Abstract
Few invasive birds are as globally successful as the Common or European Starling (Sturnus vulgaris). Native to the Palearctic, the starling has been intentionally introduced to North and South America, South Africa, Australia, and the Pacific Islands, enabling us to explore species traits that may contribute to its invasion success. Coupling the rich studies of life history and more recent explorations of genomic variation among invasions, we illustrate how eco-evolutionary dynamics shape the invasion success of this long-studied and widely distributed species. Especially informative is the comparison between Australian and North American invasions, because these populations colonized novel ranges concurrently and exhibit shared signals of selection despite distinct population histories. In this review, we describe population dynamics across the native and invasive ranges, identify putatively selected traits that may influence the starling’s spread, and suggest possible determinants of starling success world-wide. We also identify future opportunities to utilize this species as a model for avian invasion research, which will inform our understanding of species’ rapid evolution in response to environmental change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: the globally invasive starling
The ecological and economic impacts of invasive species are a growing concern in our globalized world (Bellard et al. 2016; Diagne et al. 2021). Invasive species are those that humans have introduced outside of their natural geographical range, and have documented financial or ecological impacts within their introduced range (Lockwood et al. 2013; Turbelin et al. 2017; Crystal-Ornelas and Lockwood 2020). Increasing patterns of human intercontinental travel creates and reinforces invasion pathways, resulting in a great number of species becoming established and spreading in novel ranges (Turbelin et al. 2017). In an epoch when species invasions are likely to increase, identifying how some species colonize and expand in novel environments while others do not remains a challenge, and a thorough review of factors that promote invasion success may bring us closer to this aim. Invasion success typically represents a species’ establishment of a breeding population in a new region and its subsequent expansion (Lockwood et al. 2013), although we note that a definition of invasion success depends on which factors are considered. Genotypic variation, species niche, local abundance, and environmental features all influence invasion success (Colautti and Barrett 2013). Common properties of invasions (e.g., propagule pressure, genetic bottlenecks, etc.) and adaptive evolution of novel strategies (e.g., dispersal strategies, breeding behavior) may work in concert to facilitate successful invasions (Duncan et al. 2003; Redding et al. 2019; Fristoe et al. 2021). However, climate, ecosystem composition, and human activity may also influence how invasive species establish and spread (Liu et al. 2020; Miller et al. 2021). Attempts to identify drivers of invasion success have shown that what predicts a species’ invasion includes both its ecological context and its evolutionary history (Hayes and Barry 2008; Colautti et al. 2017; Enders et al. 2020).
Few avian invaders have been as globally successful as the Common or European Starling (Sturnus vulgaris) (hereafter referred to as simply the ‘starling’). Starlings are generalists that thrive in a wide array of environments, particularly those altered by humans, and have a costly impact on agriculture and native ecosystems (Homan et al. 2017; Linz et al. 2017). Native to the Palearctic, the starling has been introduced to Australia, New Zealand (and the Pacific Islands), North America, South Africa, and South America (Table 1), and has been listed as one of the world’s 100 worst invasive alien species (Lowe et al. 2000). Detailed historical records of expansion are available in each of these invasive populations, providing documentation of the behavior, life history, and ecological context encountered during the invasion process. In addition, starlings are a widely-used model in laboratory studies (Asher & Bateson 2008), and linking such thorough studies of starling traits with wild observations may help to clarify mechanisms that support invasiveness and population persistence in general.
Nearly all studies of invasion genetics examine the paradox of invasion, where species thrive despite a loss of genetic diversity (Rollins et al. 2013; Dlugosch et al. 2015; Estoup et al. 2016). Repeated invasion success across starlings’ many introduction sites presents an opportunity to document rapid evolution post-introduction, often following genetic bottlenecks and perhaps leading to adaptive evolution. DNA sequencing advances over the last decade have made genomic approaches more accessible to non-model species such as the starling (North et al. 2021), expanding the use of genetic analyses and enabling examination of the proximate mechanisms that may contribute to this invasive species’ success. Often the focus of such studies is invasive species’ ability to undergo rapid evolution in their novel range despite apparent low genetic diversity (Verhoeven et al. 2011; Willoughby et al. 2018). In contrast to their invasion success, starling numbers are currently declining in both their native range (Smith et al. 2012; Heldbjerg et al. 2019) and in the North American range (Rosenberg et al. 2019). Understanding how starlings thrive in invasive populations may inform conservation initiatives in the native range, while also aiding control efforts in the invasive ranges.
In this review we will discuss how the starling can serve as a model for understanding invasion success and eco-evolutionary dynamics more broadly. We synthesize extensive research on starling life history with genetic and genomic evidence from the native and invasive ranges to identify factors correlated with demographic or adaptive patterns within each location. We first describe the history of native and invasive starling populations, and then suggest how eco-evolutionary feedback might continue to shape range expansion and/or population declines. The starling’s dynamic invasion history presents a wild system where concurrent, replicated invasions (into Australia, North America, New Zealand and South Africa) as well as more recent invasions (into Argentina) enable us to distinguish between population-specific and species-wide strategies that appear to support invasion success. A global review of starling invasions may help to identify how this particular invasive species now thrives on nearly every continent. Interestingly, despite their longstanding title as a prolific pest, the starling continues to decline in their native range. Comparing life history, behavior, and ecology between native to invasive populations may help to identify drivers of these changes. As a model of invasion, the starling is a dynamic system, and we articulate how factors supporting invasion success might interact as steps towards predicting future shifts in range and abundance of the starling world-wide.
Beyond invasion: the starling as an important eco-evolutionary model
The starling is not the only avian species to invade nearly every continent world-wide: in fact, the House Sparrow (Passer domesticus) is similarly successful in a wide range of environments (Hanson et al. 2020), as is the rock pigeon Columba livia (Downs and Hart 2020), and the common myna Acridotheres tristis (Magory Cohen et al. 2019). Each of these species has advantages for use as a ‘model’ species for the study of invasion (but see Hanson et al. 2020’s careful discussion of the consequences of labeling a species as a ‘model’). Because there is ample interest in studying invasive bird species (Fig. 1), and comparisons among species might yield insight into invasion biology and evolution, future directions in this field could include comparative genomics of these well-studied species.
a The starling, and other common invasive avian species with their b Web of Science (representing total published papers in which the species name is a key word) and c NCBI BioSample search result counts (which represent the total count of independent sequencing projects). Data accessed on October 29, 2021
What makes the starling nearly unique among these other potential avian models is the availability of a rich literature across biological sub-disciplines and well-developed genomic resources. In addition to this information, starling introductions are replicated across the globe and these independent introductions serve as experiments through which researchers may seek to assess divergent and parallel patterns across populations. Because of the global spread and iconic nature of this species, the starling features as an abundant, globally collected avian specimen in natural history museums around the world, enabling temporal analyses to be conducted over a wide geographic area (see Table 2 which showcases the geographical breadth of starling collections globally). Museum collections include starling skins, skeletal elements, eggs, tissues in alcohol, and nest specimens from the native and invasive ranges. Historical starling specimens range from the early 1800’s to the 2000’s and enable temporal analyses which could clarify aspects of pre- and post-invasion morphology and genetics (e.g. Stuart et al. 2022). With improving technologies for extracting and sequencing DNA from museum skins, these collection resources present even more potential than in previous decades (Raxworthy and Smith 2021). Genomic analysis of these species is helped by the existence of multi-tissue transcriptomic data (Richardson et al. 2017; Stuart et al. 2022b) and genome assemblies (North America, and Australia, Stuart et al. 2022b), providing vital genomic references for future analyses. Owing to the large amount of genetic research that exists already on this species, there is also a wealth of pre-existing starling genetic data available in public repositories (Fig. 1). Finally, there is also much basic ecological and physiological research on the starling. Indeed, the starling boasts one of the broadest range of research areas across laboratory studies conducted on passerines (Bateson and Feenders 2010). Previous laboratory and field studies have examined their interactions with agriculture (Linz et al. 2017), patterns of migration and flocking (Piersma et al. 2020), social behaviors including its song (for example, Eens et al. 1993), and extensive studies of hormone regulation of behaviour (for example, Gwinner et al. 2002), all of which provide a wealth of background knowledge that assist in the interpretation and contextualization of future study findings.
Native starling distribution and population genetics
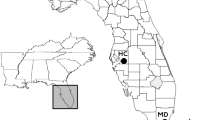
The native distribution of the starling extends across the Palearctic (Fig. 2), and is thought to be primarily a result of changes in forest coverage and aridity tied to major climate shifts across the late Miocene (Zuccon et al. 2008). The non-breeding range extends as far as Russia (Sandakova et al. 2018), whereas the breeding range extends southwest into Pakistan and Israel (Mahmood et al. 2013). Since the mid-nineteenth century, this species has been slowly expanding its Eurasian range (Feare 1984). Starling colonization of Iceland occurred in the late 1930’s (Harris 1964), while a small number of starlings have been reported to winter in Hong Kong since as early as the 1970’s (Webster 1975). This natural range expansion is likely a result of increased temperatures, anthropogenic land alteration, climate change, and possibly a decrease in competing species in newly colonized ranges (Harris 1964; Webster 1975). In one region of this recent expansion, the starling has come back into contact with its closest relative, the spotless starling (Sturnus unicolor), from which it split only ~ 1 MYA (Zuccon et al. 2008). In this contact zone in the Iberian Peninsula, the two species seem to interbreed readily: in fact, allozyme studies show that genetic distances within S. unicolor are larger than genetic distances between the two species (Cruz‐Cardiel et al. 1997; Lovette et al. 2008).
Starling distribution map according to eBird sightings data (Sullivan et al. 2009) (retrieved Feb 2018). Native marked in teal, invasive in maroon. First introduction date at an introduction site is marked with a blue circle
One of the major points of intrigue in starling biology is that in the native range, starling numbers have dwindled even as its geographical range expands. Since 1964, estimated starling numbers in Great Britain have declined by more than 50%. This decline is particularly notable in livestock farming areas of southwest Britain, with the number of breeding individuals estimated to be 8.5 million as of 2005 (Robinson et al. 2005). Finnish starlings underwent a dramatic 90% decrease in numbers from 1970 to 1985, corresponding to a widespread abandonment of cattle farming across the country (Rintala et al. 2003). Similarly, a shift to indoor cattle husbandry in Denmark may have contributed to the 60% overall decline in starling abundance between 1976 and 2015 (Heldbjerg et al. 2016). This close association between cattle farming and starling abundance may be explained by the birds’ foraging decisions. Starlings exhibit highly non-random landing sites and search efforts, and high preference for freshly mowed or grazed field (Tinbergen 1981). A move toward modern cattle rearing has resulted in changes to available pasture, which may indirectly influence surface soil availability of small invertebrate prey (Chamberlain et al. 2000; Wretenberg et al. 2006) on which starlings rely during breeding season (Feare 1984). In addition, starlings readily consume livestock feed, and modern cattle rearing processes seek to minimize feed loss to species such as starlings (Linz et al. 2017). These decreases are not isolated to agricultural areas; starling also are declining in urban areas (Robinson et al. 2005; Barton et al. 2020). Further, the starling decline is not unique: recent decades have brought a decline in farmland birds in general across the UK and Europe (Gregory et al. 2002; Wretenberg et al. 2007).
Recent declines in starling abundance in the native range may also be explained by changes in life history strategy. Studies of the declining starling population in the Netherlands identified that the main driver of changes in demography was juvenile survival (Versluijs et al. 2016). Similarly, declines in abundance in Britain from the 1970’s to the present have been linked to first-year survival rates (Freeman et al. 2007). What exactly explains lower survival rates is more challenging to identify, but may relate to how starlings adjust their foraging strategy to resource availability and other biotic factors such as predator presence. Starlings maintain a lower body mass during favorable foraging conditions when resources are abundant due to starvation-predation risk trade-offs; within the United Kingdom, starlings sampled in areas where declines in population size were greater than the mean had a higher average mass than those from areas where changes to starling population size were lower than the mean decline (Macleod et al. 2008). These results suggest that changes to land use (e.g. urbanization) that affect food webs may be related to recent population declines in areas that were once rural. However, in urban areas, declines have been linked to decreased nesting success in less natural environments (Peris et al. 2005; Siriwardena and Crick 2002).
Although studies of starling declines in their native range have increased in recent years, little genetic information exists for the native range, and what is known is largely restricted to the United Kingdom, Belgium, and north-eastern Spain. Much of the existing research that investigates starling genetic diversity in the native range has been conducted with the purpose of providing context to assist with the interpretation of genetic diversity loss within the invasive ranges (Rollins et al. 2011; Bodt et al. 2020; Hofmeister et al. 2021a; Stuart et al. 2022a). Early allozyme studies of nuclear genetic diversity in starlings reported low levels of variation across the native range (Evans 1980; Ross 1983; Neves et al. 2009); however, allozymes capture less diversity than more recently developed markers (Fig. 3). More recent microsatellite analysis indicated the presence of population genetic structure across the United Kingdom, with the northern and southern regions being differentiated from one another (Walkup 2013). Analyses of mitochondrial control region sequence data (commonly assumed to evolve faster than nuclear DNA; Brown et al. 1979) suggest very high diversity within the native range (17 haplotypes from 27 individuals sampled in one locality, Rollins et al. 2011; 13 haplotypes from 16 individuals from England and Wales, Berthouly-Salazar et al. 2013). Comparisons with the Australian invasion using reduced representation sequencing provide evidence for genetic divergence relative to historic ancestral populations (Stuart et al. 2022a). Morphology varies across the starling’s native range: subtle differences in adult plumage iridescence across the native range have led to the classification of 11–13 subspecies (Pateff & Stresemann 1947; Feare 1984). However, given the paucity of genetic and morphological evidence for within-species differentiation, most studies of this species ignore subspecies identification. It is likely that all invasive populations are S. vulgaris vulgaris, because historical records indicate the source population were in the United Kingdom (Table 1).
Additional studies to document population structure and genetic consequences of changing population size in the native range would help to clarify how starlings are responding to continued anthropogenic changes in land use. In particular, characterising demographic changes and genetic structure outside Europe (i.e., Northern Africa, Middle East, and Asia) would help resolve the dynamic relationship between environmental change and starling abundance, by providing comparisons between native populations where climate, agricultural practices and land management strategies differ widely. Certainly, environmental conditions and range expansion impact the evolutionary potential of the native starling population, but perhaps the greatest difference between native and invasive populations is in the management strategies in the different areas (e.g., culling). These strategies may lead to changes in the life history of starlings: populations that are actively managed may adjust dispersal, timing of clutches, clutch size, or other factors to increase reproductive success. Culling results in increased mortality that could lead to the evolution of new behavioral strategies to survive (Sol et al. 2002). Management strategies remain an under-examined variable that may alter the ecology and evolution of invasive populations species like the starling.
Invasive starling distribution and population genetics
Australia
The Australian introduction is the oldest documented introduction of starlings. In Australia, starlings were introduced to control invertebrate agricultural pests, and as part of efforts of acclimatization societies (Woolnough et al. 2006). Additionally, an unknown number of private introductions occurred (Jenkins 1977; Higgins et al. 2006); however, the first officially documented starling import was the 1856 introduction of an unknown number of birds to New South Wales (Higgins et al. 2006). Over the next several years, there were several introductions in Melbourne, Victoria, and the species was described as ‘well established’ in Victoria as early as 1863 (Jenkins 1977). Subsequently, there were numerous introductions during the late nineteenth century to New South Wales, Victoria, South Australia, Queensland, and Tasmania (Table 1). The releases that contributed to starlings’ widespread success in Australia are thought to have occurred near the coastal capitals of the States of Victoria, New South Wales, or South Australia (Stuart et al. 2021). From the early years of their introduction to Australia, starlings increased rapidly in both geographic range and population size (Jenkins 1977).
By the mid-twentieth century, most of the southern and eastern regions of Australia were colonized, including Tasmania, and birds were reported to be most prolific in Victoria (Long 1981). Western Australia has remained largely starling free, due jointly to the natural barrier provided by the Nullarbor Plain, and ongoing control efforts by the Western Australia Department of Primary Industries and Regional Development (DPIRD) since 1971 (Campbell et al. 2016). The latter program was intensified in the mid 2000’s in the vicinity of Esperance on the south coast where the largest incursion by starlings into Western Australia occurred (Woolnough et al. 2005). A majority of the birds culled in Western Australia originated from the southern region of the starlings’ Australian range (Rollins et al. 2011). Nevertheless, a few small incursions into locations far from the Esperance area (e.g. Broome, on the northwest coast) are presumed to have arrived via unintentional anthropogenic means (Rollins et al. 2009, 2011). While Western Australia has remained largely starling free for over a decade, the last few years have seen an increase in the number of new ‘founder’ birds spotted in the area of Munglinup and surrounds. All birds captured in this time period were females in breeding condition (Rollins, pers. comm.). Since starlings have a female-biased dispersal (Rollins et al. 2009), these individuals may represent new incursions into this area. Cost-benefit analyses of control efforts suggest that the short-term costs of control in Western Australia are worthwhile, given the large potential costs to agriculture (Campbell et al. 2016).
Australian starlings have had a relatively steady range expansion rate of 20.7 km/year since establishment (Hui and Richardson 2017), but do not undertake large-scale seasonal migration as is known in North America and their native range. It is thought the range expansion in Australia, particularly at the present day range edge, is driven by birds seeking new nesting sites, rather than seasonal visitation (Long 1981). Small-scale regional movement has been attributed to food seeking; however, banding data indicate a small number of birds disperse long distances (up to 1000 km, Waterman et al. 2008). The Australian starling invasion appears to be undergoing spatial sorting, where dispersal-enhancing traits accumulate at the leading edge of an expanding population (Phair et al. 2018), and mitochondrial sequencing indicates that while spatial expansion may be occurring across the Australian range, demographic expansion appeared to be limited to the range edge (Rollins et al. 2011).
Across the Australian range, genetic diversity is estimated to be lower than in the native range (Rollins et al. 2011; Stuart et al. 2022a, see Table 3 for summary of starling population genetic studies). Population genetic studies suggest four genetic groups of starlings are present in Australia, although the delineation of those groups differs across marker types. Microsatellite data indicate there are two small, genetically distinct incursions into Western Australia, and two larger genetically distinct groups, one in South Australia and another across Victoria, Tasmania, and New South Wales (Rollins et al. 2009). Reduced representation sequencing data (genotyping-by-sequencing) incorporating a broader sampling scheme (samples from across the entire Australian range) indicate that starlings in Victoria, South Australia, and the easternmost incursion in Western Australia form a single genetic group, those from New South Wales and Queensland form a second genetic group, and two geographically restricted genetic groups were identified: one in the westernmost incursion in Western Australia described by Rollins et al. (2011), and the second in arid regions of inland New South Wales (Stuart et al. 2021).
Across the Australian range, there is a significant relationship between time of establishment and genetic diversity (Rollins et al. 2009). The highest genetic diversity was found in sampling sites near the three primary introduction sites, and the lowest genetic diversity was found at the range edge (Rollins et al. 2009, 2011; Stuart and Cardilini 2021). In addition to signals of genetic drift often found in expanding populations, there is evidence of selection: a rapid shift in frequencies of mitochondrial DNA variants on the range edge is best explained by selection acting within heteroplasmic individuals (those that carry two mitochondrial variants; Rollins et al. 2016). Further, clinal variation in morphology indicates adaptation has occurred across this invasive range (Cardilini et al. 2016), although developmental plasticity is also shaping these morphological trends alongside genetic heritability (Stuart et al. 2022). Environmental correlations with allele frequencies in coding regions related to a range of biological functions (e.g. immune response, metabolism) have enabled the identification of putative loci under selection in this invasion (Stuart et al. 2021). Additionally, historical specimens, sequenced alongside contemporary native and invasive Australian individuals, has identified additional candidate regions of the genome that appear to have responded to selection following introduction (Stuart et al. 2022a).
New Zealand
Similar to the starling introduction of Australia, the starlings of New Zealand were introduced from Britain through acclimatization efforts (Thomson 1922). The introductions were well documented, with records of 14 major introductions from 1862 to 1883 across New Zealand, including approximately 650 individuals (Table 1, Thomson 1922). The success of starlings in New Zealand was assisted by large-scale translocations within the country (Pipek et al. 2019). Much like with the Australian introduction, interest in the species for pest control may have also led to smaller private introductions to farming properties (Thomson 1922).
The starling is now the most widespread avian species in New Zealand and their range covers the entire country, from the northern outlying island of Kermadecs, to the south outlying Macquarie Islands (Williams 1953; Flux and Flux 1981). In 1886, the species was recorded to occur in the “hundreds of thousands” (Thomson 1922). However, there was an apparent decrease in population size around the 1910’s (Thomson 1922), which is thought to be at least in part due to a decrease in nest availability (Flux and Flux 1981). The starling remains present across New Zealand (Starling-Windhof et al. 2011; Bell 2015), though no official information on breeding population estimates exists.
According to allozyme data, New Zealand starlings have retained much of the genetic diversity found in the native range (Ross 1983). There was only a slight loss of genetic variation during the colonization of New Zealand, associated with the loss of rare alleles (Ross 1983). This mild genetic bottleneck is likely a result of the large founding numbers and multiple introductions at many localities. Relatively high levels of differentiation across the New Zealand invasion suggests subpopulations are geographically isolated, owing to both the country’s mountainous terrain, and the bird’s preference for agricultural and urban areas, coupled with an absence of seasonal migratory behavior (Ross 1983). Further study of starling population genetics across New Zealand and the Pacific Islands would provide an interesting avenue to examine consecutive bottlenecks during island hopping and how this demographic process impacts adaption to novel environments.
The New Zealand invasion has also served as a stepping-stone for subsequent invasions in the Pacific islands. The Fiji starling invasion is thought to have been established in the mid 1920’s, and has now spread to Tonga (Watling and Talbot-Kelly 1982). Fijian starlings probably dispersed via natural means from the Kermadec Islands, which lie equidistant to New Zealand (Williams 1953; Flux and Flux 1981). Further spread of starlings throughout these tropical islands may be impeded by their physiological limits (Watling and Talbot-Kelly 1982), a possibility we discuss in Sect. 5.3 of this review.
North America
Acclimatization societies and private individuals attempted to introduce starlings several times in North America (Table 1) (Cooke 1928). Acclimatization enthusiast Eugene Schieffelin released 80 individuals in March 1890 and 40 more in April 1891 to Central Park in New York, where these individuals reproduced and began to spread almost immediately. This starling introduction was the first to establish a breeding population in North America according to historical records (though this is somewhat contested, see Fugate and Miller 2021), after which the species spread rapidly throughout the continent (Kalmbach et al. 1921; Linz et al. 2017). North American starlings are now found as far north as the Arctic Circle and are slowly continuing to expand their range southward (eBird 2021). Starlings have expanded into Cuba and the Bahamas (Linz et al. 2017). Additionally, starlings were purposely introduced to Jamaica in 1903 (Craig 2020) for crop damage mitigation, likely sourced from the North America introduction (Lever 2010), and starlings were first recorded in eastern Mexico in the 1930’s (Zusi et al. 1959). How starlings continue to expand into novel environments is a question that we revisit in Sect. 5 of this review.
Starlings’ movement varies across the North American landscape. Starlings in the western U.S. tend to move regionally whereas birds sampled in the eastern U.S. tend to be recovered outside of the state to which they are assigned based on molt origin. This suggests that migration and/or dispersal vary longitudinally (Werner et al. 2020). Banding efforts across North America also indicate haphazard migratory patterns, such that starlings migrate northwest to southeast and most migration occurs along the migratory flyway of the Mississippi River (Kessel 1953; Brewer 2010). These patterns of movement likely contributed to the panmixia seen in the North American introduction, which could reduce geographical differentiation and strong population structure (Cabe 1999; Hofmeister et al. 2021b).
Starling numbers within North America are estimated at 60–200 million, peaking during the fall (Homan et al. 2017; Linz et al. 2017; Rosenberg et al. 2019), though have been estimated to have declined by approximately half since the 1970’s (Rosenberg et al. 2019). Genetic data from historical samples could be used to determine whether genetic diversity has declined with the decline of population size, which could result in decreased evolutionary potential. Demographic models indicate a slight decline in effective population size, based on both reduced-representation sequencing markers (Hofmeister et al. 2021b) and whole genome sequences (Hofmeister et al. 2021a). In contrast to models that use site-frequency spectra to reconstruct demographic history, faster-evolving mitochondrial evidence indicates that the North American invasion may now be expanding (Bodt et al. 2020).
Comparisons of genetic diversity between invasive and native ranges provide another perspective on historical population size changes. While heterozygosity in allozyme data among the North American and native range populations remained similar, a loss of allelic diversity within North America provides evidence that a genetic bottleneck occurred (Cabe 1998). Mitochondrial analyses indicate that both nucleotide and haplotype diversity is lower in the North American range compared to that of the UK, further supporting the evidence for a slight genetic bottleneck in North America (Bodt et al. 2020). Mitochondrial haplotype diversity is nevertheless higher in North America in comparison to the invasive populations of Australia and South Africa (Bodt et al. 2020), which may reflect greater genetic diversity in the North American founders compared with these other introductions. This higher level of genetic diversity in the North American invasion may provide greater standing variation upon which selection can act (Hofmeister et al. 2021a, b).
The geographic expansion of North American starlings likely relied on urban and agricultural areas to support a large enough breeding population to facilitate expansion. Historical records indicate that expansion accelerated following the establishment at range-edges within cities (e.g., Philadelphia in 1910). Mountainous regions may impose a barrier to starling spread in North America, because range expansion stalled when birds reached the Allegheny Mountains in 1911, the Adirondacks of New York in 1914, and White Mountains of Vermont in 1922 (Forbush 1915; Kalmbach et al. 1921; Cooke 1928), and again when starlings reached the 1000 m elevation mark in the Midwest of the United States of America in 1930 (Hoffman 1930; Dickerson 1938). In accordance with this expansion history, a genome-wide scan found genotypic associations between environmental characteristics, particularly elevation, precipitation and temperature (Hofmeister et al. 2021b). Whether starling expansion was in fact supported by adaptive evolution to novel elevational barriers requires both more thorough genomic investigation and functional validation.
Rapid evolution in the North American starling invasion is evidenced by morphological differences in individuals sampled across the continent. For example, wing pointedness has decreased over the last 120 years since colonization, which might allow more dexterity during flight (Bitton and Graham 2015). Further, North American starlings sampled at intermediate latitudes (which may best approximate the native range climate) had the greatest lipid reserves overwinter, and this trait was correlated with mean temperature in July and January (Blem 1981). Because starlings are partial migrants, populations and/or individuals that experience the same environmental conditions may evolve different migratory strategies that are associated with changes in morphology and/or physiology. While coarse morphology is conflated with a plethora of variables, the results of these studies provide direction for future molecular and experimental investigations.
South Africa
The South African starling population resulted from just 18 birds, introduced in 1897 to Cape Town by Cecil Rhodes, the then Prime Minister of the British Cape Colony (Cooper and Underhill 1991; Harrison and Cherry) (Table 1). These 18 birds were reportedly caught in Britain during winter months (Winterbottom and Liversidge 1954). From the introduction site, starlings spread eastwards across the Cape Flats. The natural mountainous barriers plausibly contributed to the initial slow expansion rate (Rensburg 2014). By the early 2000’s, starlings reached the Kwazulu Natal Province and the species’ current range covers up to Johannesburg to the north and southern Namibia to the west (Berthouly-Salazar et al. 2013; Rensburg 2014). The range’s eastward expansion has been largely enabled by the corridor provided by human habitation (Berthouly-Salazar et al. 2013). The rate of range spread in the South African starling has increased since their introduction, from 6.1 km/year to 25.7 km/year (Hui et al. 2012). Despite this, mismatch analysis on mitochondrial sequence data do not provide evidence of demographic expansion (Bodt et al. 2020).
Mitochondrial genetic diversity in South African starlings was found to be moderate: less than that of the native range and North American starlings, but greater than that of Australian starlings (Berthouly-Salazar et al. 2013; Bodt et al. 2020). In contrast, microsatellite data suggest the South African invasion had similar levels of genetic diversity to that of the UK samples used in that study (Berthouly-Salazar et al. 2013). These conflicting results may indicate a heavily sex-biased introduction (more males than females). Analysis of mitochondrial control region sequence data indicated no population structure within South Africa, but did identify a subtle decrease in genetic diversity towards the range edge (Berthouly-Salazar et al. 2013). Despite the genetic patterns underlying the invasion gradient, the South African invasion displayed no pattern of spatial sorting, unlike the Australian invasion (Phair et al. 2018). This may be due to higher rates of long distance dispersal in South Africa, which would maintain genetic homogeneity (Berthouly-Salazar et al. 2013). Despite an absence of spatial sorting in South Africa, there was increased genetic distance between individuals from sampling sites within areas with higher winter precipitation, indicating gene flow may be limited where precipitation is high in winter and low in summer (Berthouly-Salazar et al. 2013). This has resulted in two subpopulations around George and Mossel Bay (300 km east of introduction site), despite the lack of population subdivision found elsewhere in this range. The area around George and Mossel Bay is associated with a sharp change in climatic conditions, particularly winter precipitation (Berthouly-Salazar et al. 2013).
South America
Starlings were introduced relatively recently to South America. In 1949, five individuals were transported by ship from England and alighted in Lago de Maracaibo, Venezuela, though the success of these specific individuals remains unknown (Long 1981) (Table 1). In 1987, starlings were spotted in Buenos Aires, Argentina, in the wooded areas of the Palermo district (Peris et al. 2005), thought to be the result of an introduction via birds imported from North America for the pet trade (Navas 2002; Fiorini et al. 2021).
Despite prompt eradication efforts, the species established itself and further sightings occurred in 2001 near Sante Fe, 400 km north of Buenos Aires (Peris et al. 2005; Navas 2002). Starling range expansion within South America is strongly associated with urban areas which facilitate continual range expansion into regional areas (Zufiaurre et al. 2016). Starlings make use of novel nesting sites available in the human modified environment though retaining a preference for natural nesting sites (Peris et al. 2005) and have high nesting success rates within native forests (Jauregui et al. 2022). More recently, the starling’s South American distribution is reported to cover Uruguay and reached Brazil in late 2016 (e Silva et al. 2017), and are most abundant in grasslands, mirroring the habitat preference of functionally (in terms of body mass, and dietary and foraging traits) comparable native species (Palacio et al. 2016). The Brazilian range currently covers an area greater than 65,000 km2 in the Pampas region, with the rate of range expansion having increased linearly from 7.5 km/year in 2005 to 22.2 km/year in 2016 (Zufiaurre et al. 2016). This acceleration of range expansion after establishment has also been documented in the Australian and South African invasions (Phair et al. 2018).
Mitochondrial DNA analysis of birds collected in Buenos Aires indicated reduced haplotype diversity compared to North American and native range starlings, although several novel haplotypes were identified (Fiorini et al. 2021). That study also noted increased primary wing feather asymmetry within this secondary introduction, compared to that of the North American invasive population and to native birds sampled from the UK. This morphological asymmetry is hypothesized to result from destabilized developmental processes due to reduced genetic variation (Fiorini et al. 2021).
What explains invasion success in the starling?
Invasion theory predicts that an invasive species’ successful establishment and spread depends on a dynamic orchestration of ecological and evolutionary factors. Components of invasion success include but are not limited to: climate and environmental suitability, ecological interactions, social interactions, personality, demography, dispersal patterns and genetic factors such as pre-adaptation or invasion potential (Redding et al. 2019; Fristoe et al. 2021). Distinguishing among contributors to invasion success in wild systems is a technical challenge but comparing recent and replicated invasions of the same species (e.g. starlings) may help identify which factors best explain invasion success. In the following sections, we place the burgeoning genomic studies of starlings in the context of modern invasion theory, to both highlight the utility of such genomic approaches, and to identify hypotheses yet to be tested in this species.
Dispersal and migration
Starlings may be resident (remaining in the same area year-round) or migratory (seasonal visitation to a location), with birds migrating up to 1000–1500 km (Linz et al. 2007). In general, native starlings are migratory in the Northern and Eastern portions of their European range, and partially migratory and resident in the Southern and Western regions (due to warmer temperatures) (Higgins et al. 2006). Within the North American invasive range, rates of migration vary from 3 and 100% among regions (Kessel 1953; Blem 1981). Migratory behaviour is frequently reported in the Eastern United States, though residency during colder winter months is enabled by urban landscape elements (Kessel 1953; Dolbeer 1982; Higgins et al. 2006; Werner et al. 2020). Within the Australia and New Zealand invasive populations, there is no evidence of migration (Waterman et al. 2008). Patterns of migration or lack thereof align well with the known patterns of genetic differentiation within each population of starlings, as discussed above. How migratory behaviour might support adaptation in passerine birds, and contribute to invasion success, is an active and fruitful area of research and should be extended to include starlings (Chapman et al. 2011; Winger et al. 2019; Delmore et al. 2020).
In contrast to seasonal migration, all starling populations experience dispersal strategies that impact range expansion. In every population, younger, juvenile birds or immature adults will form larger flocks in the non-breeding season, presumably as a means of additional protection during these more vulnerable periods of the bird’s life cycle (Higgins et al. 2006). This stage is essential in the species’ expansion: long-range dispersal is common when starlings are juveniles, before they have mated (Cabe 1999), as is common in many avian species (Paradis et al. 1998). Although dispersal is ubiquitous across populations, the distance dispersed as well as the timing of dispersal varies with particular environmental conditions (e.g. density, climatic conditions). Specifically, within the South African introduction, increased dispersal is associated with deteriorating environmental conditions (Hui et al. 2012). Lower spread rates have been reported in areas with higher winter precipitation, indicating that unfavorable (low rainfall) conditions may trigger greater dispersal (Berthouly-Salazar et al. 2013). The same association has not been explicitly tested within other invasive ranges. However, isotopic evidence in North America suggests region-specific movement that may be related to population density, abiotic conditions, or other factors (Werner et al. 2020). In Australia, long-distance dispersal events are heavily female-biased (Rollins et al. 2009). Considering this evidence, starling dispersal strategies differ dramatically between invasive ranges and the native one, indicating a flexible response of the species to spatial and temporal environmental variations (Hui et al. 2012) which may favor invasion ability.
Breeding behaviour
Starlings depend on existing cavities for nesting and are resourceful when selecting sites, regularly nesting in manmade structures (Mainwaring 2015), or in cavities excavated by other birds or animals (Higgins et al. 2006; Palacio et al. 2016). Starlings may expand easily where equivalent niche or cavity-nesting species already reside, because there are already nesting sites available in these areas. Nest site availability is one of many limiting factors in starling survival.
Starlings breed synchronously during spring, in response to a number of social, abiotic, and biotic cues, and when living in denser populations, starlings showed increased breeding synchrony (Evans et al. 2009). Higher population density is associated with an increase in reproduction-associated competition (for mates, nest sites, and/or prey) but also greater risks (increased predation), which presumably would encourage a decrease in breeding synchrony (Evans et al. 2009). It is likely that breeding synchrony provides group benefits such as collective predator awareness and defense in both parents and fledglings (Smith 2004). Hence the starling’s social system may facilitate the species' success, and if breeding success is positively related to high group density, then any strategy to increase local density (unseating other species, larger nests, use of anything natural or unnatural that may serve as a nest, etc.) all create a positive feedback loop that leads to greater abundance. High reproductive rates lead to increased population density, and this positive density dependence may then trigger dispersal. Such population growth during invasion may also work in concert with rapid adaptive evolution within populations that contributes to their ongoing success; indeed, many putatively adaptive loci have been identified by selection studies of invasive starlings (e.g. Hofmeister et al. 2021b; Stuart et al. 2021).
Other evidence exists of flexibility or adaptation of breeding strategies in starlings, which may support both establishment and expansion of invasive populations. Across North America, starlings lay larger clutches than the average clutch size in the native range (Dawson 1983; Ball and Wingfield 1987, see Fear 1984 for information on population specific breeding characteristics). Starlings are known for displaying a wide range of personality types (Eens et al. 1993; Garamszegi et al. 2008; Thys et al. 2017), and starling parents have evolved many strategies (e.g. monogamous, polygamous, intraspecific brood parasitism) for optimizing their effort in caring for young (Higgins et al. 2006). The flexibility the starling displays across a range of breeding behaviours likely has played a vital role in their successful establishment across the diverse environments within their native and invasive ranges.
Cognition
Starlings have high cognitive abilities (Campbell et al. 1999; Bateson and Feenders 2010), and their larger brain size compared to other birds of a similar body size may play a role in their invasion success (Sol et al. 2002). Starlings’ cognition may facilitate greater behavioral flexibility and innovation, of particular importance during initial invasive population establishment. This cognitive ability also enables great dietary flexibility (via, for example, motor diversity or social learning, see Griffin et al. 2014; Lee and Thornton 2021), which impacts the species’ persistence during invasion, and during times of stress such as food shortage (Van Berkel et al. 2018; Bateson et al. 2021). Such behaviour may be heritable within families and developmentally modulated (Nettle et al. 2015) but, regardless, the starling’s ability to learn and cope with changing conditions likely supports its invasion success. Several genes underlying novelty-seeking behaviour have been identified as putatively under selection (Stuart et al. 2021, 2022a), but no evidence of selection on the commonly-studied dopamine D4 receptor gene was found in Australian starlings (Rollins et al. 2015). Further genetic work is needed to determine if there is evidence to support whether genes underlying behavioral flexibility differ across these populations.
Rapid adaptive evolution may facilitate expansion in novel environmental conditions
Rapid expansion and evolution despite reduced genetic diversity
Invasion success may be constrained by both population size of the founders and population density during expansion. Most introductions are initially small in size, and thus subject to genetic bottlenecks; however rapid expansion can counteract diversity loss (Birzu et al. 2019). As the population expands, mutation may generate novel selectively advantageous variants (Gilbert et al. 2017; Gilbert and Whitlock 2017) and selection in new environments may favor existing variants that were found in low frequencies in the native range. During the phases of rapid population growth and range expansion, populations may experience Allee effects (such that mean individual fitness is correlated with population size or density, Allee 1931). Among starling invasions, the size of the South African introduction makes it both an outlier and the population most likely to experience such Allee effects: the founding population was only 18 individuals (Craig 2020, Table 1), which may explain the slow expansion speed in South Africa. In reality, population density and environmental conditions likely both shape range expansion in South Africa and other invasive starling populations.
Environmental conditions often shape the evolution of populations, and when an introduced environment is substantially different to that of the native range, strong selection regimes may promote rapid adaptation in the new environment. Even invasive species that have undergone severe bottlenecks are capable of rapid adaptive evolution in a novel environment (Dlugosch & Parker 2008; Facon et al. 2011; Rollins et al. 2013), perhaps via inbreeding and environment interactions (Schrieber and Lachmuth 2017). While genetic bottlenecks or founder effects may explain why starlings in Australia remain very distinct from the North American and native UK populations (Hofmeister et al. 2021a), the multiple introductions to Australia are likely to have mitigated these demographic effects. It seems likely that, because the Australian environment is so dissimilar to that of the native range, selection at the introduction sites or during range expansion in Australia may contribute to the pronounced genetic differences between this population and native range starlings. Further, elevational barriers appear to have influenced starling dispersal, and hence evolution, in the North American (Hofmeister et al. 2021b) and Australian populations (Stuart et al. 2021, 2022).
Interactions between adaptation and dispersal
Differences in genetic characteristics and substructure within each population are plausibly linked to differences in dispersal. Environmental similarity to native range environments may explain part of the dispersal variation across populations, because unlike other invasive starling populations, migration is common within North America (although not ubiquitous, see Royall et al. 1972; Royall and Guarino 1976). The presence of migration in this population, with flyways that include both north/south and east/west movement (Kessel 1953), may increase gene flow among demes and thereby decrease population structure. In comparison to the older Australian and the marginally (7 years) younger South African invasions, the North American starling range covers an area many times larger. It is possible that migration enabled faster range expansion, and continues to enable genetic exchange across the range, diluting the effects of genetic drift and spatial sorting and decreasing the strength of local adaption (Hofmeister et al. 2021b).
The Australian invasion appears to have been affected by spatial sorting, with wing length and loading strongly linked to distance from the introduction site, whereas this is not the case in the South African introduction (Phair et al. 2018). Why do some populations display spatial sorting and others do not? Higher dispersal is associated with less desirable conditions, either due to the environment or high population density. Meanwhile, the native range shows minimal dispersal at range margins, which may be due to environmental suitability or population density (Hui et al. 2012). This ‘good-stay, bad-disperse’ hypothesis (i.e. higher quality environments lead to smaller dispersal distances and more individuals remaining within the immediate habitat, Hui et al. 2012) may account for the introduction-sites to range-edge genetic gradients, and may encourage spatial sorting as seen in Australia (Phair et al. 2018). However, spatial sorting was not identified in South Africa, perhaps for two reasons: (1) founding size of the initial introduction was much smaller, providing less genetic variation, and (2) the geographic range is much smaller, such that individuals disperse more readily from introduction site to range edge. Overall, spatial sorting may increase population genetic structure; this may explain differences in genetic patterns in South Africa versus those in Australia (Table 3).
While these dispersal patterns (Hui et al. 2012; Phair et al. 2018) can at least in part explain the paradox of invasion success in starlings, determining the genetic basis for dispersal-related traits may clarify the eco-evolutionary feedback loops central to this species’ invasion success. Empirical tests of dispersal evolution have been conducted in invasive systems like the cane toad (Perkins et al. 2013) and the ladybird beetle (Lombaert et al. 2014), and models that weigh the contribution of both population densities and selection strength may yield insight into the relative importance of each factor (Lion 2018).
Persistence aided by environmental niche flexibility
The starling possesses great environmental niche flexibility as outlined above, but their biology and potential for range expansion varies dramatically among invasions. There are limits to this flexibility; for example, they are restricted from northward expansion in the northern hemisphere due to colder temperatures, and expansions towards the equator are hampered by heat and aridity extremes (e.g., inland Queensland, Australia). North American starlings appear to exhibit a wider thermal tolerance than other invasive birds in that continent (Johnson and Cowan 1974); this flexibility may have evolved in the native range, or could represent adaptive changes following introduction. Local adaptation is possible within all ranges, and will continue to interact with global climate change and anthropogenic land alterations, which may impact their distribution. Climate suitability plays a major role in determining invasion success (Redding et al. 2019). Even within established and ‘suitable’ ecosystems, the nature of the environment holds great sway over starling population characteristics. Already we see local adaptation to environmental factors developing in Australian starlings (Cardilini et al. 2016) and possibly even in North American starlings (Hofmeister et al. 2021b). However, highly varied levels of heritability across a range of morphological traits point to the role of developmental plasticity in enabling climate induced ecogeographical patterns in this species (Stuart et al. 2022). Further developing our understanding of adaptive genetic change requires more research into epigenetic variation and developmental plasticity, and the role these mechanisms play in facilitating adaptation and invasion success; this is a key research direction in invasion genetics (Ghalambor et al. 2007; Gomez-Mestre and Jovani 2013; Murren et al. 2015).
The starling, as a generalist, may successfully habituate in environments very different from those of their native range (Vall-llosera et al. 2016). This high level of behavioral flexibility may explain why starlings have so successfully co-existed with humans; for example, anthropogenic land alteration may facilitate range expansion by counteracting limitations of the natural environment (e.g. supplementing water availability and providing artificial nesting spaces; Peris et al. 2005; Zufiaurre et al. 2016). Humans, indirectly, may have assisted the starling’s colonization of cold extremes in the native range and North America, and arid areas of inland Australia. While starlings are successful in the urban environment, they prefer cleared agricultural and suburban areas to urban centers, and starlings have also been found to produce fewer young in more urbanized areas (Mennechez and Clergeau 2006). Starlings, however, do not require large habitats to settle and are capable of colonizing small remnant vegetation patches (Antos et al. 2006). Further population modeling and range estimates of this species should account for changes to anthropogenic land use, especially land associated with agriculture (Duncan et al. 2001; Baker and Bomford 2009; Magory Cohen et al. 2019). Accounting for human modification of the environment is critical because climate alone appears to not have any large scale macro-association with range distributions of many native European avian species (Beale et al. 2008). Examining further the link between anthropogenic land features and invasion success and expansion (e.g. Hill et al. 2005; Menon & Mohanraj 2016; Schmack et al. 2020) is an essential next step in understanding the interactions between this species and human populations.
Future directions & pressing questions related to starling invasiveness
To summarize the future research directions this review has discussed, we present the points below as key knowledge gaps within starling population genetics research:
-
1.
Starling demography and genetics within their native range We are currently witnessing range and demographic shifts in Europe, northern Africa, and Asia. How might climate change and anthropogenic land alteration shape these shifts, and what other factors might influence range shifts and changes in population size? Conservation of native starling populations will require explicit studies of range-wide genetic diversity, including subspecies designations. To our knowledge, there is poor knowledge of starling population genetics and diversity within their native range outside of the United Kingdom and Belgium, and improving this is a critical next step.
-
2.
Monitoring ongoing starling range expansions Documenting rapid evolution at expanding range edges may help to clarify whether and how populations adapt to local conditions. This work is critical in Australia, New Zealand, South Africa and South America, where conservation managers actively work to control starling spread. In particular, updated sampling and analysis is needed for the New Zealand invasion, for which currently only allozyme data exists. As the newest major starling incursion, the South American invasion will be a critical system to test the relative importance of intrinsic and extrinsic factors.
-
3.
Comparative studies between starlings and other avian invaders There are a few other globally successful avian invaders (Fig. 1). Comparative studies may identify genomics regions that are involved in rapid adaptation across species. This includes investigations into a variety of genetic variant types, including single nucleotide polymorphisms, structural variants and transposable elements.
-
4.
Further empirical studies into the invasion paradox. Given current debate around the existence of a genetic paradox (Estoup et al. 2016), the starling study system offer great potential to investigate genetic processes during invasion. Utilizing the replicated invasions provides a system in which we may identify which genetic bottlenecked and other genetic patterns are stochastic, and which characterize starling introductions in general.
-
5.
Investigating the role of non-genetic processes underlying adaptation during invasion The roles of developmental plasticity and environmentally induced epigenetic change in adaptation to novel environments is poorly understood. Understanding these processes often requires manipulative experiments so that the relative contributions of genetics and environment in shaping phenotype may be separated from one another. Well established laboratory protocols exist for starlings, enabling the pursuit of these research directions complimentary to exploratory field studies.
Data availability
No new data was produced as part of this manuscript.
Change history
24 February 2023
Missing Open Access funding information has been added in the Funding Note.
31 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10530-023-03066-8
References
Allee WC (1931) Animal aggregations: a study in general sociology. University of Chicago Press, Chicago
Antos MJ, Fitzsimons JA, Palmer GC, White JG (2006) Introduced birds in urban remnant vegetation: Does remnant size really matter? Austral Ecol 31:254–261. https://doi.org/10.1111/j.1442-9993.2006.01572.x
Asher L, Bateson M (2008) Use and husbandry of captive European starlings (Sturnus vulgaris) in scientific research: a review of current practice. Lab Anim 42:111–126. https://doi.org/10.1258/la.2007.007006
Baker J, Bomford M (2009) Opening the climate modelling envelope. Plant Prot Q 24:88
Ball GF, Wingfield JC (1987) Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodedness and nest-site density in male starlings. Physiol Zool 60:191–199. https://doi.org/10.1086/physzool.60.2.30158643
Barton JH, Morris K, Meritt D, Magle S, LaMontagne JM (2020) Does urbanization influence population trends of cavity-nesting birds and their relationship with European starlings? Acta Oecol 108:103636. https://doi.org/10.1016/j.actao.2020.103636
Bateson M, Feenders G (2010) The use of passerine bird species in laboratory research: implications of basic biology for husbandry and welfare. ILAR J 51:394–408. https://doi.org/10.1093/ilar.51.4.394
Bateson M, Andrews C, Dunn J, Egger CBCM, Gray F, Mchugh M, Nettle D (2021) Food insecurity increases energetic efficiency, not food consumption: an exploratory study in European starlings. PeerJ 9:e11541. https://doi.org/10.7717/peerj.11541
Beale CM, Lennon JJ, Gimona A (2008) Opening the climate envelope reveals no macroscale associations with climate in European birds. Proc Natl Acad Sci 105:14908–14912. https://doi.org/10.1073/pnas.0803506105
Bell BD (2015) Temporal changes in birds and bird song detected in Zealandia sanctuary, Wellington, New Zealand, over 2011–2015. Notornis 62:173–183
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Let 12:20150623. https://doi.org/10.1098/rsbl.2015.0623
Berthouly-Salazar C, Hui C, Blackburn TM, Gaboriaud C, van Rensburg BJ, van Vuuren BJ, Roux JJL (2013) Long-distance dispersal maximizes evolutionary potential during rapid geographic range expansion. Mol Ecol 22:5793–5804. https://doi.org/10.1111/mec.12538
Birzu G, Matin S, Hallatschek O, Korolev KS (2019) Genetic drift in range expansions is very sensitive to density dependence in dispersal and growth. Ecol Lett 22:1817–1827. https://doi.org/10.1111/ele.13364
Bitton P-P, Graham BA (2015) Change in wing morphology of the European starling during and after colonization of North America. J Zool 295:254–260. https://doi.org/10.1111/jzo.12200
Blem CR (1981) Geographic variation in mid-winter body composition of starlings. Condor 83:370–376. https://doi.org/10.2307/1367508
Bodt LH, Rollins LA, Zichello JM (2020) Contrasting mitochondrial diversity of European starlings (Sturnus vulgaris) across three invasive continental distributions. Ecol Evol 10:10186–10195. https://doi.org/10.1002/ece3.6679
Brewer D (2010) Wrens, dippers and thrashers. Bloomsbury Publishing, London
Brown WM, George M, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76:1967–1971
Cabe PR (1998) The effects of founding bottlenecks on genetic variation in the European starling (Sturnus vulgaris) in North America. Heredity 80:519–525. https://doi.org/10.1046/j.1365-2540.1998.00296.x
Cabe PR (1999) Dispersal and population structure in the European starling. Condor 101:451–454. https://doi.org/10.2307/1370014
Campbell FM, Heyes CM, Goldsmith AR (1999) Stimulus learning and response learning by observation in the European starling, in a two-object/two-action test. Anim Behav 58:151–158. https://doi.org/10.1006/anbe.1999.1121
Campbell S, Roberts EJ, Craemer R, Pacioni C, Rollins L, Woolnough AP (2016) Assessing the economic benefits of starling detection and control to Western Australia. Australas J Environ Manag 23:81–99. https://doi.org/10.1080/14486563.2015.1028486
Cardilini APA, Buchanan KL, Sherman CDH, Cassey P, Symonds MRE (2016) Tests of ecogeographical relationships in a non-native species: what rules avian morphology? Oecologia 181:783–793. https://doi.org/10.1007/s00442-016-3590-9
Chamberlain DE, Fuller RJ, Bunce RGH, Duckworth JC, Shrubb M (2000) Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and wales. J Appl Ecol 37:771–788
Chapman BB, Brönmark C, Nilsson J-Å, Hansson L-A (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775. https://doi.org/10.1111/j.1600-0706.2011.20131.x
Colautti RI, Barrett SCH (2013) Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342:364–366. https://doi.org/10.1126/science.1242121
Colautti RI, Alexander JM, Dlugosch KM, Keller SR, Sultan SE (2017) Invasions and extinctions through the looking glass of evolutionary ecology. Philos Trans R Soc b: Biol Sci 372:20160031. https://doi.org/10.1098/rstb.2016.0031
Cooke MT (1928) The spread of the European starling in North America (to 1928). U.S. Dept. of Agriculture, Washington, D.C.
Cooper J, Underhill LG (1991) Breeding, mass and primary moult of european starlings sturnus vulgaris at Dassen island, South Africa. Ostrich 62:1–7. https://doi.org/10.1080/00306525.1991.9639629
Craig AJFK (2020) Common starling (Sturnus vulgaris Linnaeus, 1758). In: Hart LA, Downs CT (eds) Invasive birds: Global trends and impacts. CAB International
Crystal-Ornelas R, Lockwood JL (2020) The ‘known unknowns’ of invasive species impact measurement. Biol Invasions 22:1513–1525. https://doi.org/10.1007/s10530-020-02200-0
Dawson A (1983) Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol 49:286–294. https://doi.org/10.1016/0016-6480(83)90146-6
de la Cruz-Cardiel PJ, Deceuninck B, Peris SJ, Elena-Rosselló JA (1997) Allozyme polymorphism and interspecific relationships in the Common starling (Sturnus vulgaris) and spotless starling (S. unicolor) (Aves: Sturnidae). J Zool Syst Evol Res 35:75–79. https://doi.org/10.1111/j.1439-0469.1997.tb00406.x
Delmore KE, Van Doren BM, Conway GJ, Curk T, Garrido-Garduño T, Germain RR, Hasselmann T, Hiemer D, van der Jeugd HP, Justen H et al (2020) Individual variability and versatility in an eco-evolutionary model of avian migration. Proc R Soc b: Biol Sci 287:20201339. https://doi.org/10.1098/rspb.2020.1339
Diagne C, Leroy B, Vaissière A-C, Gozlan RE, Roiz D, Jarić I, Salles J-M, Bradshaw CJA, Courchamp F (2021) High and rising economic costs of biological invasions worldwide. Nature 592:571–576. https://doi.org/10.1038/s41586-021-03405-6
Dickerson LM (1938) The western frontier of the European starling in the united states as of February, 1937. Condor 40:118–123. https://doi.org/10.2307/1363826
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449. https://doi.org/10.1111/j.1365-294X.2007.03538.x
Dlugosch KM, Anderson SR, Braasch J, Cang FA, Gillette HD (2015) The devil is in the details: genetic variation in introduced populations and its contributions to invasion. Mol Ecol 24:2095–2111. https://doi.org/10.1111/mec.13183
Dolbeer R (1982) Migration patterns for age and sex classes of blackbirds and starlings. J Field Ornithol. Vol 53
Downs CT, Hart LA (2020) Invasive birds: global trends and impacts. CABI
Duncan RP, Bomford M, Forsyth DM, Conibear L (2001) High predictability in introduction outcomes and the geographical range size of introduced Australian birds: a role for climate. J Anim Ecol 70:621–632. https://doi.org/10.1046/j.1365-2656.2001.00517.x
Duncan RP, Blackburn TM, Sol D (2003) The ecology of bird introductions. Annu Rev Ecol Evol Syst 34:71–98
e Silva FC, da Pinto JM, Mäder A, de Souza VAT (2017) First records of European Starling Sturnus vulgaris in Brazil. Rev Bras Ornitol 25:297–298. https://doi.org/10.1007/BF03544409
eBird 2021 eBird: an online database of bird distribution and abundance [web application]. eBird, Cornell Lab of Ornithology, Ithaca, New York
Eens M, Pinxten R, Verheyen RF (1993) Function of the song and song repertoire in the European starling (Sturnus vulgaris): an aviary experiment. Behaviour 125:51–66
Enders M, Havemann F, Ruland F, Bernard-Verdier M, Catford JA, Gómez-Aparicio L, Haider S, Heger T, Kueffer C, Kühn I et al (2020) A conceptual map of invasion biology: integrating hypotheses into a consensus network. Glob Ecol Biogeogr 29:978–991. https://doi.org/10.1111/geb.13082
Estoup A, Ravigné V, Hufbauer R, Vitalis R, Gautier M, Facon B (2016) Is there a genetic paradox of biological invasion? Annu Rev Ecol Evol Syst 47:51–72. https://doi.org/10.1146/annurev-ecolsys-121415-032116
Evans LE, Ardia DR, Flux JEC (2009) Breeding synchrony through social stimulation in a spatially segregated population of European starlings. Anim Behav 78:671–675. https://doi.org/10.1016/j.anbehav.2009.05.031
Evans P (1980) Population genetics of the European starling (Sturnus vulgaris). University of Oxford, Oxford
Facon B, Hufbauer RA, Tayeh A, Loiseau A, Lombaert E, Vitalis R, Guillemaud T, Lundgren JG, Estoup A (2011) Inbreeding depression is purged in the invasive insect Harmonia axyridis. Curr Biol CB 21:424–427. https://doi.org/10.1016/j.cub.2011.01.068
Feare CJ (1984) The starling. Oxford University Press, Shire
Fiorini VD, Domínguez M, Swaddle J (2021) Recent invasive population of the European starling Sturnus vulgaris has lower genetic diversity and higher fluctuating asymmetry than primary invasive and native populations. Biol Invasion. https://doi.org/10.21203/rs.3.rs-310175/v1
Flux JEC, Flux MM (1981) Population dynamics and age structure of starlings (Sturnus vulgaris) in New Zealand. N Z J Ecol 4:65–72
Forbush EH (1915) The starling. Wright & Potter Printing Company, Nottingham
Freeman SN, Robinson RA, Clark JA, Griffin BM, Adams SY (2007) Changing demography and population decline in the Common Starling Sturnus vulgaris: a multisite approach to integrated population monitoring. Ibis 149:587–596. https://doi.org/10.1111/j.1474-919X.2007.00684.x
Fristoe TS, Chytrý M, Dawson W, Essl F, Heleno R, Kreft H, Maurel N, Pergl J, Pyšek P, Seebens H et al (2021) Dimensions of invasiveness: links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc Natl Acad Sci USA 118:e2021173118. https://doi.org/10.1073/pnas.2021173118
Fugate L, Miller JM (2021) Shakespeare’s starlings: literary history and the fictions of invasiveness. Environ Humanit 13:301–322. https://doi.org/10.1215/22011919-9320167
Garamszegi LZ, Eens M, Török J (2008) Birds reveal their personality when singing. PLoS ONE. https://doi.org/10.1371/journal.pone.0002647
Ghalambor CK, McKAY JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407. https://doi.org/10.1111/j.1365-2435.2007.01283.x
Gilbert KJ, Whitlock MC (2017) The genetics of adaptation to discrete heterogeneous environments: frequent mutation or large-effect alleles can allow range expansion. J Evol Biol 30:591–602. https://doi.org/10.1111/jeb.13029
Gilbert KJ, Sharp NP, Angert AL, Conte GL, Draghi JA, Guillaume F, Hargreaves AL, Matthey-Doret R, Whitlock MC (2017) Local adaptation interacts with expansion load during range expansion: maladaptation reduces expansion load. Am Nat 189:368–380. https://doi.org/10.1086/690673
Gomez-Mestre I, Jovani R (2013) A heuristic model on the role of plasticity in adaptive evolution: plasticity increases adaptation, population viability and genetic variation. Proc R Soc b: Biol Sci 280:20131869. https://doi.org/10.1098/rspb.2013.1869
Gregory RD, Wilkinson NI, Clark D, Robinson JA, Brown AF, Hughes JDAP, Procter DA, Gibbons DW, Galbraith CA (2002) The population status of birds in the United Kingdom, Channel Islands and Isle of man: an analysis of conservation concern 2002–2007. Br Birds 95:410–448
Griffin AS, Diquelou M, Perea M (2014) Innovative problem solving in birds: a key role of motor diversity. Anim Behav 92:221–227. https://doi.org/10.1016/j.anbehav.2014.04.009
Gwinner H, Van’t Hof T, Zeman M (2002) Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav 42:21–31. https://doi.org/10.1006/hbeh.2002.1795
Hanson HE, Mathews NS, Hauber ME, Martin LB (2020) The house sparrow in the service of basic and applied biology. Elife 9:e52803. https://doi.org/10.7554/eLife.52803
Harris G (1964) Climatic changes since 1860 affecting European birds. Weather 19:70–79. https://doi.org/10.1002/j.1477-8696.1964.tb02074.x
Harrison J, Cherry M. The atlas of Southern African birds. Johannesburg: BirdLife South Africa
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506. https://doi.org/10.1007/s10530-007-9146-5
Heldbjerg H, Fox AD, Levin G, Nyegaard T (2016) The decline of the Starling Sturnus vulgaris in Denmark is related to changes in grassland extent and intensity of cattle grazing. Agr Ecosyst Environ 230:24–31. https://doi.org/10.1016/j.agee.2016.05.025
Heldbjerg H, Fox AD, Lehikoinen A, Sunde P, Aunins A, Balmer DE, Calvi G, Chodkiewicz T, Chylarecki P, Escandell V et al (2019) Contrasting population trends of common starlings (Sturnus vulgaris) across Europe. Ornis Fennica 96:153–169
Higgins PJ, Peter JM, Cowling SJ (2006) Handbook of Australian, New Zealand & Antarctic birds. Volume 7, Boatbill to starlings. Oxford University Press, Melbourne
Hill SJ, Tung PJ, Leishman MR (2005) Relationships between anthropogenic disturbance, soil properties and plant invasion in endangered Cumberland Plain Woodland, Australia. Austral Ecol 30:775–788. https://doi.org/10.1111/j.1442-9993.2005.01518.x
Hoffman EC (1930) The spread of the European starling in America. Wilson Bull 42:80
Hofmeister NR, Werner SJ, Lovette IJ (2021b) Environmental correlates of genetic variation in the invasive European starling in North America. Mol Ecol 30:1251–1263. https://doi.org/10.1111/mec.15806
Hofmeister NR, Stuart K, Warren WC, Werner SJ, Bateson M, Ball GF, Buchanan KL, Burt DW, Cardilini APA, Cassey P et al (2021) Concurrent invasions by European starlings (Sturnus vulgaris) suggest selection on shared genomic regions even after genetic bottlenecks. bioRxiv. https://doi.org/10.1101/2021.05.19.442026)
Homan HJ, Johnson RJ, Thiele JR, Linz GM (2017) European starlings. Wildlife damage management technical series. USDA, APHIS, WS National Wildlife Research Center. Ft. Collins, Colorado
Hui C, Richardson DM (2017) Invasion dynamics. Oxford University Press, Oxford, New York
Hui C, Roura-Pascual N, Brotons L, Robinson RA, Evans KL (2012) Flexible dispersal strategies in native and non-native ranges: environmental quality and the ‘good–stay, bad–disperse’ rule. Ecography 35:1024–1032. https://doi.org/10.1111/j.1600-0587.2012.07697.x
Jauregui A, Gerstmayer PA, Colombo MA, Segura LN (2022) Concerningly high breeding success rates for the European starling after a recent invasion in the neotropics. Review. https://doi.org/10.21203/rs.3.rs-1662353/v1
Jenkins CFH (1977) The Noah’s ark syndrome: One hundred years of acclimatization and zoo development in Australia. Zoological Gardens Board of Western Australia, Perth
Johnson SR, Cowan IM (1974) Thermal adaptation as a factor affecting colonizing success of introduced Sturnidae (Aves) in North America. Can J Zool 52:1559–1576
Kalmbach ER, Gabrielson IN, United States, & Department of Agriculture (1921) Economic value of the starling in the United States. Washington, D.C.: U.S. Dept. of Agriculture
Kessel B (1953) Distribution and Migration of the European Starling in North America. The Condor 55:49–67. https://doi.org/10.2307/1365026
Lee VE, Thornton A (2021) Animal cognition in an urbanised world. Front Ecol Evol 9:633947
Lever C (2010) Naturalised birds of the world. A&C Black, London
Linz GM, Homan HJ, Gaulker SM, Penry LB & Bleier WJ 2007 European starlings: a review of an invasive species with far-reaching impacts. Manag Vertebr Invasive Species 24.
Linz G, Johnson R, Thiele J (2017) European starlings. In Ecology and Management of Terrestrial Vertebrate Invasive Species in the United States, 1st ed, pp 311–332. Ed WC Pitt. Boca Raton : Taylor & Francis, 2018. | “A CRC title, part of the Taylor & Francis imprint, a member of the Taylor & Francis Group, the academic division of T&F Informa plc.”: CRC Press. https://doi.org/10.1201/9781315157078-15
Lion S (2018) Theoretical approaches in evolutionary ecology: environmental feedback as a unifying perspective. Am Nat 191:21–44. https://doi.org/10.1086/694865
Liu C, Wolter C, Xian W, Jeschke JM (2020) Most invasive species largely conserve their climatic niche. Proc Natl Acad Sci 117:23643–23651. https://doi.org/10.1073/pnas.2004289117
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion ecology. Wiley-Blackwell, Chichester
Lombaert E, Estoup A, Facon B, Joubard B, Grégoire J-C, Jannin A, Blin A, Guillemaud T (2014) Rapid increase in dispersal during range expansion in the invasive ladybird Harmonia axyridis. J Evol Biol 27:508–517. https://doi.org/10.1111/jeb.12316
Long JL (1981) Introduced birds of the world: the worldwide history, distribution, and influence of birds introduced to new environments. Universe Books, Terrey Hills
Lovette IJ, McCleery BV, Talaba AL, Rubenstein DR (2008) A complete species-level molecular phylogeny for the ‘Eurasian’ starlings (Sturnidae: Sturnus, Acridotheres, and allies): recent diversification in a highly social and dispersive avian group. Mol Phylogenet Evol 47:251–260. https://doi.org/10.1016/j.ympev.2008.01.020
Lowe S, Browne M, Boudjelas S (2000) 100 of the world’s worst invasive alien species. A selection from the global invasive species database. Invasive Species Specialist Group, Auckland
Macleod R, Clark J, Cresswell W (2008) The starvation–predation risk trade-off, body mass and population status in the Common Starling Sturnus vulgaris. Ibis 150:199–208. https://doi.org/10.1111/j.1474-919X.2008.00820.x
Magory Cohen T, McKinney M, Kark S, Dor R (2019) Global invasion in progress: modeling the past, current and potential global distribution of the common myna. Biol Invasions 21:1295–1309. https://doi.org/10.1007/s10530-018-1900-3
Mahmood T, Usman-Ul-Hassan SM, Nadeem MS, Kayani A (2013) Population and diet of migratory Common Starlings Sturnus vulgaris wintering in agricultural areas of Sialkot district, Pakistan. Forktail
Mainwaring MC (2015) The use of man-made structures as nesting sites by birds: a review of the costs and benefits. J Nat Conserv 25:17–22. https://doi.org/10.1016/j.jnc.2015.02.007
Mennechez G, Clergeau P (2006) Effect of urbanisation on habitat generalists: starlings not so flexible? Acta Oecol 30:182
Menon M, Mohanraj R (2016) Temporal and spatial assemblages of invasive birds occupying the urban landscape and its gradient in a southern city of India. J Asia-Pac Biodivers 9:74–84. https://doi.org/10.1016/j.japb.2015.12.005
Miller AD, Inamine H, Buckling A, Roxburgh SH, Shea K (2021) How disturbance history alters invasion success: biotic legacies and regime change. Ecol Lett 24:687–697. https://doi.org/10.1111/ele.13685
Murren CJ, Auld JR, Callahan H, Ghalambor CK, Handelsman CA, Heskel MA, Kingsolver JG, Maclean HJ, Masel J, Maughan H et al (2015) Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115:293–301. https://doi.org/10.1038/hdy.2015.8
Navas J (2002) Las aves exóticas introducidas y naturalizadas en la Argentina. Revista Del Museo Argentino de Ciencias Naturales Nueva Serie 4:191–202
Nettle D, Andrews CP, Monaghan P, Brilot BO, Bedford T, Gillespie R, Bateson M (2015) Developmental and familial predictors of adult cognitive traits in the European starling. Anim Behav 107:239–248. https://doi.org/10.1016/j.anbehav.2015.07.002
Neves VC, Griffiths K, Savory FR, Furness RW, Mable BK (2009) Are European starlings breeding in the Azores archipelago genetically distinct from birds breeding in mainland Europe? Eur J Wildl Res 56:95–100. https://doi.org/10.1007/s10344-009-0316-x
North HL, McGaughran A, Jiggins C (2021) Insights into invasive species from whole-genome resequencing. Mol Ecol. https://doi.org/10.1111/mec.15999
Palacio FX, Maragliano RE, Montalti D (2016) Functional role of the invasive European Starling, Sturnus vulgaris, in Argentina. Emu 116:387–393. https://doi.org/10.1071/MU16021
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1998) Patterns of natal and breeding dispersal in birds. J Anim Ecol 67:518–536. https://doi.org/10.1046/j.1365-2656.1998.00215.x
Pateff P, Stresemann E (1947) On the systematic position of the starlings inhabiting Bulgaria and the neighbouring countries. Ibis 89:494–507. https://doi.org/10.1111/j.1474-919X.1947.tb04367.x
Peris S, Soave G, Camperi A, Darrieu C, Aramburú RM (2005) Range expansion of the European starling Sturnus vulgaris in Argentina. Ardeola 52:359–364
Perkins TA, Phillips BL, Baskett ML, Hastings A (2013) Evolution of dispersal and life history interact to drive accelerating spread of an invasive species. Ecol Lett 16:1079–1087. https://doi.org/10.1111/ele.12136
Phair DJ, Roux JJL, Berthouly-Salazar C, Visser V, van Vuuren BJ, Cardilini APA, Hui C (2018) Context-dependent spatial sorting of dispersal-related traits in the invasive starlings (Sturnus vulgaris) of South Africa and Australia. BioRxiv. https://doi.org/10.1101/342451
Piersma T, Loonstra AHJ, Verhoeven MA, Oudman T (2020) Rethinking classic starling displacement experiments: evidence for innate or for learned migratory directions? J Avian Biol. https://doi.org/10.1111/jav.02337
Pipek P, Blackburn TM, Pyšek P (2019) The ins and outs of acclimatisation: imports versus translocations of skylarks and starlings in 19th century New Zealand. Biol Invasions 21:1395–1413
Raxworthy CJ, Smith BT (2021) Mining museums for historical DNA: advances and challenges in museomics. Trends Ecol Evol 36:1049–1060. https://doi.org/10.1016/j.tree.2021.07.009
Redding DW, Pigot AL, Dyer EE, Şekercioğlu ÇH, Kark S, Blackburn TM (2019) Location-level processes drive the establishment of alien bird populations worldwide. Nature. https://doi.org/10.1038/s41586-019-1292-2
Rensburg MJ van (2014) Reconstructing the range expansion of the European starling in Southern Africa using a hybrid method of niche modelling and individual based modelling. Stellenbosch University
Richardson MF, Sherwin WB, Rollins LA (2017) De novo assembly of the liver transcriptome of the European starling, Sturnus vulgaris. J Genomics 5:54–57. https://doi.org/10.7150/jgen.19504
Rintala J, Tiainen J, Pakkala T (2003) Population trends of the Finnish starling Sturnus vulgaris, 1952–1998, as inferred from annual ringing totals. Ann Zool Fenn 40:365–385
Robinson RA, Siriwardena GM, Crick HQP (2005) Status and population trends of Starling Sturnus vulgaris in Great Britain. Bird Study 52:252–260. https://doi.org/10.1080/00063650509461398
Rollins LA, Woolnough AP, Wilton AN, Sinclair R, Sherwin WB (2009) Invasive species can’t cover their tracks: using microsatellites to assist management of starling (Sturnus vulgaris) populations in Western Australia. Mol Ecol 18:1560–1573. https://doi.org/10.1111/j.1365-294X.2009.04132.x
Rollins LA, Woolnough AP, Sinclair R, Mooney NJ, Sherwin WB (2011) Mitochondrial DNA offers unique insights into invasion history of the common starling. Mol Ecol 20:2307–2317. https://doi.org/10.1111/j.1365-294X.2011.05101.x
Rollins LA, Moles AT, Lam S, Buitenwerf R, Buswell JM, Brandenburger CR, Flores-Moreno H, Nielsen KB, Couchman E, Brown GS et al (2013) High genetic diversity is not essential for successful introduction. Ecol Evol 3:4501–4517. https://doi.org/10.1002/ece3.824
Rollins LA, Whitehead MR, Woolnough AP, Sinclair R, Sherwin WB (2015) Is there evidence of selection in the dopamine receptor D4 gene in Australian invasive starling populations? Curr Zool 61:505–519. https://doi.org/10.1093/czoolo/61.3.505
Rollins LA, Woolnough AP, Fanson BG, Cummins ML, Crowley TM, Wilton AN, Sinclair R, Butler A, Sherwin WB (2016) Selection on mitochondrial variants occurs between and within individuals in an expanding invasion. Mol Biol Evol 33:995–1007. https://doi.org/10.1093/molbev/msv343
Rosenberg KV, Dokter AM, Blancher PJ, Sauer JR, Smith AC, Smith PA, Stanton JC, Panjabi A, Helft L, Parr M et al (2019) Decline of the North American avifauna. Science 366:120–124. https://doi.org/10.1126/science.aaw1313
Ross HA (1983) Genetic differentiation of starling (Sturnus vulgaris: Aves) populations in New Zealand and Great Britain. J Zool 201:351–362. https://doi.org/10.1111/j.1469-7998.1983.tb04281.x
Royall WC, Guarino JL, Zajanc A, Siebe CC (1972) Movements of starlings banded in California. Bird-Banding 43:26–37. https://doi.org/10.2307/4511824
Royall WC, Guarino JL (1976) Movements of starlings banded in north-central Colorado, 1960–74. 1 5
Sandakova SL, Kuksina DK-O, Saaya AT-O, Matveeva OA, Seveley SS, Toushkin AA, Toushkina AF, Tarazanova IS (2018) The fauna and nature of birds stay of residential landscapes of northern part of Central Asia. Eurasian J Biosci 12:105–112
Schmack JM, Schleuning M, Ward DF, Beggs JR (2020) Biogeography and anthropogenic impact shape the success of invasive wasps on New Zealand’s offshore islands. Divers Distrib 26:441–452. https://doi.org/10.1111/ddi.13021
Schrieber K, Lachmuth S (2017) The Genetic Paradox of Invasions revisited: the potential role of inbreeding × environment interactions in invasion success. Biol Rev 92:939–952. https://doi.org/10.1111/brv.12263
Siriwardena GM, Crick HQP (2002) National trends in the breeding performance of Starlings Sturnus vulgaris. In H.Q.P. Crick, R.A. Robinson, G.F. Appleton, N.A. Clark & A.D. Rickard (Eds) Investigation into the Causes of the Decline of Starlings and House Sparrows in Great Britain, BTO Research Report No 290, p pp 91–120. DEFRA, Bristol
Smith HG (2004) Selection for synchronous breeding in the European starling. Oikos 105:301–311. https://doi.org/10.1111/j.0030-1299.2004.10543.x
Smith HG, Ryegård A, Svensson S (2012) Is the large-scale decline of the starling related to local changes in demography? Ecography 35:741–748. https://doi.org/10.1111/j.1600-0587.2011.06310.x
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502. https://doi.org/10.1006/anbe.2001.1953
Starling-Windhof A, Massaro M, Briskie JV (2011) Differential effects of exotic predator-control on nest success of native and introduced birds in New Zealand. Biol Invasions 13:1021–1028. https://doi.org/10.1007/s10530-010-9886-5
Stuart KC, Cardilini APA, Cassey P, Richardson MF, Sherwin WB, Rollins LA, Sherman CDH (2021) Signatures of selection in a recent invasion reveal adaptive divergence in a highly vagile invasive species. Mol Ecol 30:1419–1434. https://doi.org/10.1111/mec.15601
Stuart KC, Sherwin WB, Austin JJ, Bateson M, Eens M, Brandley MC, Rollins LA (2022a) Historical museum samples enable the examination of divergent and parallel evolution during invasion. Mol Ecol 31:1836–1852. https://doi.org/10.1111/mec.16353
Stuart KC, Edwards RJ, Cheng Y, Warren WC, Burt DW, Sherwin WB, Hofmeister NR, Werner SJ, Ball GF, Bateson M et al (2022b) Transcript- and annotation-guided genome assembly of the European starling. Mol Ecol Resour 22:3141-3160. https://doi.org/10.1111/1755-0998.13679
Stuart KC, Sherwin WB, Cardilini APA., Rollins LA (2022) Genetics and plasticity are responsible for ecogeographical patterns in a recent invasion. Front Genet. https://doi.org/10.3389/fgene.2022.824424
Sullivan BL, Wood CL, Iliff MJ, Bonney RE, Fink D, Kelling S (2009) eBird: a citizen-based bird observation network in the biological sciences. Biol Cons 142:2282–2292. https://doi.org/10.1016/j.biocon.2009.05.006
Taylor RG (1953) Starlings in Jamaica. Ibis 95:700–701. https://doi.org/10.1111/j.1474-919X.1953.tb01902.x
Thomson GM (1922) The naturalisation of animals & plants in New Zealand. The University Press, Cambridge
Thys B, Eens M, Aerts S, Delory A, Iserbyt A, Pinxten R (2017) Exploration and sociability in a highly gregarious bird are repeatable across seasons and in the long term but are unrelated. Anim Behav 123:339–348. https://doi.org/10.1016/j.anbehav.2016.11.014
Tinbergen JM (1981) Foraging decisions in starlings (Sturnus vulgaris L.). Ardea 69:1–67
Turbelin AJ, Malamud BD, Francis RA (2017) Mapping the global state of invasive alien species: patterns of invasion and policy responses. Glob Ecol Biogeogr 26:78–92. https://doi.org/10.1111/geb.12517
Vall-llosera M, Llimona F, de Cáceres M, Sales S, Sol D (2016) Competition, niche opportunities and the successful invasion of natural habitats. Biol Invasions 18:3535–3546. https://doi.org/10.1007/s10530-016-1246-7
Van Berkel M, Bateson M, Nettle D, Dunn J (2018) Can starlings use a reliable cue of future food deprivation to adaptively modify foraging and fat reserves? Anim Behav 142:147–155. https://doi.org/10.1016/j.anbehav.2018.06.015
Verhoeven KJF, Macel M, Wolfe LM, Biere A (2011) Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc R Soc b: Biol Sci 278:2–8. https://doi.org/10.1098/rspb.2010.1272
Versluijs M, van Turnhout CAM, Kleijn D, van der Jeugd HP (2016) Demographic changes underpinning the population decline of starlings Sturnus vulgaris in the Netherlands. Ardea 104:153–165. https://doi.org/10.5253/arde.v104i2.a7
Walkup JA (2013) Small scale genetic and morphological structure in an island population of European starlings (Sturnus vulgaris). University of Aberdeen, Aberdeen
Waterman M, Fuller C, Murray MD (2008) Studies of roosting common starlings Sturnus vulgaris in South Australia. Corella 32:25–29
Watling D, Talbot-Kelly C (1982) Birds of Fiji, Tonga and Samoa
Webster MA (1975) Hong Kong’s trade in wildlife. Biol Cons 8:203–211
Werner SJ, Fischer JW, Hobson KA (2020) Multi-isotopic (δ2H, δ13C, δ15N) tracing of molt origin for European starlings associated with U.S. dairies and feedlots. PLOS ONE 15:e0237137. https://doi.org/10.1371/journal.pone.0237137
Williams GR (1953) The dispersal from New Zeal- and Australia of some introduced European passerines. Ibis 95:676–692. https://doi.org/10.1111/j.1474-919X.1953.tb01895.x
Willoughby JR, Harder AM, Tennessen JA, Scribner KT, Christie MR (2018) Rapid genetic adaptation to a novel environment despite a genome-wide reduction in genetic diversity. Mol Ecol 27:4041–4051. https://doi.org/10.1111/mec.14726
Winger BM, Auteri GG, Pegan TM, Weeks BC (2019) A long winter for the Red Queen: rethinking the evolution of seasonal migration. Biol Rev 94:737–752. https://doi.org/10.1111/brv.12476
Winterbottom JM, Liversidge R (1954) The European starling in the south west cape. Ostrich 25:89–96. https://doi.org/10.1080/00306525.1954.9633410
Woolnough AP, Lowe TJ, Rose K (2006) Can the Judas technique be applied to pest birds? Wildl Res 33:449–455. https://doi.org/10.1071/WR06009
Woolnough AP, Massam MC, Payne RL, Pickles GS (2005) Out on the border: keeping starlings out of Western Australia. Out on the border: keeping starlings out of Western Australia. 183–189
Wretenberg J, Lindström Å, Svensson S, Thierfelder T, Pärt T (2006) Population trends of farmland birds in Sweden and England: similar trends but different patterns of agricultural intensification. J Appl Ecol 43:1110–1120. https://doi.org/10.1111/j.1365-2664.2006.01216.x
Wretenberg J, Lindström Å, Svensson S, Pärt T (2007) Linking agricultural policies to population trends of Swedish farmland birds in different agricultural regions. J Appl Ecol 44:933–941. https://doi.org/10.1111/j.1365-2664.2007.01349.x
Zuccon D, Pasquet E, Ericson PGP (2008) Phylogenetic relationships among Palearctic-oriental starlings and mynas (genera Sturnus and Acridotheres: Sturnidae). Zoolog Scr 37:469–481. https://doi.org/10.1111/j.1463-6409.2008.00339.x
Zufiaurre E, Abba A, Bilenca D, Codesido M (2016) Role of landscape elements on recent distributional expansion of European Starlings (Sturnus vulgaris) in agroecosystems of the Pampas, Argentina. Wilson J Ornithol 128:306–313. https://doi.org/10.1676/wils-128-02-306-313.1
Zusi RL, Stott K, Sams JR, Novaes FC, Coffey BB, Davis J, Longhurst WM, Yocom CF, Morley A, Small A et al (1959) From field and study. Condor 61:298–303. https://doi.org/10.2307/1365501
Acknowledgements
Megan Bishop created the starling illustration in Fig. 3. We thank the editor and reviewers for comments that improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. LAR was supported by the Scientia program at UNSW. Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature review, design, and writing.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stuart, K.C., Hofmeister, N.R., Zichello, J.M. et al. Global invasion history and native decline of the common starling: insights through genetics. Biol Invasions 25, 1291–1316 (2023). https://doi.org/10.1007/s10530-022-02982-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02982-5