Abstract
It has become increasingly apparent that even mature forests are susceptible to plant invasions. However, invasive plants are often more abundant in younger forest stands. It is difficult to disentangle possible mechanisms that would explain this pattern due to the scarcity of long-term studies in successional forests. Several mechanisms have been proposed to explain patterns of invasions as forests age, including biotic resistance, window of opportunity, historical legacies, and invasion debt. We explored patterns and potential mechanisms of plant invasions over 70 years in a regenerating forest with different land-use histories in the Bolleswood Natural Area, Connecticut, USA. We examined how environmental factors related to colonization patterns of invasive and non-invasive introduced species over time, and whether these patterns were consistent with the proposed mechanisms. Non-invasive introduced species declined rapidly with canopy closure, while many invasive plants persisted or even increased over time as the forest aged. Colonization was focused in younger post-agricultural areas, although this declined with time. Dispersal distance, soil conditions, and land-use history played important roles in patterns of colonization, while the effect of shading was less clear. There was some evidence for each mechanism, but the relative importance of each mechanism was species-dependent, making generalizations about how invasive plants invade forests difficult. We found that land-use history impacted invasion more strongly than forest age, but over time even mature forests were slowly being invaded by some species. Thus, invasive species management may be required even in mature forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Closed-canopy forests have long been considered resistant to invasive plants due to shading and competition with native trees (Simberloff et al. 2002), the long-lived nature of many forest trees (Von Holle et al. 2003), and because invasive species often have disturbance-adapted traits (Rejmánek and Richardson 1996). Indeed, surveys of introduced species presence and abundance have shown fewer introduced plant species in closed-canopy forests in both temperate (Von Holle and Motzkin 2007; Chytrý et al. 2008; Rejmánek et al. 2013) and tropical regions (Waddell et al. 2020) compared to other plant communities. However, it is increasingly clear that forests are being invaded, and that some invasive species are shade-tolerant, and thus able to invade even closed-canopy forests (Martin et al. 2009). While there is now widespread recognition that some closed-canopy forests are being invaded, it is unclear if all forests are equally invasible and what factors may influence the degree of invasion in different forests.
Forests may be more susceptible to invasion earlier in successional development (Holmes and Matlack 2019). Although patterns and mechanisms may differ for introduced species as a whole compared to shade-tolerant invasive species (Martin et al. 2009), numerous chronosequence studies from the northeastern USA have shown that younger forests have much greater frequency and/or abundance of both introduced species generally (Parker et al. 2010; Matlack and Schaub 2011; Holmes and Matlack 2019) and invasive species specifically (Lundgren et al. 2004; Flory and Clay 2006; Mosher et al. 2009; Calinger et al. 2015). A regional scale analysis of factors influencing the distribution of invasive species also showed that invasives declined with forest age, although at this scale factors such as mean annual temperature and landscape openness were much better predictors (Golivets et al. 2019). Studies have found similar patterns of lower frequency and/or abundance of introduced species in older regenerating forests in California (Dudney et al. 2021), Puerto Rico (Pascarella et al. 2000), and the southeastern USA (Wang et al. 2012). This repeated pattern of fewer introduced and invasive species in older forests could support the idea that older forests are more resistant to invasion.
However, given that these studies are a snapshot of different-aged forests at a specific time, it is not possible to tell whether young forests have more invasive plants because they are at a different stage of development or because of confounding variables, such as the time period when they established or the land-use history prior to forest regeneration. In fact, we do not have a good understanding of how invasive plant populations change through succession in developing forests (Holmes and Matlack 2019), in part due to a relative paucity of long-term studies that follow the same forest over time (Vilà and Ibáñez 2011).
Some long-term studies have looked at general patterns of invasion over time in forests. In the most detailed study to date, Meiners et al. (2002) found that introduced species abundance generally declined over the first 40 years following abandonment of old fields in New Jersey. At the same sites, Rosa multiflora, a common invasive shrub increased for 30 years, but then started to decline for the next 14 years (Banasiak and Meiners 2009). In contrast, (Huebner 2020) found that both mature (> 80 years) and young (10–15 years) forests in West Virginia showed an increase in invasive richness over a 16 year period, although the mature forests generally had lower richness than the young forests. Similarly, Rogers et al. (2008) found that richness and abundance of introduced species increased in forest stands in Wisconsin that were resampled after a 50-year period, although this study did not report on the successional stage of the forest stands.
Several mechanisms have been proposed that could lead to a pattern of greater numbers of introduced non-invasive or invasive species in younger forests than older forests. Each of these mechanisms has distinct implications for the future of invasion in these forests, and thus understanding the prevalence of different mechanisms is critical for informing management and conservation decisions.
Biotic resistance
First, increasing biotic resistance as forest succession progresses may cause younger forests to have greater numbers of introduced species. Biotic resistance indicates the ability of the resident community to resist invasion (Levine et al. 2004). Research on biotic resistance has focused on the impact of native diversity (Nunez-Mir et al. 2017), but the concept can include all aspects of a community’s resistance to invasion (Mack et al. 2000; Levine et al. 2004). In forests, dense canopy cover may increase resistance, with or without higher diversity (Gomez et al. 2019). Thus, biotic resistance could increase as forests develop because of increasing richness of native species, increasing canopy cover and shading, or increased competition for limited resources in late succession.
The biotic resistance hypothesis is often focused on the diversity or richness of native plant communities, which have long been proposed to reduce invasion by introduced species (Elton 1958). This hypothesis has been extensively studied and is clearly scale and context dependent (Traveset and Richardson 2020; Gioria et al. 2023). Many studies have found the opposite pattern, a positive relationships between native richness and invasion, although these patterns may be impacted by other factors such as propagule pressure (Levine 2000) and overall resource availability (Stohlgren et al. 1999). Generally, a negative relationship between invasion and native diversity has been found to be more common at small spatial scales (Fridley et al. 2007) and when controlling for these other factors (Beaury et al. 2020). Given that species richness commonly increases at least through the early and middle stages of succession (Anderson 2007), this mechanism may allow older forests to have greater resistance to invasion. Because invasion may also impact native richness, measuring native richness prior to invasion can provide a clearer understanding about how native diversity impacts invasion than a snapshot of native and introduced diversity at a single time (Ernst et al. 2022).
In forests, tree biomass or canopy cover may play a role in biotic resistance independent from native richness by reducing available light (Mack et al. 2000; Gomez et al. 2019; Petri and Ibañez 2023). As the canopy closes and shading increases, colonization by shade-intolerant species that dominate the introduced species pool is likely to decline (Meiners et al. 2002; Martin et al. 2009). Competition for resources, especially light, is expected to increase through succession (Walker and Chapin III 1987) which could also lead to a decline in introduced species as a forest ages. If increasing biotic resistance with succession is driving patterns of invasion, we would expect introduced species to be negatively associated with canopy cover and/or tree basal area. In a regional analysis of forests in the eastern USA, tree biomass was negatively related to both richness and cover of invasive species, even while tree richness had a positive relationship with degree of invasion (Iannone et al. 2015). This pattern has been found at more local scales as well. In Montana, Jang et al. (2021) found that 23 years after thinning and burning, introduced forb cover and richness and introduced graminoid cover were negatively related to tree basal area. In old fields in New Jersey, declines in introduced species over the first few decades of forest development was negatively related to the increases in woody cover associated with canopy closure (Meiners et al. 2002). There is some evidence, however, that this mechanism may not be as strong when focusing on shade-tolerant invasive species (Martin et al. 2009). For example, even with the overall abundance of introduced species declining, Meiners et al. (2002) noted increases in individual shade-tolerant invasive species after 40 years of forest succession.
If biotic resistance is the primary driver of invasion patterns through succession, we would expect declining invasion over time, especially during the early stages of forest succession (Fig. 1). Thus, the pattern of greater invasive abundance in younger than in older forest stands found in chronosequence studies (e.g. Lundgren et al. 2004; Mosher et al. 2009) would accurately represent a trajectory of declining invasion through succession.
Schematic representations of hypothesized patterns of invasion over time expected based on four mechanisms. Patterns between forests of different initial age at the same time (e.g. new, young or old) may represent different points on the same trajectory (biotic resistance, historical legacies for forests with the same history at different times) or may have different patterns based on timing of species introduction (window of opportunity) or past land-use history (historical legacies). For invasion debt, initial age of the forest is much less important than the proximity of forest to seed sources (although those may be correlated)
Window of opportunity
The timing of when forest development begins may be more important than actual forest age in creating a pattern of greater introduced species abundance in younger forests. Successful invasions may be dependent on the invasive species arriving at a time when sufficient resources are available, and these resources are likely to vary over time creating “windows of opportunity” for invasion (Johnstone 1986; Davis et al. 2000; Shea and Chesson 2002). The early stages of forest development may be one example of this type of window of opportunity (Hobbs 2000; Degasperis and Motzkin 2007; Calinger et al. 2015). Thus, areas that are still open when an invasive species arrives in an area may be colonized and then the species may persist, while older forests that already had a closed canopy when the species arrived may be resistant to invasion. For example, land use after introduction was the best single predictor of Berberis thunbergii presence in Massachusetts forests (Degasperis and Motzkin 2007), indicating that invasive species distributions in a forested landscape may reflect timing of past land use relative to introduction of the species. The intensity of land use and how much it initially opened up niches for colonization may also play a critical role, with higher intensity disturbances, even decades in the past, leading to increased current abundance of invasives (Holmes et al. 2021). This mechanism would show a trend of greater invasion in younger forests across a landscape, but for individual forests over time it would show an initial increase followed by persistence in the already-invaded sites (Fig. 1). Older forests that developed prior to the arrival of the invasives would have persistently low invasion.
Historical legacies
Historical land use may not only provide a window of opportunity for colonization, but also have long-term effects on soil and other environmental variables in a developing forest (Von Holle and Motzkin 2007; Kuhman et al. 2013; Holmes et al. 2021). Past land use, especially agriculture, can alter soil characteristics for decades or even a century after abandonment (Verheyen et al. 1999; Compton and Boone 2000; Flinn and Marks 2007; Parker et al. 2010). Given that the distribution of invasive species in forests is related to patterns in these soil characteristics (e.g. soil richness, McDonald et al. 2008), forests may be more invasible as long as these legacies of past land use persist. Although there is considerable variation among studies, these legacies may decline over time and after 60 + years may be indistinguishable from much older forests (Holmes and Matlack 2017). However, land-use history can also impact native community development, which in turn may continue to impact soil characteristics over the long term (Kuhman et al. 2013). If these legacies play an important role in invasion, then we might expect to see a decline in introduced species several decades to a century after forest establishment as these legacies diminish (Fig. 1). Sites without historical land use causing these soil legacies would have persistently lower levels of invasion. We would also expect to see a strong relationship between invasive species’ distribution and soil characteristics both spatially and over time.
Invasion debt
All three of the previous mechanisms may contribute to the general pattern that younger forests are more invaded than older ones. However, they may also help mask a mechanism that could actually lead to increased invasion as forests age. Older forests may be currently less invaded, not because they are completely resistant to invasion, but because there is a longer lag-time for invasions in older forests. Thus, these forests may be facing a delayed “invasion debt” rather than a resistance to invasion per se (Essl et al. 2012). This invasion debt idea suggests impending future invasion due to invasive species that are already present in the landscape and that will expand into uninvaded forests over time. Relative to more open habitat types, it is clear that forest invasions are slower (Martin et al. 2009) and may develop after a significant time lag either because of the longer-lived nature of many forest species or because of more limited propagule pressure in closed-canopy forests lacking large-scale disturbances (Essl et al. 2012).
Propagule pressure has been shown to be a more consistent predictor of invasions than characteristics of the invading species or habitats being invaded (Von Holle and Simberloff 2005; Colautti et al. 2006; Simberloff 2009). Multiple studies in temperate forests have shown a pattern of increased introduced species abundance in forests nearer to invasion sources, suggesting that propagule pressure may play a key role in forest invasions (Lundgren et al. 2004; Essl et al. 2012; Iannone et al. 2015; Wagner et al. 2021). Similarly, a number of studies have shown increased invasions near the edges of forests (e.g., Yates et al. 2004; Riitters et al. 2018), which may reflect greater propagule pressure near the edges due to proximity to seed sources outside the forests. Thus, forest interiors may be less invaded because of an invasion debt–the invasive species have simply not arrived in these areas yet. However, invasion near forest edges may also be due to environmental differences in forest edge habitat rather than dispersal distance, and may not always be indicative of invasion debt, although these factors may interact (Vilà and Ibáñez 2011). Propagule pressure could also explain the pattern of lower invasion in older forests if older forests are on average farther from seed sources than younger post-agricultural forests. In fragmented landscapes, landscape-scale land-use change may lead to future increases in invasive species surrounding many forests. Thus, propagule pressure is likely to increase over time, leading to invasion debt even in older forest patches in these fragmented landscapes (Vilà and Ibáñez 2011; Lebbink et al. 2022).
This invasion debt mechanism may be strengthened due to the structure of closed canopy forests. For example, if the bird species commonly dispersing invasive seeds in edge or open habitats are less likely to enter closed-canopy older forests, dispersal into these forests may be slowed (McCay and McCay 2009; Holmes et al. 2021). In addition, invasive plants that are able to disperse into closed-canopy forests may establish at low levels but then “sit and wait” until small-scale disturbances, such as canopy gaps, allow increased establishment (Greenberg et al. 2001). If an invasion debt is a driving mechanism, then we would expect to see a gradual increase in invasive species over time in forests of all different initial ages, with distance from potential seed sources being more important than forest age (Fig. 1). We would also likely see spread of invasive species from the edge into the interior of the forests over time.
Disentangling these mechanisms requires a long-term dataset with forests that initiated at different times (Vilà and Ibáñez 2011). Such a dataset was begun in the 1950s in the Bolleswood Natural Area (BNA) of the Connecticut College Arboretum when permanent plots were sampled in recently abandoned fields, post-agricultural forest, and mature uncleared forest (Niering and Goodwin 1962). These plots have been resampled every 10 years for the past 70 years which allows an exploration of the patterns and mechanisms of introduced plant spread in developing forests of different ages and land-use histories.
We use this long-term dataset to address three major questions. (1) What are the patterns of spread of invasive and introduced non-invasive species in forests with different land-use histories over time in the Bolleswood Natural Area? (2) To what extent can factors relating to canopy cover, propagule pressure, land-use history and soil characteristics explain these patterns and how do these relationships change over time? (3) To what extent do these patterns in spread and associated factors support one or more of the mechanisms described above?
Methods
Study area
The Bolleswood Natural Area is a 23.1 ha unmanaged tract in the Connecticut College Arboretum in New London and Waterford, Connecticut, USA. The western end of the BNA was used for agriculture up until the natural area was created in 1951. Other areas had previously been cleared for agriculture but abandoned earlier. The eastern portion of the BNA contains rocky ridges and a ravine and was likely never completely cleared, but had been subject to localized fires and windthrow as well as selective cutting in the decades prior to 1952 (Niering and Goodwin 1962). The natural area is bordered by a residential neighborhood to the west, a powerline corridor to the south and a road to the north, all of which currently contain populations of multiple invasive species (C. Jones personal observation) and thus are potential sources of dispersal. Forests in BNA are dominated by Quercus spp., Tsuga canadensis (L.) Carr. and Fagus grandifolia Ehrh., with Tsuga canadensis increasing in abundance until 1992 followed by a steep decline after the invasion of Adelges tsugae (hemlock wooly adelgid) in the late 1980s (Small et al. 2005). Since its establishment, the BNA has been allowed to develop without management or human disturbance, with the exception of surrounding it with a deer exclusion fence in 1989 to reduce herbivory (Buchanan et al. 2016).
Data collection
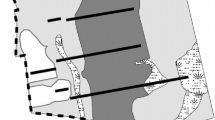
In 1952, four parallel permanent transects were established in the BNA (Fig. 2). The transects are 122 m apart and range from 265–439 m long. Each transect is 6 m wide and made up of two rows of contiguous 3 × 3 m plots (Niering and Goodwin 1962). The western ends of three of the transects were offset to avoid field edges. For this study, we focused on the upland areas (without standing water during the growing season; 750 of the 890 total plots).
Map of the Bolleswood Natural Area in New London and Waterford, Connecticut, USA. Thick black lines indicate position of transects. Dashed line indicates the edge of the natural area. Uncleared forests were never cleared for agriculture, while older post-agricultural areas had been abandoned for at least two decades before the start of the study and younger post-agricultural areas were abandoned less than ten years before the start of the study
All plots have been surveyed every ten years beginning in 1952. In each survey year, the presence of each species of vascular plant was recorded in each of the plots. In addition, the diameter at breast height (DBH) of all trees was recorded. Trees < 2.5 cm DBH were categorized as either < 1 cm or > 1 cm DBH. In 2012 and 2022, we photographed the canopy from the center of each plot using a Sigma SD14 camera with a 4.5 mm circular fisheye lens at ~ 1 m height. In these same years we also collected a soil sample from each plot. We collected soils from the top 10 cm below the O horizon from three locations in each plot and then mixed them to produce one sample per plot.
All species were categorized as native, introduced non-invasive, or invasive based on Dreyer et al. (2014), which follows the official Connecticut list of invasive species. Using the species presence data, we determined which plots were colonized for the first time by a given invasive species in each survey year (newly colonized plots). For each year, we defined plots that had never been previously colonized by a given species as uncolonized plots for that species.
For all sample years, we determined five characteristics of each upland plot. We assigned plots to three land-use history categories: younger post-agricultural fields (abandoned within 10 years prior to the start of the study), older post-agricultural fields (shrublands or thickets in 1952 that had previously been used for agriculture but had been abandoned in previous decades), and uncleared forest (closed-canopy forest in 1952 that was never used for agriculture but subjected to small-scale natural and human disturbances in the decades prior to 1952) (Niering and Goodwin 1962). We used total basal area (in m2 per hectare) as a measure of tree density and light availability. Because the 3 × 3 m plots are too small to adequately represent the tree density surrounding a point, we calculated total basal area for 6 × 15 m sections containing ten plots (75 total sections) and then assigned that basal area to each plot in the section. Species richness was measured as the number of species present in the plot in the previous survey. We calculated distance to the edge of the natural area for each plot using ArcMap 10.8.2. For each species, we also used ArcMap 10.8.2 to calculate the distance from the nearest plot in the previous survey where the target species occurred.
In 2012 and 2022 we also measured canopy openness and seven soil variables. We calculated canopy openness as the percentage of open sky in the hemispherical photos using Gap Light Analyzer (Canham et al. 1999). We calculated percent soil moisture (percent of dry weight) by weighing soils immediately on return to the lab and then drying them at 105 °C for 24 h. Soils were collected following at least 24 h of dry conditions. We calculated soil organic matter using loss on ignition test at 500 °C for 12 h. For pH and soil nutrient analysis, we mixed soils from the 10 plots in a section and sent them for analysis to the Cornell Nutrient Analysis Laboratory in 2012 and the University of Connecticut Soil Nutrient Analysis Laboratory in 2022. We assigned each plot the value of pH, nitrate nitrogen, phosphorus, calcium, and potassium for its section.
Statistical analysis
To assess invasion over time, we calculated the average number of introduced non-invasive and invasive species per plot in each of the three land-use history categories in each survey year. We also calculated the number of plots where each invasive species occurred in each survey year and the total number of plots where at least one invasive species was present. We determined the proportion of newly colonized plots (for all species combined) in each survey year that occurred in each of the land-use history categories.
We used linear regression to assess changes in the characteristics of newly colonized plots over time. For each species we used time since the beginning of the study as the predictor variable for each of the four response variables separately (distance to nearest individual, distance to edge, basal area and species richness).
We then compared each of the four variables between newly colonized plots and uncolonized plots for each species in each year in which at least 10 plots were newly colonized (N = 15). Colonization for most species was focused on the western half of the natural area. We were interested in the differences in colonized and uncolonized plots within the range in which colonization might occur, rather than larger scale spatial patterns that might correlate with distance from the seed source. Thus, we limited the uncolonized plots used in this analysis to those within the colonization range. For each species in each year we noted the maximum distance that a newly colonized plot was from a plot containing that species in the previous survey and removed all uncolonized plots beyond this maximum distance from analysis. Because Lonicera morrowii Gray colonization occurred in a very limited area of the natural area and in only one land-use history category, it was not included in further analyses.
For each species and year, we used t-tests to compare the mean values for each variable (distance to nearest individual, distance to edge, basal area, and species richness) between newly colonized and uncolonized plots. Canopy openness and the seven soil variables were only available for species spreading to ten or more new plots in 2012 or 2022 (N = 4). For each variable, significance of comparisons was adjusted for multiple tests using the Holm-Bonferroni procedure. For species richness we compared the mean values for total species richness and for native and introduced (invasive and non-invasive together) richness separately.
To understand the relative importance of the variables, we used relative weights analysis using the rwa package in R (Tonidandel and LeBreton 2011; Chan 2022). Relative weights analysis addresses multicollinearity among variables in a multiple regression model by separating the total variance into weights that reflect the relative contributions of each predictor variable (Tonidandel and LeBreton 2011). We conducted this analysis using multiple logistic regression models for each species in each year with colonization status as the response variable and six predictor variables: distance to nearest individual, distance to edge, basal area, species richness and two dummy variables representing land-use history (younger post-agricultural and older-post agricultural).
Results
Although correlations among variables were generally low (|r|< 0.55), the land-use history categories were different in many respects (Online Supplement S1). Younger post-agricultural plots had consistently higher richness, although richness fluctuated over time in all land-use histories. Basal area increased over time in all land-use history categories, but then decreased starting in 2002 in uncleared forests and 2022 in the older post-agricultural forests. Basal area increased more rapidly in the younger post-agricultural plots but remained slightly lower in these areas than in plots in the other land-use history categories at the end of the study. Younger post-agricultural plots were nearer to the edge of the natural area and had higher canopy openness, nitrate and calcium levels, and pH and lower soil moisture, organic matter, and potassium. The pattern for phosphorus varied by year (Online Supplement S1).
Non-invasive introduced species were initially much more abundant in the younger post-agricultural plots compared to other land-use history categories but declined rapidly over time (Fig. 3). By 1992, very few non-invasive introduced species occurred anywhere on the transects. In contrast, invasive species increased dramatically over time in younger post-agricultural plots (Fig. 3). Invasive species were much less common in the uncleared forest plots but also increased over time, while invasive richness fluctuated in the older post-agricultural plots. Ten invasive species were found in the upland plots. One of these, Rumex acetosella L., was initially present primarily in the younger post-agricultural plots (that were initially open) and declined rapidly, disappearing completely from the transects by 1982. Fallopia japonica (Houtt.) R. Decr. and Rubus phoenicolasius Maxim. first appeared on the transects in 2012 and 2022 respectively and occurred in no more than four plots. The other seven species were analyzed in more detail. The frequency of these invasive species over time varied by species (Fig. 4). Several species (Lonicera japonica Thunb., Lonicera morrowii Gray, and Celastrus orbiculatus Thunb.) initially increased in abundance and then declined. Berberis thunbergii DC. and Rosa multiflora Thunb. ex Murr. increased and then leveled off while Ligustrum vulgare L. and Euonymus alatus (Thunb.) Sieb. continued to increase. For all species, colonization was concentrated in younger post-agricultural plots, even after 70 years, with Lonicera morrowii colonization completely restricted to these areas. However, the proportion of the new occurrences that occurred in uncleared forests has increased over time. Prior to 2002, no more than 7% of the new occurrences for all species were in the uncleared forests. This increased to 12% in 2002, 14% in 2012 and 30% in 2022. The number of newly colonized plots peaked in 1982, but new plots continue to be colonized in every survey since (Online Supplement S2).
Mean number of introduced (non-invasive) and invasive species per plot over time. Introduced species counts do not include those species listed as invasive by Dreyer et al. (2014). PA = post-agricultural areas
Frequency of invasive species over time by land-use history. There were 750 total plots with 86 in younger post-agricultural sites (Younger PA), 180 in older post-agricultural sites (Older PA), and 484 in uncleared forests. The All species graph shows the number of plots where at least one invasive species is present
The mean distance of newly colonized plots from individuals of the same species in the previous survey was always less than 40 m, and usually less than 20 m (Fig. 5). The maximum distance ranged from 21.7 m for R. multiflora in 1992 to 256.1 m for Lonicera japonica in 1982. Even when removing uncolonized plots farther away than the maximum colonized distance from the analysis, colonized plots were closer to plots with previously existing individuals than uncolonized plots for most species and years (Fig. 5). The mean distance declined over time for R. multiflora but not for any other species (Table 1).
Distance of colonized and uncolonized plots to the nearest plot that contained the same species in the previous survey. Only years for which at least 10 new plots were colonized were included. Numbers above the bars indicate the furthest distance (in m) a colonized plot was from a plot containing the same species in the previous survey and uncolonized plots at greater distances were removed from further analysis. Error bars represent ± 1 SE. For comparisons between colonized and uncolonized plots that were still significant after a Holm-Bonferroni correction for multiple tests: ***p < 0.001, **p < 0.01, *p < 0.05
Colonized plots were closer to the edge of the natural area for all species and years except for Ligustrum vulgare in 1992 (Fig. 6). When considering colonization across all years, only C. orbiculatus colonized plots increasingly distant from the edge over time (Table 1). Ligustrum vulgare, Lonicera japonica, and R. multiflora actually colonized plots closer to the edge over time. This pattern appears to be driven by years when only few plots were colonized (Supplement S3). A few plots distant from the edge were initially colonized by Ligustrum vulgare and R. multiflora in the early stages of the study, while the bulk of later invasion in the years with enough data for complete analysis were very near the edge (Fig. 6). For three of the species, the maximum distance from the edge did increase over time–but only a few individuals colonized at distances greater than 200 m from the edge (Online Supplement S3).
Distance of colonized and uncolonized plots from the edge of the natural area over time. Only years for which at least 10 new plots were colonized were included. Error bars represent ± 1 SE. For comparisons between colonized and uncolonized plots that were still significant after a Holm-Bonferroni correction for multiple tests: ***p < 0.001, **p < 0.01, *p < 0.05
In most years and for most species, colonization occurred in plots with higher species richness in the previous survey (Fig. 7). This pattern was most obvious for introduced species richness, but four of the six analyzed species colonized areas with higher native richness in at least one year (see Online Supplement S4 for full statistical analysis). There were no obvious trends over time, except for Lonicera japonica, which colonized plots with lower richness over time (Table 1).
Species richness of colonized and uncolonized plots over time. Only years for which at least 10 new plots were colonized were included. Patterned sections of the bars represent richness of all introduced species (both invasive and non-invasive) while sections without patterns represent richness of native species. Error bars represent ± 1 SE (of total richness). Letters above bars indicate significant differences between colonized and uncolonized plots (p < 0.05 after a Holm-Bonferroni correction for multiple tests): T = total richness, I = richness of all introduced species (invasive and non-invasive), N = native species richness
In at least one survey year for each species except B. thunbergii, colonized plots had lower basal area than uncolonized plots (Fig. 8). However, this pattern was not consistent. C. orbiculatus colonized plots with lower basal area initially but not later on, while Ligustrum vulgare and E. alatus showed the opposite pattern. All of the species except E. alatus and Ligustrum vulgare colonized plots with higher basal area over time as the overall basal area in the forest increased (Table 1). Canopy openness in 2012 and 2022 did not show a similar pattern (Fig. 9). Only for E. alatus in 2022 was there a difference in canopy openness between colonized and uncolonized plots and the colonized plots had a lower canopy openness.
Basal area of 6 × 15 m sections containing colonized and uncolonized plots over time. Only years for which at least 10 new plots were colonized were included. Error bars represent ± 1 SE. For comparisons between colonized and uncolonized plots that were still significant after a Holm-Bonferroni correction for multiple tests: ***p < 0.001, **p < 0.01, *p < 0.05
Comparison of colonized and uncolonized plots for variables only measured in 2012 and 2022. Only years for which at least 10 new plots were colonized were included, so data from C. orbiculatus (Cel.), Ligustrum vulgare (Lig.) and R. multiflora (Rosa) is from 2012 and for E. alatus (Euon.) is from 2022. Error bars represent ± 1 SE. For comparisons between colonized and uncolonized plots that were still significant after a Holm-Bonferroni correction for multiple tests: ***p < 0.001, **p < 0.01, *p < 0.05
Soil variables did differ between uncolonized and colonized plots in 2012 and 2022 (Fig. 9). Soil pH was higher and potassium lower in colonized plots for all species. For some species, soil moisture and organic matter were lower, and nitrate and calcium were higher in colonized plots. Phosphorus was lower in colonized plots only for Ligustrum vulgare in 2012.
When including all of the explanatory variables, the importance of each variable changed by species and year with few obvious patterns (Fig. 10). The younger post-agricultural land-use category was the most important variable in many cases (as high as 82% for R. multiflora in 2012). Basal area generally had low relative weights, while the importance of the other factors varied among species and year.
Discussion
Non-invasive introduced species and invasive species generally had opposite abundance patterns as forests developed over time. Non-invasive introduced species and one disturbance-adapted invasive species, Rumex acetosella, occurred almost exclusively in younger post-agricultural plots and rapidly declined as forests developed on these sites. While patterns varied somewhat among the rest of the invasive species, colonization was generally concentrated in the younger post-agricultural plots and increased over time, particularly between 1972 and 2002. Uncleared forests showed the lowest level of invasion, but invasion has increased in these areas in the past 30 years.
Shading and competition, as measured by basal area, canopy cover, and existing species richness, played a somewhat ambiguous role in colonization patterns. We found some evidence that colonization in a given year was more common in areas with lower basal area, but when multiple explanatory variables were considered, basal area explained only a small proportion of the total variation. In addition, most invasive species colonized areas with increasing basal area over time, suggesting that basal area by itself may not be the driving factor. Basal area was previously shown to have little association with site-level invasion patterns in this region (Howard et al. 2004), although it has been associated with invasion elsewhere (Jang et al. 2021). We also did not find evidence that colonization was greater in areas with greater canopy openness, which is a more direct measure of shading. Likewise, species richness was usually higher in colonized vs non-colonized plots, supporting previous studies that have found a positive relationship between richness and invasion (Stohlgren et al. 1999; Levine 2000). This positive relationship was particularly strong for richness of pre-existing introduced species, which could suggest evidence of invasional meltdown (Simberloff and Von Holle 1999) or simply that these invasive species are colonizing similar locations, but not necessarily interacting (Kuebbing and Nuñez 2015). The lack of a clear role of shading and competition for colonization patterns of species in this study may not be surprising given that several of these species have been shown to tolerate shaded conditions (Greenberg et al. 2001; Martin et al. 2009; Driscoll et al. 2016). For species such as these, canopy closure and forest density may not be the primary driver of invasion patterns in forests. We only focused on invasive species’ presence, however, so light availability, such as canopy gaps, may play a greater role in these species’ growth rates and abundance within plots (Greenberg et al. 2001; Driscoll et al. 2016).
Dispersal clearly plays a role in patterns of colonization. Newly colonized plots were consistently closer to the edge of the natural area and to previously existing individuals of the same species than uncolonized plots, supporting propagule pressure as a key factor in invasion patterns (Von Holle and Simberloff 2005; Colautti et al. 2006). The average newly colonized plot was within about 100 m of the edge of the natural area for all species and years and only for C. orbiculatus did this distance increase over time. We did see an increase in the maximum distance from the edge for three of the species over time, but the other three never occurred more than 130 m from the edge. Thus, while invasive species are more common near the edge as found in previous studies (Yates et al. 2004; Riitters et al. 2018), there is moderate evidence that the species continue to move farther into the natural area. This evidence could be limited because many of the colonizing individuals are still small and are not reproductive, so more time may be necessary for this pattern to become more apparent for all species. Although it has been hypothesized that invasive species will continue to move into forests that are distant from propagule sources over time (Essl et al. 2012), this may be a very slow process in closed-canopy forests in the absence of major disturbances.
Soil conditions were related to colonization patterns, with pH, nitrate, and calcium generally higher in colonized plots and soil moisture, organic matter, potassium, and to some extent phosphorus lower in colonized plots. Nitrate and calcium have previously been shown to be positively associated with invasion in forests in the eastern USA, while the patterns for potassium and phosphorus have been less consistent (Howard et al. 2004; Degasperis and Motzkin 2007; Von Holle and Motzkin 2007). Increased pH has also been associated with plant invasions in post-agricultural soils (Kuhman et al. 2011, 2013). Because these variables were only measured recently, and thus were not included in the multivariate analysis, it is unclear how important they are relative to other variables.
Land-use history continued to be an important predictor of invasion throughout all years. Most new occurrences of invasive species were in younger post-agricultural areas—even decades after canopy closure. However, the other measured variables are connected to land-use history, with the younger post-agricultural areas having lower basal area, being closer to both the edge of the natural area and the initially invaded plots, and having higher richness and distinct soil conditions. Nevertheless, land-use history was often the most important variable even with these other variables included in the analysis. Thus, land-use history may be an important proxy for several variables that together influence colonization patterns. Land-use history has often been found to be among the strongest predictors of invasion in northeastern USA forests (Lundgren et al. 2004; Degasperis and Motzkin 2007; Von Holle and Motzkin 2007), and this study has demonstrated that that pattern can persist over time even as forests age. However, the importance of land-use history does not necessarily help us separate between windows of opportunity and historical legacies (Kuhman et al. 2011).It is also critical to note that over the past 20 years, colonization into uncleared, and thus oldest, forest areas has increased.
Evidence for different invasion mechanisms
Biotic resistance
It is clear that biotic resistance due to canopy closure is a strong mechanism for reducing the number of non-invasive introduced species in these forests. These species were initially abundant in the sites that had been cleared for agriculture and rapidly declined as has been found previously (Meiners et al. 2002). Very few non-invasive introduced species occurred in closed-canopy forests. However, there was little evidence supporting biotic resistance for the invasive species other than Rumex acetosella. All of the invasive species found in closed-canopy forests were most common in the youngest forest (the younger post-agricultural plots), but these invasions largely occurred after canopy development started and generally did not decline over time. Even though colonized plots often had lower basal area than uncolonized plots within a given year, most species colonized plots with increasing basal area over time, which is not consistent with biotic resistance (sensu lato) causing a decline in invasion over time. Thus, we lack good reason to believe that biotic resistance is the primary cause of older forests being less heavily invaded. However, because this study focused on presence of invasive species rather than abundance, we may not have captured the full effect of biotic resistance. Indeed, biotic resistance may reduce the abundance of invasive species while not fully preventing colonization (Levine et al. 2004).
We also did not find evidence of a negative relationship between existing richness and invasion (the biotic resistance hypothesis sensu stricto). Thus, this hypothesis does not explain patterns of invasion at our site. Even within forested habitat, we found that invasion occurred in sites with higher existing species richness, similar to patterns found in many different ecosystems (Stohlgren et al. 1999; Levine 2000). In particular, the younger post-agricultural sites generally had higher richness and were also the most invaded. However, since the analysis here focused on site-scale patterns, this study may reflect processes at a spatial scale more likely to show a positive relationship between species richness and invasion (Fridley et al. 2007). For diversity to convey resistance to invasion to older forests, richness would have to increase during succession and be greatest in the oldest forests, and invasive species would tend to colonize sites with lower richness. However, none of these were true in our study system. Thus, species richness is not a form of biotic resistance that explains why these younger forests sites have more invasive species.
Window of opportunity
Some of the species, especially B. thunbergii and R. multiflora, showed patterns consistent with the window of opportunity model which predicts greater invasion in younger forest sites with an initial increase in invasion followed by persistence. Between 1972 and 1992, both species spread into the younger post-agricultural areas while the tree canopy was still developing. Since 1992 the species have persisted but not really expanded into older forests, even those in close proximity. Lonicera japonica showed a similar pattern although it has declined in frequency since its peak. This pattern is consistent with the pattern found for B. thunbergii by DeGasperis and Motzkin (2007), where the species was found primarily in areas where forest development occurred after the species was already present in the area. B. thunbergii has previously demonstrated the ability to persist even in closed-canopy forests once present (Mosher et al. 2009). In contrast, previous studies for R. multiflora have shown a pattern more consistent with biotic resistance, with the species most common in early successional forests (Mosher et al. 2009) and then declining in time as the forests develop (Banasiak and Meiners 2009). Our results for R. multiflora are more consistent with the window of opportunity and consistent with the finding that both B. thunbergii and R. multiflora are less dependent on treefall gaps for persisting in forests than other invasive species (Driscoll et al. 2016). For these species, the pattern of lower abundance in older forests found in previous chronosequence studies may be due to younger forests being invaded in their early stages while older forests were not, instead of indicating that the species will actually become less common as forests age.
Historical legacies
It is clear that historical legacies still persist and differentiate the areas with varying land-use history even after 70 years of forest development. Some soil nutrients remained twice as high in the younger post-agricultural plots compared to the uncleared forest plots in both 2012 and 2022. This is consistent with studies showing that soil legacies after agriculture can persist for as much as a century (Verheyen et al. 1999). It is possible that these differences are becoming less pronounced over time (as is the case with basal area), but because we do not have soil data from earlier surveys this is uncertain. Thus, the continued colonization in these younger post-agricultural forests could be impacted by these historical legacies. Both Lonicera morrowii and C. orbiculatus have seen a steep decline in the number of colonized plots in the younger post-agricultural areas in the past 20 years, which could be consistent with the historical legacies model if the legacies are in fact declining. Several other species, Ligustrum vulgare, R. multiflora, and B. thunbergii, are largely restricted to these younger post-agricultural plots, so even though they are not declining, their pattern of spread could be a result of the continuing biological legacies and it is possible that these species may decline in the future if these legacies fade over time. This study reinforces the finding that land-use history, particularly agriculture, can impact invasion patterns in forests in the northeastern USA (Von Holle and Motzkin 2007; Kuhman et al. 2011; Holmes and Matlack 2019; Holmes et al. 2021).
Invasion debt
While invasion outside of the post-agricultural forests has been limited at this site, there are some initial indications of an ongoing invasion debt. A pattern of declining invasion with distance from seed sources is one indication of invasion debt (Essl et al. 2012) and as predicted, early colonization was restricted to plots nearer the edge. Even after decades, most colonization still occurred near the edge of the natural area, suggesting the continued importance of propagule pressure from outside the natural area. Nevertheless, we found some evidence that the invasive species were spreading farther from the edge and into the oldest forests. While the average distance of newly colonized plots from the edge only increased over time for C. orbiculatus, three of the seven species increased the maximum distance from the edge by more than 100 m over the course of the study. Perhaps even more telling, over time the proportion of newly colonized plots in the uncleared forest plots has increased, even though this is the oldest forest. In particular, E. alatus and C. orbiculatus have been spreading into this habitat, even while C. orbiculatus has been declining in the younger post-agricultural plots. Thus, there is an indication that the lack of invasion throughout the study area was not due to resistance to invasion in older sites, but because of delayed dispersal to these areas. This is consistent with predictions of slow spread of invasive species into closed-canopy forest, especially those that are more distant from seed sources (Martin et al. 2009; Essl et al. 2012). As the invasive individuals within the site mature, propagule pressure in the interior of the forest should increase over time, likely leading to increased invasion in these oldest forests.
Conclusion
We found some support for all four mechanisms for invasion into developing forests. It is likely that all are happening simultaneously but that their importance varies by species (Petri and Ibañez 2023). Our results also clearly indicate that while developing forests may be resistant overall to introduced species (including disturbance-dependent invasive species), there is a set of shade-tolerant invasive species that can colonize forests even with closed canopies (Martin et al. 2009). Given the species-specific and likely site-specific nature of these mechanisms, it will be difficult to generalize predictions. In particular, we recognize that this study represents invasion patterns at only a single site. Nevertheless, it is critical to recognize that all of these mechanisms may be happening simultaneously.
Long-term studies are critical to be able to disentangle these mechanisms and patterns, as forest age is often confounded with land-use history in chronosequence studies. In this study, we showed different patterns in colonization among forests with different land-use histories. As found repeatedly in other studies, older forests without a history of agriculture had fewer overall invasive species (Degasperis and Motzkin 2007; Holmes and Matlack 2019; Holmes et al. 2021). However, we also found that overall the abundance of invasive species increased over time in both the post-agricultural and the older uncleared forests, suggesting that abundance of invasive species may not decline as forests age. In particular, in this study we found that both C. orbiculatus and E. alatus originally invaded the younger post-agricultural forests that had been open at the beginning of the study but then over the past few decades have increasingly shifted into older, uncleared forest.
Our results suggest that while the rate of colonization in forests without a history of agriculture is slow (Martin et al. 2009; Essl et al. 2012), at least some species are continuing to spread. These older, less disturbed forests may be resistant, but not impervious, to invasion. Managers thus must recognize that despite the strong pattern found in many studies of younger forests being more invaded, monitoring and management of invasive species in older forests may be critical. The slower nature of these invasions, however, may offer some hope in successfully intervening to prevent widespread colonization.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Anderson KJ (2007) Temporal patterns in rates of community change during succession. Am Nat 169:780–793. https://doi.org/10.1086/516653
Banasiak SE, Meiners SJ (2009) Long term dynamics of Rosa multiflora in a successional system. Biol Invasions 11:215–224. https://doi.org/10.1007/s10530-008-9226-1
Beaury EM, Finn JT, Corbin JD, et al (2020) Biotic resistance to invasion is ubiquitous across ecosystems of the United States. Eco Lett 23:476–482. https://doi.org/10.1111/ele.13446
Buchanan ML, Askins RA, Jones CC (2016) Response of bird populations to long-term changes in local vegetation and regional forest cover. Wilson J Ornithol 128:704–718. https://doi.org/10.1676/15-108.1
Calinger K, Calhoon E, Chang H-C et al (2015) Historic mining and agriculture as indicators of occurrence and abundance of widespread invasive plant species. PLoS ONE 10:e0128161. https://doi.org/10.1371/journal.pone.0128161
Canham CD, Frazer GW, Sallaway P, Marinakis D (1999) Gap light analyzer. Simon Fraser University, Cary Institute of Ecosystem Studies
Chan M (2022) Package “rwa”: perform a relative weights analysis. Comprehensive R Archive Network. https://CRAN.R-project.org/package=rwa
Chytrý M, Maskell LC, Pino J et al (2008) Habitat invasions by alien plants: a quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J Appl Ecol 45:448–458. https://doi.org/10.1111/j.1365-2664.2007.01398.x
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037. https://doi.org/10.1007/s10530-005-3735-y
Compton JE, Boone RD (2000) Long-term impacts of agriculture on soil carbon and nitrogen in New England forests. Ecology 81:2314–2330. https://doi.org/10.1890/0012-9658(2000)081[2314:LTIOAO]2.0.CO;2
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
Degasperis BG, Motzkin G (2007) Windows of opportunity: historical and ecological controls on Berberis thunbergii invasions. Ecology 88:3115–3125. https://doi.org/10.1890/06-2014.1
Dreyer GD, Jones CC, Capers R et al (2014) Native and naturalized vascular plants of Connecticut checklist. Connecticut Botanical Society, New Haven
Driscoll AG, Angeli NF, Gorchov DL et al (2016) The effect of treefall gaps on the spatial distribution of three invasive plants in a mature upland forest in Maryland. J Torrey Bot Soc 143:349–358
Dudney J, York RA, Tubbesing CL et al (2021) Overstory removal and biological legacies influence long-term forest management outcomes on introduced species and native shrubs. For Ecol Manag 491:19149. https://doi.org/10.1016/j.foreco.2021.119149
Elton C (1958) The ecology of invasions by animals and plants. Methuen, London
Ernst AR, Barak RS, Hipp AL et al (2022) The invasion paradox dissolves when using phylogenetic and temporal perspectives. J Ecol 110:443–456. https://doi.org/10.1111/1365-2745.13812
Essl F, Mang T, Moser D (2012) Ancient and recent alien species in temperate forests: steady state and time lags. Biol Invasions 14:1331–1342. https://doi.org/10.1007/s10530-011-0156-y
Flinn KM, Marks PL (2007) Agricultural legacies in forest environments: tree communities, soil properties, and light availability. Ecol Appl 17:452–463. https://doi.org/10.1890/05-1963
Flory SL, Clay K (2006) Invasive shrub distribution varies with distance to roads and stand age in eastern deciduous forests in Indiana, USA. Plant Ecol 184:131–141. https://doi.org/10.1007/s11258-005-9057-4
Fridley JD, Stachowicz JJ, Naeem S et al (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17. https://doi.org/10.1890/0012-9658(2007)88[3:TIPRPA]2.0.CO;2
Gioria M, Hulme PE, Richardson DM, Pyšek P (2023) Why are invasive plants successful? Annu Rev Plant Biol 74:635–670. https://doi.org/10.1146/annurev-arplant-070522-071021
Golivets M, Woodall CW, Wallin KF (2019) Functional form and interactions of the drivers of understory non-native plant invasions in northern US forests. J Appl Ecol 56:2596–2608. https://doi.org/10.1111/1365-2664.13504
Gomez P, Murua M, Martin JS et al (2019) Maintaining close canopy cover prevents the invasion of Pinus radiata: basic ecology to manage native forest invasibility. PLoS ONE 14:e0210849. https://doi.org/10.1371/journal.pone.0210849
Greenberg CH, Smith LM, Levey DJ (2001) Fruit fate, seed germination and growth of an invasive vine—an experimental test of “sit and wait” strategy. Biol Invasions 3:363–372. https://doi.org/10.1023/A:1015857721486
Hobbs RJ (2000) Land-use changes and invasions. In: Mooney HA, Hobbs RJ (eds) Invasive species in a changing world. Island Press, Washington, pp 55–64
Holmes MA, Matlack GR (2019) Non-native plant species show a legacy of agricultural history in second-growth forests of southeastern Ohio. Biol Invasions 21:3063–3076. https://doi.org/10.1007/s10530-019-02029-2
Holmes MA, Matlack GR (2017) Forest micro-environment develops through time: changes in physical and structural heterogeneity follow abandonment from two forms of agriculture. For Ecol Manag 404:55–64. https://doi.org/10.1016/j.foreco.2017.08.034
Holmes MA, Whitacre JV, Bennion LD et al (2021) Land-use history and abiotic gradients drive abundance of non-native shrubs in Appalachian second-growth forests with histories of mining, agriculture, and logging. For Ecol Manag 494:119296. https://doi.org/10.1016/j.foreco.2021.119296
Howard TG, Gurevitch J, Hyatt L et al (2004) Forest invasibility in communities in southeastern New York. Biol Invasions 6:393–410. https://doi.org/10.1023/B:BINV.0000041559.67560.7e
Huebner CD (2020) Patterns of invasive plant abundance in disturbed versus undisturbed forests within three land types over 16 years. Divers Distrib 27:130–143. https://doi.org/10.1111/ddi.13175
Iannone BV III, Oswalt CM, Liebhold AM et al (2015) Region-specific patterns and drivers of macroscale forest plant invasions. Divers Distrib 21:1181–1192. https://doi.org/10.1111/ddi.12354
Jang W, Crotteau JS, Ortega YK et al (2021) Native and non-native understory vegetation responses to restoration treatments in a dry conifer forest over 23 years. For Ecol Manag 481:118684. https://doi.org/10.1016/j.foreco.2020.118684
Johnstone IM (1986) Plant invasion windows: a time-based classification of invasion potential. Biol Rev 61:369–394. https://doi.org/10.1111/j.1469-185X.1986.tb00659.x
Kuebbing SE, Nuñez MA (2015) Negative, neutral, and positive interactions among nonnative plants: patterns, processes, and management implications. Glob Chang Biol 21:926–934. https://doi.org/10.1111/gcb.12711
Kuhman TR, Pearson SM, Turner MG (2013) Why does land-use history facilitate non-native plant invasion? A field experiment with Celastrus orbiculatus in the southern Appalachians. Biol Invasions 15:613–626. https://doi.org/10.1007/s10530-012-0313-y
Kuhman TR, Pearson SM, Turner MG (2011) Agricultural land-use history increases non-native plant invasion in a southern Appalachian forest a century after abandonment. Can J for Res 41:920–929. https://doi.org/10.1139/x11-026
Lebbink G, Dwyer JM, Fensham RJ (2022) ‘Invasion debt’ after extensive land-use change: an example from eastern Australia. J Environ Manag 302:114051. https://doi.org/10.1016/j.jenvman.2021.114051
Levine JM (2000) Species diversity and biological invasions: relating local process to community pattern. Science 288:852–854. https://doi.org/10.1126/science.288.5467.852
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Lundgren MR, Small CJ, Dreyer GD (2004) Influence of land use and site characteristics on invasive plant abundance in the Quinebaug Highlands of southern New England. Northeast Nat 11:313–332. https://doi.org/10.1656/1092-6194(2004)011[0313:IOLUAS]2.0.CO;2
Mack RN, Simberloff D, Lonsdale MW et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Martin PH, Canham CD, Marks PL (2009) Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ 7:142–149. https://doi.org/10.1890/070096
Matlack GR, Schaub JR (2011) Long-term persistence and spatial assortment of nonnative plant species in second-growth forests. Ecography 34:649–658. https://doi.org/10.1111/j.1600-0587.2010.06654.x
McCay TS, McCay DH (2009) Processes regulating the invasion of European buckthorn (Rhamnus cathartica) in three habitats of the northeastern United States. Biol Invasions 11:1835–1844. https://doi.org/10.1007/s10530-008-9362-7
McDonald RI, Motzkin G, Foster DR (2008) Assessing the influence of historical factors, contemporary processes, and environmental conditions on the distribution of invasive species. J Torrey Bot Soc 135:260–271. https://doi.org/10.3159/08-RA-012.1
Meiners SJ, Pickett STA, Cadenasso ML (2002) Exotic plant invasions over 40 years of old field successions: community patterns and associations. Ecography 25:215–223. https://doi.org/10.1034/j.1600-0587.2002.250209.x
Mosher ES, Silander JA Jr, Latimer AM (2009) The role of land-use history in major invasions by woody plant species in the northeastern North American landscape. Biol Invasions 11:2317–2328. https://doi.org/10.1007/s10530-008-9418-8
Niering WA, Goodwin RH (1962) Ecological studies in the Connecticut Arboretum Natural Area I. introduction and a survey of vegetation types. Ecology 43:41–54. https://doi.org/10.2307/1932038
Nunez-Mir GC, Liebhold AM, Guo Q et al (2017) Biotic resistance to exotic invasions: its role in forest ecosystems, confounding artifacts, and future directions. Biol Invasions 19:3287–3299. https://doi.org/10.1007/s10530-017-1413-5
Parker JD, Richie LJ, Lind EM, Maloney KO (2010) Land use history alters the relationship between native and exotic plants: the rich don’t always get richer. Biol Invasions 12:1557–1571. https://doi.org/10.1007/s10530-009-9568-3
Pascarella JB, Aide TM, Serrano MI, Zimmerman JK (2000) Land-use history and forest regeneration in the Cayey Mountains, Puerto Rico. Ecosystems 3:217–228. https://doi.org/10.1007/s100210000021
Petri L, Ibañez I (2023) Assessing the mechanisms and impacts of shrub invasion in forests: a meta-analysis. J Appl Ecol 60:2314–2326. https://doi.org/10.1111/1365-2664.14496
Rejmánek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661. https://doi.org/10.2307/2265768
Rejmánek M, Richardson DM, Pyšek P (2013) Plant invasions and invasibility of plant communities. In: van der Maarel E, Franklin J (eds) Vegetation Ecology. Wiley, pp 387–424. https://doi.org/10.1002/9781118452592.ch13
Riitters K, Potter K, Iannone BV III et al (2018) Landscape correlates of forest plant invasions: a high-resolution analysis across the eastern United States. Diversity Distrib 24:274–284. https://doi.org/10.1111/ddi.12680
Rogers DA, Rooney TP, Olson D, Waller DM (2008) Shifts in southern Wisconsin forest canopy and understory richness, composition, and heterogeneity. Ecology 89:2482–2492. https://doi.org/10.1890/07-1129.1
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176. https://doi.org/10.1016/S0169-5347(02)02495-3
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102. https://doi.org/10.1146/annurev.ecolsys.110308.120304
Simberloff D, Relva MA, Nuñez M (2002) Gringos en el bosque: introduced tree invasion in a native Nothofagus/Austrocedrus forest. Biol Invasions 4:35–53. https://doi.org/10.1023/A:1020576408884
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32. https://doi.org/10.1023/A:1010086329619
Small MJ, Small CJ, Dreyer GD (2005) Changes in a hemlock-dominated forest following woolly adelgid infestation in southern New England. J Torrey Bot Soc 132:458–470. https://doi.org/10.3159/1095-5674(2005)132[458:CIAHFF]2.0.CO;2
Stohlgren TJ, Binkley D, Chong GW et al (1999) Exotic plant species invade hot spots of native plant diversity. Ecol Monogr 69:25–46. https://doi.org/10.2307/2657193
Tonidandel S, LeBreton JM (2011) Relative importance analysis: a useful supplement to regression analysis. J Bus Psychol 26:1–9. https://doi.org/10.1007/s10869-010-9204-3
Traveset A, Richardson DM (2020) Plant invasions: the role of biotic interactions–an overview. In: Traveset A, Richardson DM (eds) Plant invasions: the role of biotic interactions. CAB International, Wallingford, UK, pp 1–25
Verheyen K, Bossuyt B, Hermy M, Tack G (1999) The land use history (1278–1990) of a mixed hardwood forest in western Belgium and its relationship with chemical soil characteristics. J Biogeogr 26:1115–1128. https://doi.org/10.1046/j.1365-2699.1999.00340.x
Vilà M, Ibáñez I (2011) Plant invasions in the landscape. Landscape Ecol 26:461–472. https://doi.org/10.1007/s10980-011-9585-3
Von Holle B, Delcourt HR, Simberloff D (2003) The importance of biological inertia in plant community resistance to invasion. J Veg Sci 14:425–432. https://doi.org/10.1111/j.1654-1103.2003.tb02168.x
Von Holle B, Motzkin G (2007) Historical land use and environmental determinants of nonnative plant distribution in coastal southern New England. Biol Conserv 136:33–43. https://doi.org/10.1016/j.biocon.2006.10.044
Von Holle B, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218. https://doi.org/10.1890/05-0427
Waddell EH, Chapman DS, Hill JK et al (2020) Trait filtering during exotic plant invasion of tropical rainforest remnants along a disturbance gradient. Funct Ecol 34:2584–2597. https://doi.org/10.1111/1365-2435.13679
Wagner V, Večeřa M, Jiménez-Alfaro B et al (2021) Alien plant invasion hotspots and invasion debt in European woodlands. J Veg Sci 32:e13014. https://doi.org/10.1111/jvs.13014
Walker L, Chapin FS III (1987) Interactions among processes controlling successional change. Oikos 50:131–135. https://doi.org/10.2307/3565409
Wang HH, Wonkka CL, Grant WE, Rogers WE (2012) Potential range expansion of Japanese honeysuckle (Lonicera japonica Thunb.) in southern U.S. forestlands. Forests 3:573–590. https://doi.org/10.3390/f3030573
Yates ED, Levia DF, Williams CL (2004) Recruitment of three non-native invasive plants into a fragmented forest in southern Illinois. For Ecol and Manag 190:119–130. https://doi.org/10.1016/j.foreco.2003.11.008
Acknowledgements
Our thanks to William A. Niering and Richard H. Goodwin, who initiated the long-term dataset, and to many students and faculty who collected the data over the years. Martin Dovciak and an anonymous reviewer provided helpful comments on drafts of this manuscript.
Funding
This work was supported by long-term research funding specifically for this dataset administered by the Connecticut College Arboretum.
Author information
Authors and Affiliations
Contributions
Chad Jones conceived and designed the study. Both authors contributed to data collection and analysis. Both authors contributed to drafting and revising the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, C.C., Yamamoto, M.H. Long-term patterns and mechanisms of plant invasions in forests: the role of forest age and land-use history. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03365-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03365-8