appendix 3). Another option would be a negative binomial distribution, but this more highly weights the influence of low-count samples in determining the influence of potential factors (Ver Hof and Boveng 2007), which there seems no reason to do. All GLM analyses were carried out in R (R Core Team 2017) using the glm function.
First, we predicted both richness and abundance using month and treatment, as factors each time, with data from both 2011 and 2012 combined. We were not able to use location as a factor because IVM_old is only found at the Patuxent Wildlife Research Center (PWRC), so any such analyses would be uninformative. In each analysis, we preformed the following sequence of tests: (1) test of the whole model including an interaction term, (2) assuming the whole model test is significant, test for significant interaction term, (3) if the interaction term is significant, test each factor (month, treatment) by comparing whole model with interaction to a model without that factor. If the interaction term is not significant, test each factor (month, treatment) by comparing whole model without interaction to a model without that factor. We note that most month-treatment combinations include transect data from more than one site, which introduces additional variation on top of expected between-transect variation. That extra ‘noise’ makes these tests conservative. For clarity we also combined the data across months and years, giving us an average richness and abundance estimate per transect for each site across the entire study period, and performed 1-way GLM ANOVAs in order to detect treatment effects across the duration of the study.
Because the IVM_old treatment is found at only one site (PWRC), and because this site was surveyed, and species identified, by a separate team of people, we repeated the analyses above with PWRC data excluded. Although our collecting techniques were passive and therefore not subject to collector bias, individuals do differ in how they classify species based on experience and access to references collections and literature. Therefore, it is important to verify that differences between IVM_old and the other sites are not inflated due to the experience gap between investigators. In addition, this exclusion allows us to isolate the effect of IVM from the potentially confounding effect of location, as IVM_old sites are clustered in space. If we still see a treatment effect when such a large portion of our data is removed (21 out of 38 sites), that would speak to the strength of the effect.
Hypothesis 2
Sites managed with IVM will have bee communities with a more even distribution of nesting preferences. We classified each species of bee according to its nesting preference, using both a two-way classification (cavity, ground) and a four-way classification (cavity, soil, stem, wood), which were analyzed separately. Bumblebees were excluded from these analyses because their complex nesting habits do not fit neatly into these categories (Hatfield et al. 2012). Species were classified individually where data were available, and using closest taxonomic relatives otherwise. We used a Chi square analysis (with intrinsic expected values) to test whether the proportions differed significantly between treatment types.
To detect if different treatments led to distinct vegetation profiles, and to characterize those profiles, quadrat data on vegetation characteristics that are likely correlates of nest site diversity were analyzed using Canonical Correspondence Analysis with randomization tests (Legendre and Legendre 1998) coded in Mathematica (Wolfram Research Inc. 2017). Coverage data from three quadrats were averaged to give one value for each site. Stem data by treatment were analyzed separately with a 1-way ANOVA.
Hypothesis 3
Sites managed with IVM will have a higher proportion of small bodied species. Because we were most interested in nesting behavior, we excluded males from this analysis. The mean body length (l) for females of most species was obtained from the literature. For morphospecies, or species where size information was not available, we either excluded these individuals or measured those that were available in our collections. Based on the overall distribution of lengths, we classified bees into the following categories: l < 6 mm “small”; 6 ≤ l < 9 “medium”; 9 ≤ l < 12 “large”; l ≥ 12 “extra-large”. We used a Chi square analysis (with intrinsic expected values) to test whether the proportions of bees in each size class differed significantly between treatment types.
Hypothesis 4
Sites managed with Integrated Vegetation Management will have more social species. Bee species were classified as social, solitary or parasitic based on data from the literature. Once again, we used Chi Square analysis (with intrinsic expected values), to test whether the proportions of these groups differed significantly between treatment types.
Hypothesis 5
Sites managed with IVM will have more rare species, including parasitic species and floral specialist. Parasitic species were identified as stated above. Species were classified as specialists primarily using criteria set forth by Fowler and Droege (2016), employing a mixture of weighing the literature, information from museum collections, our direct field experience, and vetting the list with other field experts to identify bee species that use pollen from only one or two genera of plants. The difficulty here is that our IVM_old sites were sampled much more intensively than either IVM_new or Controls, with 126 transects versus 45 and 46, respectively, making direct comparisons of species presence suspect. To correct for this, we used rarefaction to down-sample the IVM_old sites to 46 transects prior to making statistical comparisons.
Hypothesis 6
Any differences between IVM and other treatments (see hypotheses 1–5 above) will rapidly increase after the beginning of implementation. As we expected changes in vegetation within the IVM_new sites as they matured, we performed a 2-way GLM ANOVA on each year separately for all study areas and treatment types, comparing abundance and richness. If this maturation produces better habitat for wild bees, we would expect to see divergence between the controls and the IVM sites. For similar reasons, we performed CCA analysis on species relative abundance by treatment separately for 2011 and 2012 to see if IVM_new sites were becoming more distinct from Controls.
Trait correlations
Because a number of traits (nesting preference, body size, floral specialism, sociality) are analyzed across hypotheses 2 through 5, our interpretation of the results could be limited by correlations between them. For example, if body size is tightly correlated with sociality and both are more common in one treatment type, how can we meaningfully discuss the underlying mechanisms that produce each pattern separately? To examine the association between our life-history traits, we used Cramér’s V, which has the same range (0–1) and a similar interpretation to Pearson’s correlation coefficient, but can be applied to categorical data.
Results
Bee collections
9084 bees were collected, and 8638 were used in the species-level analyses (see Supplemental Materials: Appendix 2 for details of omitted specimens). 146 species were collected in all (of which only three were left as morphospecies), including ten new county records (Ann Arundel co.), one new state record and a species that has yet to be described in the literature. See Supplemental Materials: Appendix 4 for our full species list with classifications and counts.
As is the case in most studies making use of passive collecting techniques, especially bee bowls (Sam Droege, personal communication), we had a number of bowl tips or crushed bowls (or “tidied” bowls in one case) that may alter the uniformity of collection intensity at each site. Data were collected on bowl tips for all but one sampling period/site location combination from which the data on tips was lost. Although it is straightforward to standardize by the number of bowls when looking at abundance estimates, analyses involving corrected richness and species identity can quickly become complicated, as they rely on richness estimators and repeated randomized sampling to reveal potential corrected species lists. Since only a subset of samples had tipped bowls, comparisons between samples would quickly become statistically problematic. To investigate whether there were significant differences in total number of intact bowls by treatment type, we performed a simple 1-way ANOVA with all the bowl tip data we had and found that although the differences were significant (F2,193 = 3.83; p = 0.023), the magnitude of the difference was small with Control sites having an average of 43.13 bowls per collection period, IVM_new sites 42.8, and IVM_old 41.11. And as these < 5% differences in sampling intensity would only contribute to results in the opposite direction of our predictions, with Controls being oversampled and IVM_old being undersampled, we concluded that it was a conservative approach to use the uncorrected raw abundance and richness in all subsequent analyses. As a check on our decision, we did perform the overall analysis (2-way GLM ANOVA with month and treatment) using both adjusted and non-adjusted abundance measures and found it made only small differences to the magnitude of the treatment effect, leaving statistical significance unchanged. This being the case, we felt comfortable moving forward using the raw data.
See Supplemental Materials: Appendix 3 for output tables of all analyses organized by hypothesis.
Hypothesis 1
Sites managed with Integrated Vegetation Management will have a higher richness and abundance. First, the only analysis that allowed us to use all of the data collected (as sites were added over the course of the first year of the study to increase sample size) was to perform a 2-way GLM ANOVA with month and treatment, using data from both 2011 and 2012 as replicates. The whole model was significant for both bee richness (F8,216 = 8.70; p ≪ 0.0001) and bee abundance (F8,216 = 18.6; p ≪ 0.0001). There was highly significant variation from month to month in both richness (F6,214 = 10.7; p ≪ 0.0001) and abundance (F6,214 = 22.0; p ≪ 0.0001), with August having far fewer species and individuals than collections in May and July (Fig. 1a, b). There were also significant interactions between month and treatment (F4,212 = 2.91; p = 0.0225 and F4,212 = 5.30; p = 0.000441 respectively), perhaps indicating that vegetation management had different effects on the early versus late summer bee fauna. Treatment itself was a significant predictor of both richness (F6,214 = 2.78; p = 0.0126) abundance (F6,214 = 6.60; p ≪ 0.0001), with IVM_new sites hosting the highest numbers of bees.
Mean number of bee species per transect (a) and the mean number of individual bee specimens per transect (b) collected in each site category by month in Maryland over the two-year span of the project. Error bars show one standard error from the mean. IVM_new refers to sites for which integrated vegetation management had been implemented in 2009. IVM_old refers to sites for which integrated vegetation management had been in place for over three decades. Control refers to sites under standard company management, in this case, annual mowing. 2-wayANOVA results indicate that Control sites differ significantly from IVM_new and IVM_old and that there were significant seasonal effects for both richness and abundance. 1-way ANOVA results on combined data across all six sampling periods indicate that Control sites differ significantly from IVM_new and IVM_old in both per transect richness (25.6, 30, 31.9, respectively; F2,34 = 3.70, p = 0.0352) and abundance (146.5, 229.75 and 260.4, respectively; F2,34 = 4.544, p = 0.0178)
After combining all the data across months and years, giving us an average richness and abundance estimate per transect for each site across the entire study period, we saw clear and significant differences, with control sites having the least species (25.6) and individuals (146.5) and IVM_old the most (31.9 and 260.4, respectively).
Excluding IVM_old and therefore comparing just IVM_new and Controls, we found that although the whole model was highly significant for both abundance and richness, only abundance showed a significant treatment effect (richness: F1,88 = 3.57, p = 0.062; abundance: F1,88 = 7.87; p = 0.00621 respectively) with no interaction between month and treatment.
Hypothesis 2
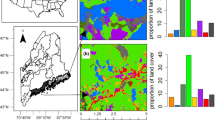
Sites managed with IVM will have bee communities with a more even distribution of nesting preferences. Results of the Chi Square analysis demonstrated that the proportions of bees across nesting preference types was significantly associated with treatment type for both the 2-category (X2 = 16.9, df = 1, p < 0.0001) and 4-category classification (X2 = 24.2, df = 3, p < 0.0001), with IVM_old having the most even distribution across nesting types. Differences between IVM_new and Controls were not significant. Looking at the vegetation data, the first CCA axis is a significant predictor of cover types (CCA randomization test, p = 0.0068), and separates herbaceous vegetation and grasses from woody species. Overlaying the treatments, it is clear that that IVM eventually (IVM_old) results in more woody plants, especially dead ones (Fig. 2). ANOVA results indicate that the IVM_old and IVM_new sites also had significantly more dead stems > 3 mm (F = 5.653; p = 0.0295), but not live stems, compared to Control sites. Intriguingly, the second vegetation axis suggests that the main difference between control (mowed) sites and recently-started IVM_new treatments is that the latter sites have more bare ground exposed, but the bee collections do not show a different proportion of ground-nesting individuals.
Site differences in potential nesting substrate correlates based on quadrat data. Visual results of Canonical Correspondence Analysis performed to evaluate differences in percent cover by treatment type illustrating the most important variables separating IVM_old from the other site types. Coverage data from three quadrats were averaged to give one value for each site
Hypothesis 3
Sites managed with IVM will have a higher proportion of small bodied species. The proportion of bees in each size class was significantly associated with treatment type (X2 = 323, df = 3, p < 0.0001), with IVM_old having the highest percentage of small bees and Controls the lowest (Fig. 3).
Percentage of female individuals categorized by size class. Species were categorized into size classes (< 6 mm “small”; = 6<9 “medium”; = 9<12 “large”; ≥ 12 “extra-large”) based on mean body length estimates for females as documented in the literature. For morphospecies, or species where size information was not available, we either excluded these individuals or measured those that were available in our collections. We found that treatment types did vary in the proportion of bees in each size class (X2 = 323; p < 0.0001), with IVM_old having the highest percentage of small bees
Hypothesis 4
Sites managed with Integrated Vegetation Management will have more social species. The proportions of social, solitary and parasitic bees in each treatment type also differed significantly (X2 = 43.2, df = 2, p < 0.001). IVM_old had the highest proportion of individuals belonging to social species. Because of this result, we consider the possibility that the significant differences in abundance by treatment given above could be attributable to the social species swamping out the solitary species, as we would expect social species to occur at higher densities. To explore this further, we omitted 31 species considered to be partially or fully social and repeated the 2-way GLM analyses of richness and abundance from Hypothesis 1 on the remaining solitary species. Interestingly, despite the reduction in sample size this entailed, the richness differences between treatment types was even more significant, with IVM sites again having higher average richness per transect than controls (F2,205 = 6.96; p = 0.00119). The abundances, however, became statistically indistinguishable.
Hypothesis 5
Sites managed with IVM will have more rare species, including parasitic species (due to the increased density of their hosts) and floral specialists (due to increase floral diversity). Across the entire collection, there were seventeen species classified as specialist and eighteen as parasitic. The raw data show that IVM_old sites combined have both more parasitic and specialist individuals and species than either IVM_new or Control sites [parasitic: 45 (14) vs. 13 (6) vs. 5 (4); specialist: 186 (12) vs. 21 (4) vs. 34 (9), respectively]. This is to be expected, however, because the IVM_old treatment was sampled by 126 transects, whereas IVM_new and Control sites were sampled by 45 and 46 transects respectively. We therefore used rarefaction to down-sample the IVM_old sites to 46 transects. After performing 1000 resamplings, the number of specialist species in IVM_old sites was not significantly different than in the other treatment types, however, the number of individuals representing these species was still higher compared with IVM_new and Control sites (average 65.9 individuals with only three samples out of 1000 dipping to 34 individuals). As for parasitic species, after rarefaction, IVM_old retained both more species (average 7.5) and more individuals (average 16.1) than the other treatments. To test the significance of these differences, we calculated a p value as the frequency of rarefied IVM_old values that were the same or smaller than the observed values from the Control (one-tailed test). The results were 44/1000 (p = 0.044) for species and 19/1000 (p = 0.019) for the individuals. Using the same method, differences between IVM_new and IVM_old were found to not be significant.
Hypothesis 6
We predict that the newer IVM sites will exhibit directional change in the bee communities over time (in addition to seasonal changes) as these sites are undergoing rapid ecological succession. Results of the Canonical Correspondence Analysis showed differences between all site types, with the first CCA axis clearly separating sites managed for the longest time (IVM_old) from the others (CCA randomization test, p < 0.001), and the second axis separating IVM_new from mowed Control sites. When performed by year, the separation of IVM_new from Control sites was much clearer in 2012 than 2011 (Fig. 4a, b), as predicted by the hypothesis of rapid initial divergence through succession. Repeating the analyses of richness and abundance data from Hypothesis 1, but using data only from 2011, the significant predictors were the same as for both years combined, except that there was no longer a significant interaction between month and treatment in predicting richness, likely because the effect of treatment itself on richness was now negligible. Treatment still affected bee abundance (F6,101 = 17.4; p ≪ 0.0001). In 2012, month was again a significant predictor of richness (F2,110 = 29.5, p ≪ 0.0001) and abundance (F2,110 = 27.193, p ≪ 0.0001), but this time so was treatment, with IVM sites hosting greater numbers of species (F2,110 = 6.96, p = 0.00144) and individuals (F2,110 = 6.52, p = 0.00213). There were no significant interactions between month and treatment. It seems likely that changes in the IVM_new sites were driving this pattern, as we would expect IVM_old to be in a much less dynamic state (having been managed with IVM for many decades). To explore this idea, we combined the data across months to calculate total richness and abundance by transect for each year. This time performing a 1-way GLM ANOVA separately for 2011 and 2012, we saw that the small differences in richness and abundance between treatments and controls apparent in 2011 became significant only in 2012 (2011 Richness: F1,14 = 0.103, p = 0.753; 2012 Richness: F1,14 = 6.876, p = 0.019; 2011 Abundance: F1,15 = 0.319, p = 0.5812; 2012 Abundance: F1,15 = 7.545, p = 0.015). Taken together, these results show a progressive divergence of bee communities once IVM treatment begins, with increases in both richness and abundance detectable after 2 years.
Graphical results of the Canonical Correspondence Analysis on bee species collected in Maryland by treatment type in 2011 (a) 2012 (b). Each symbol is data from a single site and symbol type (circle, triangle, star) represents treatment type. IVM_new refers to sites for which integrated vegetation management had been implemented in 2009. IVM_old refers to sites for which integrated vegetation management had been in place for over three decades. Control refers to sites under standard company management, in this case, annual mowing. Although statistically significant in both cases (p < 0.001), the degree of overlap across the axes appears to decrease between 2011 and 2012, illustrating the divergence of the Control and IVM_new sites
Trait correlations
Associations between species traits as quantified by Cramér’s V ranged from 0.133 (nest preference and specialization), which is very low, to 0.45 (specialization and body size), which is moderate (all values are shown in Supplemental Materials: Appendix 3). In fact, the relatively moderate but consistent association between body size and other traits is typical of data from across the animal kingdom (Peters 1983), but none of the associations here were strong enough to make a trait redundant.
Discussion
The method in which vegetation under transmission lines is managed clearly impacts the resident bee communities. In areas that have been managed in an integrated way—with topping of tall vegetation and selective extraction of tall growing species and problematic invasive species—richness of wild bees is higher and bees are more abundant (Fig. 1). Clearly, when IVM is practiced in the long run, as it has been in the Patuxent Wildlife Research Center, the bee community flourishes and includes rare and unique species, as well as species that make use of the greater diversity of nesting habitats available (Fig. 4). Russell and colleagues (2005) demonstrated that these areas had higher bee richness than mowed areas on the reserve itself, though the mowed areas were not directly comparable due to the fact that they were not part of the easement. The study detailed here complements this work by showing that the IVM-managed sites on PWRC do, in fact, provide superior habitat for wild bees compared with other nearby easements managed with the more traditional method of episodic mowing.
Interestingly, even after a few years of alternative management, changes in the bee communities become statistically significant as the vegetation begins to diverge from those areas managed through episodic mowing. Analyses show that these areas are diverging from control sites (mowed areas) in terms of richness, abundance and species identity (Fig. 4). This is likely due to the combined effect of an increase in floral diversity and abundance, as well as an increase in potential nesting habitat. Lonsdorf and colleagues (2009), in developing their model of pollination services across agricultural landscapes, found that their model’s predictions depended largely on the availability of nesting and floral resources. Although it was beyond the scope of this study to quantify floral abundance throughout the season beyond anecdotal observations, other studies have shown a significant correlation between bee and plant diversity (Ebeling et al. 2008, 2012; Frund et al. 2010; Potts et al. 2003) therefore we expect that floral resources are a likely contributor to the high diversity of bees found at the IVM sites. Less well documented is the significance of nesting substrate diversity as a determinant of bee species richness. A study by Steffan-Dewenter and Schiele (2008) comes the closest to demonstrating nest-site limitation in a population of a solitary bee, Osmia rufa (now synonymized with O. bicornis), but community-level studies of this nature have yet to be carried out. However, those relating potential nest site availability to bee richness have found similar evidence for bottom-up influences on community structure (Grundel et al. 2010; Potts et al. 2005).
Our analyses showed that the IVM_old sites had a more even distribution of bees across nesting guilds, with a noted increase in the proportion of cavity and wood nesting bees relative to control or IVM_new sites. Multiple studies have documented how nesting guilds are differently affected by disturbance (Williams et al. 2010; Winfree et al. 2009; Ricketts et al. 2008), so we expected that differences would exist based on these major differences in habitat management. Quadrat surveys employed to measure nest site diversity illustrate that IVM_old sites had larger numbers of dead woody stems present and more live and dead woody cover overall (Fig. 2), all of which help explain the higher diversity of cavity nesting bees, thereby increasing the overall diversity of bees at these sites. The IVM_new sites have not diverged detectably from Controls for these measures, which is not particularly surprising given that these sites are only 3 years post mowing and it takes time for litter and woody debris to accumulate. Ricketts and colleagues (2008) showed that bees which nest above ground level (mostly cavity nesters) were negatively affected by fire disturbance in the short-term, but that this effect disappears within 5 years. This is likely due to the immediate destruction of nest sites, but also the burning of woody debris. In time, the burns facilitate the growth of woody shrubs and eventually litter and woody stems accumulate again.
We expect this process to be accelerated in IVM sites due to the use of selective herbicides and tree topping, as woody debris and standing dead are created immediately. We suggest that in the short-term, IVM creates an abundance of floral resources that are exploited quickly by both resident species and long-distance foragers. As woody species move in and litter and woody debris accumulate, more diverse nesting substrates become available and the diversity of the resident bee community increases. Of course, we cannot discount the possibility that the surrounding landscape may have an impact on nesting guilds (Neame et al. 2013), as all of the IVM_old sites are located within a habitat preserve. However, our quadrat data do suggest real differences in nesting materials available, and many of the IVM_new and Control sites were also bordered by trees and semi-natural landscapes, at least in part.
The spatial context of the three easements studied here differs profoundly, ranging from the relatively undisturbed in the Patuxent Wildlife Research Center to the highly urbanized around the section of line in Columbia. Davidsonville provides an interesting intermediate context that is part rural, part suburban. Landscape context is important for two reasons. First, it determines the pool of species available to colonize appropriate habitats. It is possible that context may swamp the effects of vegetation management, therefore it is important to look at within-region differences. Unfortunately, our only example of long-term IVM management comes from PWRC so we are unable to separate the impact of the management from the context of the habitat preserve. Previous work suggests that PWRC does have an overall high abundance of bees, but also that there still is a statistically detectable difference in bee richness between collection sites located within the easement as compared to other collection sites on the reserve (Russell et al. 2005). This suggests that the vegetation management is important for promoting bee richness, and perhaps other areas benefit from proximity to the ROW. In this study, eliminating PWRC from the analysis allows us to look at the effects of location and treatment (IVM vs. mow) using data only from Davidsonville and Columbia. This is where we see an interesting trend where differences in bee richness went from being non-significant in 2011 to a highly significant difference in 2012, with these newer IVM sites having more species than mowed sites. This is despite the fact that the urban setting of the Columbia sites introduced many sources of error (e.g., dogs tipping bowls, people throwing bowls away) that resulted in low collection yields. It is also possible that collection yields in Columbia were low also due to its urban context and small area (~ 2 km), i.e., there were few source populations for bees to colonize from despite the high quality of the habitat. In this case, we would expect the build-up of bee species to take place over a longer period of time than in the Davidsonville sites, where natural areas are more abundant in the landscape surrounding the easement. Only future sampling will allow us to parse this out. Still, the strong emerging differences between the IVM and mowed sites in Davidsonville speaks to the importance of vegetation management in providing quality habitat for wild bees in the long run.
As the average flight time for most solitary bees is roughly six weeks, seasonality in bee communities is expected and found in prior work in this system (Russell et al. 2005). In this study, we see that although June/July had the highest average richness and abundance of bees, the biggest difference between the treatment types was in May (Fig. 1). This was consistent across both years of study, despite 2012 being strikingly more hot and dry early in the season than 2011. This suggests that differences in management most affect the spring bee fauna, perhaps by providing early season floral resources that may be lacking in grass dominated control sites. Buri and colleagues (2014) showed that for meadows, timing of mowing was significant, with early mowing being detrimental to bee populations both within and between years. Interestingly, by August, the oldest IVM sites had the lowest raw abundance and richness of bees, though these differences are not statistically significant (we suggest, however, that the small sample sizes due to the lower catch-rate overall may partly explain the inability to detect significance). This may simply illustrate that the benefit of alternative management disappears by the late summer, when the overall abundance of bees is typically low. It would be interesting to know how other open sites in the environment vary in this regard, i.e., is habitat that provides for the early season fauna more limited than mid to late summer throughout the landscape? By July, the overall abundance of bees is very high and the differences between treatment types more subtle; bees may be flying further from their nests (e.g., bumblebee cohorts get larger as the season progresses), so due to the passive nature of our collection techniques, we may not be sampling as locally in July as we are in May.
It is intriguing that the easement sites with the longest history of integrated vegetation management also had the largest number of both small (Fig. 3) and social bees. Small bees are unable to travel great distances to forage and must position their nests close to adequate floral resources to survive (Zurbuchen et al. 2010a, b; Greenleaf et al. 2007; Gathmann and Tscharntke 2002). We interpret this as a sign that these bees are residents of the easement and not just foragers coming from the surrounding landscape, something that cannot necessarily be ruled out for bigger species. If this inference is true, then the IVM managed sites are supporting more resident bees than control sites, potentially due to nest site availability or floral resources (see above). An alternate explanation is that mowing causes a population crash and that it simply takes longer for small bees to recolonize, hence their being the fewest in the most recently mowed sites. The proportion of large bees was greater in Control and IVM_new sites, again suggesting that some of these bees are foragers coming from the surrounding landscape. Some would argue that from a physiological standpoint, it takes higher quality habitat with more floral resources to support large bees. If that were true, then it is surprising how few we found in the IVM_old sites. However, conflicting results abound when studies attempt to correlate body size to habitat loss and disturbance (Kremen et al. 2007; Winfree et al. 2007; Cane et al. 2006). Certainly, the patterns that we see are better explained by relative mobility and foraging range of small and large bees.
In terms of sociality, other studies have documented that social species are more sensitive to habitat area (Jauker et al. 2013) and landscape context than solitary species (Jauker et al. 2013; Williams et al. 2010; Winfree et al. 2009; Ricketts et al. 2008; Klein et al. 2002). Most attribute this to correlated differences in nesting substrate and nest location (Winfree et al. 2009; Ricketts et al. 2008), which would be consistent with our findings, although perhaps premature to discount the importance of floral resources. These resources must be more abundant and more consistent across the growing season to support a social colony compared to solitary species, most of which are univoltine. Although these groups (small bees and social bees) are not entirely independent, the abundance of both at the PWRC sites indicates that the easements located there can adequately support these bees, whereas recently mowed areas cannot.
The disproportionate presence of parasitic species in these older habitats also supports the idea that these bee communities have a resource base that can support multiple trophic levels. Sheffield and colleagues (2013a) argue that (clepto)parasitic bees are important indicators of ecological health and are a stabilizing force in bee communities. Their research along a management gradient in Canada, from commercial orchards to abandoned old fields showed an inverse relationship between parasite diversity and agricultural management intensity. They also found that the sites with higher parasite abundance tended to have a more even species distribution, further supporting the idea that parasites may increase species diversity, like predators, by modulating the population size of the dominant species. In contrast and contrary to expectations, Jauker and colleagues (2013) in their study of calcareous grasslands in central Germany failed to find a similar relationship in that parasite species were less impacted by habitat size and landscape context compared to nesting species. However, these studies were quite different in methodology, in the habitats examined and in the questions asked. Sheffield and colleagues (2013b) sampled with bowl traps (in place continuously for multiple weeks), whereas Jauker and colleagues performed timed observational transects (20–60 min per site) with net collection of species. This is relevant, as parasitic species are less efficiently sampled due to their limited foraging needs and consequently over-represented among singletons (Oertli et al. 2005). As such, sampling method may influence abundance measures (moreso than for nesting species), making comparisons problematic. Also, the calcareous grasslands sampled by Jauker and colleagues are themselves highly managed habitats, so may not be comparable to the successional gradients examined by Sheffield et al. and in the study presented here. Although our study supports the idea that parasitic bees are a good indicator of overall bee community health and in agreement with Sheffield and colleagues (2013a), future work should investigate the role of sampling methods on estimates of parasite diversity, as we cannot adequately separate out this effect from the impact of other potential explanatory variables. Regardless, we find it encouraging that the new IVM sites appear to be quickly catching up in terms of numbers of parasitic species and individuals after only 3 years of management. The fact that we do not see this pattern with the specialist species may simply be an artifact of patchy plant distributions that are more tightly correlated with soil chemistry or topology than management protocols. However, because floral specialist bees are thought to be most at risk in modern landscapes (Bartomeus et al. 2013), it is important to investigate this lack of correlation in future studies. It was beyond the scope of this paper to conduct species level plant surveys at all sites, but such work would likely illuminate the patterns found.
Conclusions
The creation and maintenance of bee-friendly habitat is a critical step in promoting healthy bee communities that are capable of pollinating native and agricultural plants. Bees need flowers for food and to provision for their young, and they need appropriate places to build their nests. Transmission line easements provide these resources, particularly when they are managed in a dynamic, integrated way that promotes a healthy mix of shrubs and herbaceous plants. This study documents that easements managed in this way do, in fact, provide habitat that supports a greater diversity of bee species as compared to the more traditional management of episodic mowing. These easements have the potential to be of even greater value to the surrounding landscape than other open areas because they have high connectivity and are maintained over the long-term. Future research will seek to quantify this value by looking for evidence of increased pollination services to neighboring habitats. There will always be stretches of easement that must be mowed or must be treated with general herbicides due to location and topology, and the details of optimal management may be biome dependent. However, if transmission companies would consider managing even 25% of their lines using IVM, they would create more than 1 million hectares of important habitat in the US alone (Goodrich-Mahony 2017) that could benefit not only bees, but other species that have similar requirements including butterflies, birds and small mammals. As such, from the perspective of these creatures at least, transmission line easements should stop being viewed solely as scars on the landscape and instead be viewed as potential linear wildlife preserves.
References
Ascher JS, Pickering J (2017) Discover life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). http://www.discoverlife.org/mp/20q?guide=Apoidea_species
Askins RA (2001) Sustaining biological diversity in early successional communities: the challenge of managing unpopular habitats. Wildl Soc Bull 29:407–412
Askins RA, Folson-O’Keefe CM, Hardy MC (2012) Effects of vegetation, corridor width and regional land use on early successional birds on powerling corridors. PLoS ONE 7:e31520
Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R (2013) Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc Natl Acad Sci 110(12):4656–4660
Berg Å, Ahrné K, Öckinger E, Svensson R, Söderström B (2011) Butterfly distribution and abundance is affected by variation in the Swedish forest-farmland landscape. Biol Conserv 144(12):2819–2831
Berg Å, Ahrné K, Öckinger E, Svensson R, Wissman J (2013) Butterflies in semi-natural pastures and power-line corridors–effects of flower richness, management, and structural vegetation characteristics. Insect Conserv Divers 6(6):639–657
Bezemer TM, Harvey JA, Cronin JT (2014) Response of native insect communities to invasive plants. Annu Rev Entomol 59:119–141. https://doi.org/10.1146/annurev-ento-011613-162104
Buri P, Humbert JY, Arlettaz R (2014) Promoting pollinating insects in intensive agricultural matrices: field-scale experimental manipulation of hay-meadow mowing regimes and its effects on bees. PLoS ONE 9(1):e85635
Cane JH, Minckley RL, Kervin LJ, Roulston TAH, Williams NM (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol Appl 16(2):632–644
Chensheng LU, Warchol KM, Callahan RA (2014) Sub-lethal exposure to neonicotinoids impaired honey bees winterization before proceeding to colony collapse disorder. Bull Insectol 67(1):125–130
Confer JL (2002) Management, vegetative structure and shrubland birds of rights-of-way. In: Goodrich-Mahony JW, Mutrie DF, Guild CA (eds) The seventh international symposium on environmental concerns in rights-of-way management. Elsevier, Oxford, pp 373–381
Confer JL, Pascoe SM (2003) Avian communities on utility rights-of-way and other managed shrublands in the northeastern United States. For Ecol Manage 185:193–205
Cresswell JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20:149–157
DeGraaf RM, Yamasaki M (2003) Options for managing early-successional forest and shrubland bird habitats in the northeastern United States. For Ecol Manage 185(1):179–191
Dixon KW (2009) Pollination and restoration. Science 325:571–573
Drake KK, Bowen L, Nussear KE, Esque TC, Berger AJ, Custer NA, Waters SC, Johnson JD, Miles AK, Lewison RL (2016) Negative impacts of invasive plants on conservation of sensitive desert wildlife. Ecosphere 7(10):e01531
Ebeling A, Klein AM, Schumacher J, Weisser WW, Tscharntke T (2008) How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117(12):1808–1815
Ebeling A, Klein AM, Weisser WW, Tscharntke T (2012) Multitrophic effects of experimental changes in plant diversity on cavity-nesting bees, wasps, and their parasitoids. Oecologia 169(2):L453–L465
Fontaine C, Dajoz I, Meriguet J, Loreau M (2005) Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4(1):e1
Fowler J, Droege S (2016) Specialist bees of the Mid-Atlantic and Northeastern United States (Website). Available online
Freeman ED, Sharp TR, Larsen RT, Knight RN, Slater SJ et al (2014) Negative effects of an exotic grass invasion on small-mammal communities. PLoS ONE 9(9):e108843. https://doi.org/10.1371/journal.pone.0108843
Fründ J, Linsenmair KE, Blüthgen N (2010) Pollinator diversity and specialization in relation to flower diversity. Oikos 119(10):1581–1590
Gallai N, Salles JM, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68(3):810–821
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71:757–764
Gibbs J (2011) Revision of the metallic Lasioglossum (Dialictus) of eastern North America (Hymenoptera: Halictidae: Halictini). Zootaxa 3073:1–216
Goodrich-Mahony J (2017) EPRI Project Manager. Insights from the literature and stepwise guidance for vegetation managers interested in promoting pollinators on electric transmission line rights-of-way. Technical Report, March 2017
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153(3):589–596
Grundel R, Jean RP, Frohnapple KJ, Glowacki GA, Scott PE, Pavlovic NB (2010) Floral and nesting resources, habitat structure, and fire influence bee distribution across an open-forest gradient. Ecol Appl 20(6):1678–1692
Hatfield R, Jepsen S, Black SH, Shepherd M (2012) Conserving bumble bees: guidelines for creating and managing habitat for Americas declining pollinators. The Xerces Society for Invertebrate Corporation, Portland, p 32
Henry M, Beguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Hill B, Bartomeus I (2016) The potential of electricity transmission corridors in forested areas as bumblebee habitat. R Soc Open Sci 3:160525. https://doi.org/10.1098/rsos.160525
Hokkanen HMT, Menzler-Hokkanen I, Keva M (2017) Arthropod-Plant Interact 11:449. https://doi.org/10.1007/s11829-017-9527-3
Jauker B, Krauss J, Jauker F, Steffan-Dewenter I (2013) Linking life history traits to pollinator loss in fragmented calcareous grasslands. Landsc Ecol 28:107–120
Johnson WC, Schreiber RK, Burgess RL (1979) Diversity of small mammals in a powerline right-of-way and adjacent forest in east Tennessee. Am Midl Nat 101(1):231–235
Johnstone RA, Haggie MR (2014) Integrated vegetation management partnership approach to meeting NERC electric transmission vegetation standards. In: Doucet J (ed) Environmental Concerns in rights-of-way management 10th International Symposium. Elsevier, Oxford, pp 371–384
Kaiser-Bunbury CN, Mougal J, Whittington AE, Valentin T, Gabriel R, Olesen JM, Bluthgen N (2017) Ecosystem restoration strengthens pollination networks. Nature 542:223–227
King DI, Byers BE (2002) An evaluation of powerline rights-of-way as habitat for early-successional shrubland birds. Wildl Soc Bull 30(3):868–874
King DI, Schlossberg S (2014) Synthesis of the conservation value of the early-successional stage in forrests of eastern North America. For Ecol Manage 324(2014):186–195
Kleijn D, Winfree R, Bartomeus I et al (2015) Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat Commun. https://doi.org/10.1038/ncomms8414
Klein A, Steffan-Dewenter I, Buchori D, Tscharntke T (2002) Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv Biol 16:L1003–L1014
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Br 274:303–313
Knight RL, Kawashima JY (1993) Responses of raven and red-tailed hawk populations to linear rights-of-way. J Wildl Manage 57(2):266–271
Komonen A, Lensu T, Kotiaho JS (2013) Optimal timing of power line rights-of-ways management for the conservation of butterflies. Insect Conserv Divers 6(4):522–529
Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. PNAS 99:1–5
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Legendre P, Legendre L (1998) Numerical Ecology, 2nd edn. Elsevier, Oxford
Litvaitis JA (2001) Importance of early successional habitats to mammals in eastern forests. Wildl Soc Bull 29(2):466–473
Litvaitis JA, Wagner DL, Confer JL, Tarr MD, Snyder EJ (1999) Early-successional forests and shrub-dominated habitats: land-use artifact or critical community in the northeastern United States? Northeast Wildl 54:101–118
Lonsdorf E, Kremen C, Ricketts T, Winfree R, Williams N, Greenleaf S (2009) Modelling pollination services across agricultural landscapes. Ann Bot 103(9):1589–1600
Macreadie J, Wallis RL, Adams R (1998) A small mammal community living in a powerline easement at Bunyip State Park, Victoria. Vic Nat 115(4):120–123
Marshall JS, VanDruff LW (2002) Impact of selective herbicide right-of-way vegetation treatment on birds. Environ Manage 30(6):801–806
Marshall JS, VanDruff LW, Shupe S, Neuhauser E (2002) Effects of powering right-of-way vegetation management on avian communities. In: Goodrich-Mahony JW, Mutrie DF, Guild CA (eds) The Seventh International Symposium on Environmental Concerns in Rights-of-Way Management. Elsevier, Oxford, pp 355–362
Mayer DF, Lunden JD (1997) Effects of imidacloprid insecticide on three bee pollinators. Hortic Sci 29:93–97
Mitchell TB (1960) Bees of the Eastern United States, vol I. The North Carolina Agricultural Experiment Station, Technical Bulletin
Mitchell TB (1962) Bees of the Eastern United States, vol II. The North Carolina Agricultural Experiment Station, Technical Bulletin
Neame LA, Griswold T, Elle E (2013) Pollinator nesting guilds respond differently to urban habitat fragmentation in an oak-savannah ecosystem. Insect Conserv Divers 6(1):57–66
Oertli S, Muller A, Dorn S (2005) Ecological and seasonal patterns in the diversity of a species-rich bee assemblage (Hymenoptera: Apoidea: Api- formes). Eur J Entomol 102:53–63
Oliver I, Beattie AJ (1996) Designing a cost-effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecol Appl 6:594–607
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Perfectti F, Gómez JM, Bosch J (2009) The functional consequences of diversity in plant–pollinator interactions. Oikos 118(9):1430–1440
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P (2003) Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84(10):2628–2642
Potts SG, Vulliamy B, Roberts S, O’Toole C, Dafni A, Ne’eman G, Willmer P (2005) Role of nesting resources in organizing diverse bee communities in a Mediterranean landscape. Ecol Entomol 30(1):78–85
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org/
Rader R, Bartomeus I, Garibaldi LA, Garratt MPD, Howlett BG, Winfree R, Cunningham SA, Mayfield MM, Arthur AD, Andersson GLU et al (2016) Non-bee insects are important contributors to global crop pollination. Proc Natl Acad Sci 113(1):146–151
Ricketts TH, Regetz J, Steffan-Dewenter I, Cunningham SA, Kremen C, Bogdanski A et al (2008) Landscape effects on crop pollination services: are there general patterns? Ecol Lett 11(5):499–515
Roulston TH, Goodell K (2011) The role of resources and risks in regulating wild bee populations. Annu Rev Entomol 56:293–312
Russell KN, Ikerd H, Droege S (2005) The potential conservation value of unmowed powerline strips for native bees. Biol Conserv 124(1):133–148
Schweitzer DF, Minno MC, Wagner DL (2010) Rare, declining, and poorly known butterflies and moths (Lepidoptera) of forests and woodlands in the eastern United States. US Forest Service, Forest Health Technology Enterprise Team, FHTET-2011-01, Morgantown
Scott-Dupree CD, Conroy L, Harris CR (2009) Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J Econ Entomol 102:177–182
Sheffield CS, Pindar A, Packer L, Kevan PG (2013a) The potential of cleptoparasitic bees as indicator taxa for assessing bee communities. Apidologie 44:501–510
Sheffield CS, Kevan PG, Pindar A, Packer L (2013b) Bee (Hymenoptera: Apoidea) diversity within apple orchards and old fields habitats in the Annapolis Valley, Nova Scotia, Canada. Can Entomol 145:94–114
Simon-Delso N, San Martin G, Bruneau E, Minsart L-A, Mouret C, Hautier L (2014) Honeybee colony disorder in crop areas: the role of pesticides and viruses. PLoS ONE 9(7):e103073
Stark JD, Jepson PC, Mayer DF (1995) Limitations to use of topical toxicity data for predictions of pesticide side effects in the field. J Econ Entomol 88(5):1081–1088
Steffan-Dewenter I, Schiele S (2008) Do resources or natural enemies drive bee population dynamics in fragmented habitats? Ecology 89(5):1375–1387
Steffan-Dewenter I, Westphal C (2008) The interplay of pollinator diversity, pollination services and landscape change. J Appl Ecol 45(3):737–741
Sydenham MAK, Moe SR, Stanescu-Yadav DN, Totland Ø, Eldegard K (2016) The effects of habitat management on the species, phylogenetic and functional diversity of bees are modified by the environmental context. Ecol Evol 6(4):961–973. https://doi.org/10.1002/ece3.1963
Tonietto RK, Ascher JS, Larkin DJ (2016) Bee communities along a prairie restoration chronosequence: similar abundance and diversity, distinct composition. Ecol Appl. https://doi.org/10.1002/eap.1481
Vanbergen AJ, Initiative Insect Pollinators (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259
Ver Hoef JM, Boveng PL (2007) Quasi-poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88:2766–2772. https://doi.org/10.1890/07-0043.1
Vidau C, Diogon M, Aufauvre J, Fontbonne R, Viguès B, Brunet J-L et al (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 6(6):e21550
Wagner DL (2007) Butterfly conservation. In: O’Donnell JE, Gall LF, Wagner DL (eds) Connecticut butterfly atlas. Connecticut Department of Environmental Protection, Hartford, pp 287–307
Wagner DL, Ascher JS, Bricker NK (2014a) A transmission right-of-way as habitat for wild bees (Hymenoptera: Apoidea: Anthophila) in connecticut. Ann Entomol Soc Am 107(6):1110–1120
Wagner DL, Metzler KJ, Leicht-Young SA, Motzkin G (2014b) Vegetation composition along a New England transmission line corridor and its implications for other trophic levels. For Ecol Manage 327:231–239
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SPM, Szentgyörgyi H, Tscheulin T, Vaissière BE, Woyciechowski M, Biesmeijer JC, Kunin WE, Settele J, Steffan-Dewenter I (2008) Measureing bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671. https://doi.org/10.1890/07-1292.1
Westrich P (1996) Habitat requirements of central European bees and the problems of partial habitats. In: Matheson A, Buchmann SL, O’Toole C, Westrich P, Williams H (eds) The conservation of bees. Linnean Society of London and the International Bee Research Association by Academic Press, London, pp 1–16
Williams NM, Kremen C (2007) Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol Appl 17:910–921
Williams NM, Minckley RL, Silveira FA (2001) Variation in native bee faunas and its implications for detecting community changes. Conserv Ecol 5(1):7
Williams NM, Crone EE, T’ai HR, Minckley RL, Packer L, Potts SG (2010) Ecological and life-history traits predict bee species responses to environmental disturbances. Biol Conserv 143(10):2280–2291
Winfree R, Griswold T, Kremen C (2007) Effect of human disturbance on bee communities in a forested ecosystem. Conserv Biol 21(1):213–223
Winfree R, Williams NM, Gaines H, Ascher JS, Kremen C (2008) Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J Appl Ecol 45(3):793–802
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90(8):2068–2076
Wojcik VA, Buchmann S (2012) Pollinator conservation and management on electrical transmission and roadside rights-of-way: a review. J Pollinat Ecol 7(3):16–26
Wolfram Research Inc. (2017) Mathematica, Version 11.2. Champaign, IL
Woodcock BA, Isaac NJB, Bullock JM, Roy DB, Garthwaite DG, Crowe A, Pywell RF (2016) Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat Commun 7:12459
Zurbuchen A, Cheesman S, Klaiber J, Müller A, Hein S, Dorn S (2010a) Long foraging distances impose high costs on offspring production in solitary bees. J Anim Ecol 79(3):674–681
Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S (2010b) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143(3):669–676
Acknowledgements
We would like to thank Sam Droege and his team of interns and associates at the Patuxent Wildlife Research Center for help in collecting, processing and identifying bees. Thanks also to Rick Johnstone from IVM Partners for coordinating with us and pushing this work forwards. Thanks to William Rees from Baltimore Gas and Electric for help identifying control sites and allowing us access to BGE transmission line sites. We also appreciate the support from Mike Silva of Enertech, who championed this project from the beginning. Funding for this research was provided by the Electric Power Research Institute (http://www.epri.com/), EPRI Project ID 1-070744-01-01 and the New Jersey Institute of Technology Undergraduate Summer Research Program. Study design, data collection and analysis, and preparation of the manuscript were all exclusively conducted by the study authors with no input from the funding agency. Permission for sampling on the Columbia (Howard County) and Davidsonville (Ann Arundel County) sites was given by William T. Rees, Jr. (Forestry Management—Director, Engineering and Standards, Baltimore Gas & Electric). Permission for sampling on the Patuxent Wildlife Research Center was granted by Sandy C. Spencer, USFWS, Supervisory Wildlife Biologist, Patuxent Wildlife Research Refuge.
Author information
Authors and Affiliations
Contributions
Conceived and designed experiments: KNR, SK, SM, KK. Species identification: KNR. Collected data in the field: KNR, SK, SM, KK. Analyzed the data: KNR, GJR SK, KK, SM. Wrote the paper: KNR, SK, GJR.
Corresponding author
Additional information
Communicated by David Hawksworth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Russell, K.N., Russell, G.J., Kaplan, K.L. et al. Increasing the conservation value of powerline corridors for wild bees through vegetation management: an experimental approach. Biodivers Conserv 27, 2541–2565 (2018). https://doi.org/10.1007/s10531-018-1552-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1552-8