Abstract
Islands tend to be more prone to plant invasions than mainland regions, with the Mediterranean ones not being an exception. So far, a large number of studies on comparing leaf morphological and physiological traits between native and non-native plants in Mediterranean environments have been performed, although none of them on Mediterranean islands. To fill this gap, this study focuses on 14 plant species grown in a controlled growth chamber in the absence of stress. The goal was (1) to differentiate leaf morpho-physiological traits between native and non-native plants on a Mediterranean island and (2) to deepen in the underlying causes of the differential photosynthetic traits displayed by non-native species. Results showed that in Mediterranean islands, non-native plant species show on average larger values of net CO2 assimilation, stomatal conductance (gm), photosynthetic nitrogen-use efficiency, among others, and lower leaf mass per area (LMA) and leaf thickness, compared to the native species. Among the assessed traits, this study reports for the first time larger gm, and lower mesophyll conductance limitation in non-native species, which seems to be linked to their lower LMA. These novel traits need to be added to the ‘leaf physiological trait invasive syndrome’. It was also found that on a Mediterranean island, native and non-native species are placed on opposite sides of the leaf economics spectrum, with non-native species being placed on the ‘‘fast-return’’ end. In conclusion, this study demonstrates that non-native species inhabiting a Mediterranean island possess distinct leaf morphological and physiological traits compared to co-occurring native species, at least during the favorable growth season, which increases the chances of a successful invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant invasions are considered to be one of the strongest global ecological threats during the twenty-first century, having a wide range of impacts on ecosystems and their functioning (Vilà and Hulme 2017; Castro-Díez et al. 2019). It has been shown that the level of invasions varies among regions, biomes, and habitats (Chytrý et al. 2008). Notably, islands tend to be more prone to invasions than mainland regions (Dawson et al. 2017) with this pattern being also observed in Mediterranean islands (Gimeno et al. 2006; Celesti-Grapow et al. 2016; Guarino et al. 2021).
There are multiple characteristics that confer a given non-native species its invasive capacity, ranging from biotic interactions to many other ecological features (Abdallah et al. 2021; Traveset and Richardson 2021). Among those suggested to play an important role in plant invasiveness are the physiological traits. Non-native plants have shown to possess higher rates of leaf net CO2 assimilation (A) (van Kleunen et al. 2010; Oliveira et al. 2014; Le et al. 2019), stomatal conductance (gs) (Durand and Goldstein 2001; Caplan and Yeakley 2010), photosynthetic nitrogen-use efficiency (PNUE) (Durand and Goldstein 2001; Heberling and Fridley 2013; Petruzzellis et al. 2021), among others. Moreover, leaf morphological traits have also been suggested to be responsible for the success of non-native plants. Several studies have demonstrated that non-native species generally possess lower leaf mass per area (LMA). These studies have also found correlations between lower LMA and some physiological traits, indicating that non-native species with lower LMA tend to have higher leaf nitrogen (N), nitrogen concentration per unit mass (Nmass), and higher PNUE (Peñuelas et al. 2010; Morais et al. 2021). Another important morphological trait is leaf area (LA), which tends to be higher in non-native plants than co-occurring native species (Daehler et al. 2004; Petruzzellis et al. 2021). To the present, none of the studies comparing leaf morphological and physiological traits variations between native and non-native plants in Mediterranean environments (Zunzunegui et al. 2020; Morais et al. 2021; Petruzzellis et al. 2021) have been performed in Mediterranean islands.
Combining leaf morphological and physiological traits, Wright et al (2004) described a universal spectrum of leaf economics reflecting coordinated changes in leaf structure and function relationships. This leaf economics spectrum (LES) was described as running from a “slow-return” end, encompassing species characterized by high LMA, low nutrient contents (Nmass and Pmass, respectively), low respiration rates, and lower maximum net assimilation rate per unit dry mass (Amass) to a “fast-return” end with the opposite suite of traits. Surprisingly, its use in the field of invasion ecology is rare. The very few studies that applied the LES have suggested that non-native and native species are aligned on its opposite sides, placing non-native species cluster on the ‘‘fast-return’’ end, with higher values of Nmass and Amass, and lower values of LMA relative to native species (Peñuelas et al. 2010; Ordonez and Olff 2013; Petruzzellis et al. 2021).
Based on the above evidences, it appears that photosynthesis and photosynthesis-related parameters may have a crucial role in favoring the invasive character of non-native species. Mechanistically, photosynthesis can be limited by either diffusional (i.e., stomatal and/or mesophyll conductance) or photo-biochemical limitations. Grassi and Magnani (2005) proposed a method to quantify photosynthetic limitations by separating the relative controls on the net CO2 assimilation rate (A) resulting from stomatal (ls), mesophyll conductance (lm) and biochemical limitations (lb)—the latter pooling together potential photochemical and biochemical factors. To the best of our knowledge, no study has previously quantified the photosynthetic limitations of native and non-native species. Quantifying these limitations is important, as a different sharing of photosynthetic limitations could confer some advantages to the latter. In particular, it has been shown that increased photosynthetic capacity along the land plant’s phylogeny is associated to a progressive reduction from a predominant lm in bryophytes to an almost matched co-limitation by ls, lm, and lb in angiosperms (Gago et al. 2019). Decreased lm over ls is associated with increasing both water-use efficiency (WUE) and PNUE along plant’s phylogeny (Flexas and Carriquí 2020), but also with increased WUE within angiosperms themselves (Flexas et al. 2013). Large WUE and PNUE are to be important traits conferring competitive advantage in source-poor environments, such as those encountered in Mediterranean islands, especially if achieved by means of reducing stomatal conductance and transpiration on one hand and nitrogen content on the other, rather than by increasing photosynthesis itself. Since a high mesophyll conductance is often associated with a low LMA, it could happen that non-native species have large photosynthesis due to low lm.
Based on the described gaps in the current knowledge, the aims of this study (1) to differentiate leaf morpho-physiological traits between native and non-native plants on a Mediterranean island and (2) to deepen in the underlying causes of the differential photosynthetic traits displayed by non-native species. To do so, 14 plant species were grown in a controlled growth chamber in the absence of stress. The specific hypotheses studied were:
-
1.
On a Mediterranean island, non-native plant species present advantageous leaf physiological traits as compared to co-occurring native species.
-
2.
In association with leaf morphological traits, decreased lm may contribute to larger A, WUE and PNUE in non-native species.
-
3.
Native and non-native plant species are aligned on the opposite sides of the leaf economics spectrum, placing non-native species cluster on the ‘‘fast-return’’ end of the spectrum (in accordance with previous studies on non-island species).
Materials and methods
Species selection
14 plant species were chosen: eight natives and six non-natives (see details Table 1). Those plant species were chosen based on their abundant presence as coexisting native and non-native species growing side by side across different sites of the island of Mallorca (Balearic Islands, Spain, Western Mediterranean Sea). Sites differed in their environmental conditions and topographic positions, including mountain areas, riparian sites of intermittent streams, and coastal areas. Therefore, plant species were not randomly selected, but chosen to represent a diversity of habitats inhabited by both native and non-native plant species. Seeds of the selected species were collected during summer-autumn 2018 and preserved in paper bags in a dry, cool place to be later grown under controlled conditions.
Experimental site and growth conditions
The study was carried out in the laboratory of the plant physiology deparment at the University of the Balearic Islands (UIB). For this study, six individuals per species (84 plant individuals in total, 14 species × 6 replicates), were grown individually, each in a plastic pot (11 cm diameter top; 8.8 cm depth; capacity: 0.52 Liters) containing peat (Silver Torf, Agrochimica, Bolzano, Italy) and Agriperlite® (70:30 v: v). Each pot was irrigated with 0.2 L of 100% Hoagland solution, three times a week. Plants were grown for 90 days in a plant growth chamber under controlled growing conditions (60% air humidity, 400 μmol m−2 s−1 of photosynthetic active radiation (white light), 12/12 h light/dark regime and temperature fixed at 27/22 °C day/night) before starting measurements.
Leaf morphological traits and Carbon/Nitrogen contents and stable isotopes
Choosing one young fully expanded leaf per individual (n = 6 leaves per species), leaf morphological traits and Carbon/Nitrogen contents and isotopes were fully characterized by implementing the following steps. Leaf area (cm2) was scanned using Canon CanoScan LiDE 220 where its projected leaf area was determined using IMAGEJ software (Schneider et al. 2012), leaf thickness (cm) was assessed using a caliper. Thereafter, the same leaf was dried to calculate the dry leaf mass per unit leaf area (LMA, g m−2) by placing it in a paper envelope inside a ventilation oven (70-80ºC) until constant dry weight (g) was reached. After estimating LMA, the maximum net assimilation rate per unit dry mass (Amass, nmol g−1 s−1) was calculated as the ratio of net CO2 assimilation (A) (see details below) to LMA.
On the other hand, the obtained dry leaves were grinded into fine and uniform powder using a mixer mill (MM-200; Retsch, Haan, Germany) from which 2 mg were used to analyze Carbon and Nitrogen isotopes ratios. Carbon isotope ratios (δ13C) has been used as a proxy for long-term WUE (Farquhar et al. 1989), while nitrogen isotope ratios (δ15N) are an integrated measure of nitrogen fluxes, assimilation, and allocation in plants, allowing to distinguish N2-fixing from non-fixing species (Kalcsits et al. 2014). Isotope ratios were determined with a Continuous-Flow Isotope Ratio Mass Spectrometry (CF-IRMS) combined with gas chromatography (Thermo Delta XPlus, Bremen, Germany) to analyze gas of mass 44/45/46 (for CO2) and 28/29 (for N2) after separation on a GC column (Flash EA 1112 Thermo Finnigan elemental analyzer). Each measurement of the isotope ratios (δ13C and δ15N, %) of a given sample with the CF-IRMS was referred to PDB following the known value of PLS standards run in parallel every 6 samples during the analysis (here Peach-leaf control samples), obtained from the National Institute of Standards & Technology (NIST 1547). For determination of mass-based leaf carbon and nitrogen content (Cmass and Nmass, %), a linear relationship was established between the area of the peak of Carbon 44 or Nitrogen 28 and the weight of C or N present in the standards (Peach-Leaf) used during the measurements. From these obtained weights, the ratio of Carbon to Nitrogen (C:N) was then estimated. Finally, to assess the photosynthetic nitrogen-use-efficiency (PNUE) (see details below), the area-based nitrogen content (Narea, g m−2) was calculated as: Nmass X LMA.
Leaf physiological traits
Leaf physiological traits were conducted using a portable photosynthesis system (Li-6400; Li-Cor, Inc., Lincoln, NE, USA) with an infrared gas analyzer (IRGA) coupled with a 2 cm2 leaf fluorescence chamber (Li − 6400–40 leaf chamber fluorometer; Li-Cor, Inc.) Choosing one young fully expanded leaf per individual (n = 6 leaves per species), instantaneous measurements were carried out between 09:00 to 13:00 h (Central European summer time), at 400 μmol CO2 mol−1 air and saturating photosynthetic photon flux density (PPFD) at 1200 μmol m−2 s−1, and 90:10 red:blue light. This light intensity was used to ensure saturation and was chosen after performing light response curves for all species (Supplementary Fig. S1). Net CO2 assimilation (A, μmol m−2 s−1) and stomatal conductance (gs, mol m−2 s−1) were taken from instantaneous measurements. Combining these obtained data with the data recorded for (Narea), both the intrinsic water-(WUE) and photosynthetic nitrogen-use efficiency (PNUE) were assessed. Intrinsic WUE (µmol mol−1) was calculated as the ratio of A to gs and PNUE (µmol mol−1 s−1) as the ratio of A to Narea. Despite very different leaf morphologies, no significant differences were observed for either leaf temperature or leaf-to-air vapor pressure deficit among species (Supplementary Table 1), making all measurements comparable.
The electron transport rate (ETR) was estimated as ETR = PPFD × ΦPSII × αβ (Genty et al. 1989), ΦPSII being the quantum efficiency of photosystem II, α the leaf absorbance, and β the partitioning of absorbed photons between photosystems I and II. ΦPSII was estimated as ΦPSII = (Fm′-Fs)/Fm′ (Genty et al. 1989), with Fm′ as maximum fluorescence under illumination, and Fs as steady-state fluorescence. The αβ parameter was estimated from A/Ci curves preformed under low oxygen (1% O2), following Valentini et al. (1995). The ratio ETR to A was calculated (µmol mol−1) after previous correction of ETR. Finally, mesophyll conductance (gm) was estimated following Harley et al. (1992), as:
where Γ* is the CO2 compensation point in absence of Rd (here assumed a value of 37.4 μmol mol−1 (following Sharkey et al. 2007), and Rd (μmol m−2 s−1) is the non-photorespiratory respiration during the day, which was estimated as half the respiration rate measured after 2 h of darkness (Niinemets et al. 2005; Martins et al. 2013; Veromann-Jürgenson et al. 2017). The ratio between net CO2 assimilation rate and respiration was also calculated (A/Rd, Pattison et al. 1998; Galmés et al. 2007). After calculation, gm data were filtered following the reliability criterion established by Harley et al. (1992):
Data in the range of 10 < ∂Cc/∂A < 50 were considered as reliable. Aberrant values of gm following this criterion were discarded, together with all the other photosynthetic parameters of that replicate. Photosynthetic limitations—i.e., stomatal limitation (ls), mesophyll conductance limitation (lm) and biochemical limitation (lb)—were estimated following Grassi and Magnani (2005), as follows:
where gtot is the total CO2 diffusion conductance, calculated as gtot = 1(1/gs + 1/gm) and ∂Cc/∂A is considered as the initial slope of the A − Cc response curve.
Data analysis
Differences in leaf morphological traits, Carbon/Nitrogen contents and stable isotopes, and leaf physiological traits between native and non-native plant species, were tested with a non-parametric Kruskal–Wallis test performed using each trait as response variable and status (native and non-native) as explanatory variable. In order to identify general trends in those variables that were significantly affected by the plant ‘status, and to understand how these traits were related in a multivariate context, a Principal Component Analysis (PCA) was performed. Lastly, correlations among parameters were performed using Spearman correlation. All data have been analyzed using R v.3.4.3 (R Core Team 2017).
Results
Leaf morphological traits and Carbon/Nitrogen contents and stable isotopes
Statistically significant differences between native and non-native species were found for all leaf morphological traits (p < 0.001; Table 2). The present results show that on a Mediterranean island, non-native plants possess on average larger values of leaf area (LA), while native ones registered on average the highest values for both leaf thickness (LT) and leaf mass per area (LMA) (Fig. 1). The largest values of LA corresponded to the non-native Solanum linnaeanum, whereas the smallest to the native Mesembryanthemum nodiflorum. Both largest and smallest LT values were reported for native species, Crithmum maritimum and Solanum nigrum, respectively. C. maritimum also had the largest LMA, whereas the smallest values were found in non-native Physalis peruviana (Table 3).
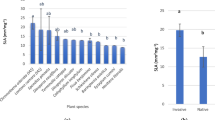
Box plots of the leaf morphological traits and Carbon/Nitrogen contents and stable isotopes. A Leaf area (LA), B leaf thickness (LT), C leaf mass per area (LMA), D mass-based carbon content (Cmass), E mass-based nitrogen content (Nmass), F carbon:nitrogen ratio (C:N), G carbon isotope ratios (δ13C), and H nitrogen isotope ratios (δ15N) for native (in yellow) and non-native (in green) plant species studied. *Denotes statistically significant difference at p < 0.05 and *** p < 0.001
Regarding to Carbon/Nitrogen contents and their stable isotopes, plant status had a significant effect on most of this category traits except for mass-based nitrogen content (Nmass) and the carbon:nitrogen (C:N) ratio (p > 0.05; Table 2). Non-native species showed the highest values for both mass-based carbon content (Cmass) and nitrogen isotope ratios (δ15N), whereas the carbon isotope ratios (δ13C) was highest for native ones, although all the species showed values typical of non-N2-fixing species (Fig. 1). Non-native P. peruviana showed the highest values of Cmass, while native M. nodiliforum presented the lowest. Both the largest and the lowest LT values were reported for non-native species, S. linnaeanum and Arundo donax, respectively. C:N, δ13C and, δ15N followed the same trend in having their highest and lowest values for native species exclusively. C:N was highest for Withania somnifera and lowest for M. nodiflorum, δ13C was highest for Glaucium flavum and lowest for P. peruviana and, δ15N was highest for S. nigrum and lowest for G. flavum (Table 3).
Leaf physiological traits
Plant status had a significant effect on most leaf physiological traits except on intrinsic water-use efficiency (WUE), respiration (Rd) and both stomatal (ls) and biochemical limitations (lb) (p > 0.05; Table 2). All traits differing significantly, except for the mesophyll conductance limitation (lm), presented on average larger values in non-native plants (Fig. 2). Native species, S. nigrum and C. maritimum showed the largest and smallest values for both the net CO2 assimilation (A) and the electron transport rate (ETR). Maximum net assimilation rate per unit dry mass (Amass), stomatal conductance (gs), mesophyll conductance (gm) and photosynthetic nitrogen-use efficiency (PNUE) were highest in non-native species (P. peruviana (for both Amax and PNUE), Tropaeolum majus (for gs), and A. donax for (gm)), while the lowest values were observed in native species (P. australis (for both Amax and gs) and C. maritimum (for both gm and PNUE)) (Table 4). The highest values for Rd were observed in the native C. maritimum, whereas the smallest were for the non-native Nicotiana glauca. Lastly, the largest and smallest values of WUE, the photosynthetic CO2 assimilation over respiration (A/Rd), ls, lm and lb were reported in non-native species exclusively. WUE was highest for Mesembryanthemum crystallinum and lowest for P. peruviana, δ13C was highest for A. donax and lowest for M. crystallinum. N. glauca presented the highest ls values, while M. crystallinum presented the lowest values. lm was highest for M. crystallinum and lowest for both N. glauca and A. donax, while lb was highest for T. majus and lowest for N. glauca (Table 4).
Box plots of the leaf physiological traits. A net CO2 assimilation (A), B maximum net assimilation rate per unit dry mass (Amass), C electron transport rate (ETR), D stomatal conductance (gs), E mesophyll conductance (gm), F intrinsic water-use efficiency (WUE), G photosynthetic nitrogen-use efficiency (PNUE), H respiration (Rd), and I photosynthetic CO2 assimilation over respiration (A/Rd), J stomatal limitation (ls), K mesophyll conductance limitation (lm) and L biochemical limitation (lb) for native (in yellow) and non-native (in green) plant species studied. *Denotes statistically significant difference at p < 0.05 and *** p < 0.001
Principal component analysis and correlations among traits
Native and non-native species showed different patterns in the bidimensional space generated by the horizontal and vertical axes. These axes stood for the first and second Principal Components of the PCA, which account for 57.72 percent of the variance of the assessed parameters (Fig. 3). The first axis of PCA (PC1) represented by the leaf physiological traits, morphological traits, and Carbon/Nitrogen contents and stable isotopes, characterized by high A/Rd, PNUE, gm and A on the upper right and higher LMA and LT on the upper left. The second (PC2) was represented as well by all these traits characterized by high ETR, Amass, gs on the lower right and high lm on the lower left. Non-natives presented high PNUE, A, Amass and gm, while native species had higher LMA and LT and were more represented on the upper left of the PCA.
Principal Component Analysis (PCA) of the significant variables for the leaf morphological traits, Carbon/Nitrogen contents and stable isotopes, and leaf physiological traits between for native (in yellow) and non-native (in green) plant species studied. The 2 big points represent the centroids of both native and non-native plant species
While the PCA suggests some positive and negative relations between some leaf physiological traits and morphological traits, Spearman correlation only showed a significantly negative correlation between Amass and LMA (r = − 0.75; p < 0.001), placing non-native and natives species at a different end, with non-natives possessing larger values in Amass and lower values in LMA, in contrast to natives (Fig. 4A). However, no significant correlation was found between both Amass and Nmass (r = 0.08; p = 0.55) (Fig. 4B) or A and Rd (r = − 0.21; p = 0.11) (Fig. 4C) and, in fact, no other significant correlation was found between any of the studied parameters, except the obvious ones (e.g., A vs gs and gm, A vs PNUE, etc.—data not shown).
Discussion
Our results showed that non-native plant species present in a Mediterranean island tend to have advantageous values in their leaf physiological traits, such as large A, Amass, gs, PNUE, among others (Fig. 2), while possessing lower leaf construction cost in terms of LT and LMA, when comparing them to co-occurring native species (Fig. 1). Many of these traits partly explain the success of non-native plant species (Baruch and Goldstein 1999; Funk and Vitousek 2007) since they may contribute to a faster growth rates and confer a competitive advantage over native species (Peñuelas et al. 2010; Matzek 2011; Funk 2013; Kunstler et al. 2015), at least under favorable weather conditions which, in the Mediterranean, usually coincide with the plant’s growing season. Of course, traits conferring tolerance to environmental stresses occurring especially in summer may also contribute to different species fitness, yet this is out of the scope of the present study. The results presented here are in accordance with previous views for native and non-native plants comparisons from other environments. Peñuelas et al. (2010) have reported that alien species throughout Oahu Island (Hawai´i) had enhanced capacities in terms of productivity (photosynthetic capacity) and lower LMA, causing such species to have higher photosynthetic returns from their foliar biomass investment. More recent studies also showed that the high photosynthetic rates of non-native species reflect their potential to accumulate more biomass, which convey higher competitive ability leading to invasion success (Tordoni et al. 2019; Zunzunegui et al. 2020; Morais et al. 2021).
Along with the long list of distinguished physiological traits between native and non-native plants, the present study reveals a novel trait which is mesophyll conductance (gm), being on average higher in non-native species. It can be observed that gm plays a major role in setting the difference of the photosynthetic capacity between native Mediterranean plants and non-native species. Such difference is usually correlated with leaf anatomy (Flexas et al. 2008; Terashima et al. 2011). Lower gm appears to be related to a significantly higher LMA observed in native species (Fig. 1C). Large LMA is associated to high leaf thickness, which appears to be an adaptation to stressful environments like those in the Mediterranean climate (Niinemets 1999). Besides LMA, sub-cellular anatomical traits, not studied here, had been shown to determine low gm, such as thick cell walls and low chloroplast coverage of intercellular air spaces (Tomás et al. 2013; Tosens et al. 2016; Carriquí et al. 2019; Gago et al. 2019). When performing the photosynthetic limitations analysis, only mesophyll conductance limitation (lm) differed significantly, being on average higher in native species (Fig. 2K). Such finding is in accordance with the fact that lm tends to be larger in native Mediterranean species (Flexas et al. 2014). Regardless of the mechanisms, which remain unknown, this study reports, for the first time, that a lower lm may be a significant physiological trait for contributing to the ‘invasive syndrome’ of non-native species. Despite this, these results were not in full accordance with the 2nd hypothesis, as WUE did not significantly differ between native and non-native species. This result is in contrary to the findings of Zunzunegui et al. (2020) and Morais et al. (2021). Such result with significantly lower δ13C for non-native plants (Fig. 1G), suggest lower WUE in the long term. Considering this, it could be argued that under water stress non-native species may not perform as well as natives, making them less adaptive to substantial warmings and prolonged periods of water shortage predicted to occur in Mediterranean ecosystems (Cramer et al. 2018). To test this, however, further studies should be performed under stress conditions.
When it comes to leaf structure and function relationships described in the leaf economics spectrum, the present results were in accordance with a few other studies that used it in the field of invasion ecology (Ordonez and Olff 2013; Petruzzellis et al. 2021). We report that non-native species on a Mediterranean island, as a group, differ from natives in both individual traits and their positioning along the LES, locating them predominantly at the “fast-return” end of it, with faster investment strategies than co-occurring native species, associated with higher values of A, PNUE and lower values of LMA. This high PNUE in ‘fast’ strategy plants may be due to a greater proportional allocation of C and N to structural than metabolic components of the leaf in low LMA leaves (Poorter et al. 2009). Such finding highlights the importance of the particular ecological-abiotic background a species faces once introduced. Consequently, introduction success is not only dependent of area of origin, but also on introduced species having a suite of traits that enable them to use the new habitat (Thompson and Davis 2011). However, and surprisingly, only the relationship between Amass vs LMA was significant, while Amass vs Nmass and A vs Rd relationships were not, contrarily to what was described for LES for plants in general (Wright et al. 2004) and native/non-native comparisons in particular (Petruzzellis et al. 2021). McDowell (2002) attributed the higher PNUE observed in non-native species to their lower N content, and this is confirmed in our study since leaf N value in our assessed non-native plants was significantly lower (p < 0.05) to that in native species when expressed on an area basis, although not significant when expressed on a mass basis (p = 0.39).
Conclusion
A leaf physiological trait syndrome is described for non-native species invading a Mediterranean island that is similar to that previously described for non-native species in other environments, consisting of larger A, Amass, gs, PNUE and lower LMA and LT, among others. While the vast majority of the assessed traits followed the same trend in non-native species possessing advantageous values, WUE was not significant. This lack of advantage on a key parameter under semi-arid conditions may perhaps reduce the success of non-native species when subjected to water stress. Hence, this should be confirmed in further experiments. Among the assessed traits, we report for the first-time larger gm, and lower lm in non-native species, which seems to be linked to their lower LMA. These novel traits need to be added to the ‘leaf physiological trait invasive syndrome’. Finally, our results confirm that non-native plants on a Mediterranean island are also positioned on the “fast-return” end of the LES. However, that only one significant LES relationship (Amass vs LMA) was found here might be explained by the fact that these plants were not grown in the field but in a growth chamber with addition of nutrient solution containing large amounts of N. This is interesting although it deserves confirmation. If so, it would mean that the Amass vs LMA relationship is the most robust among studied LES traits, for which it possibly is a central or primary one, while others being secondary or consequences of it.
Data availability
Derived data supporting the findings of this study are available from the corresponding author (M.A.) on request.
References
Abd El-Gawad AM, Shehata HS (2014) Ecology and development of Mesembryanthemum crystallinum L. in the deltaic Mediterranean coast of Egypt. Egypt J Basic Appl Sci 1:29–37. https://doi.org/10.1016/j.ejbas.2014.02.003
Abdallah M, Hervías-Parejo S, Traveset A (2021) Low pollinator sharing between coexisting native and non-native plant pairs: the effect of corolla length and flower abundance. Front Ecol Evol 9:709876. https://doi.org/10.3389/fevo.2021.709876
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawai’i. Oecologia 121:183–192. https://doi.org/10.1007/s004420050920
Caplan JS, Yeakley JA (2010) Water relations advantages for invasive Rubus armeniacus over two native ruderal congeners. Plant Ecol 210:169–179. https://doi.org/10.1007/s11258-010-9747-4
Carriquí M, Douthe C, Molins A et al (2019) Leaf anatomy does not explain apparent short-term responses of mesophyll conductance to light and CO2 in tobacco. Physiol Plant 165:604–618. https://doi.org/10.1111/ppl.12755
Castro-Díez P, Vaz AS, Silva JS et al (2019) Global effects of non-native tree species on multiple ecosystem services. Biol Rev 94:1477–1501. https://doi.org/10.1111/brv.1251110.1111/brv.12511
Cedeño M, Montenegro D (2004) Plan Exportador, Logístico y Comercialización de Uchuva al Mercado de Estados Unidos para FRUTEXPO SCI Ltda. Dissertation, Pontificia Universidad Javeriana
Celesti-Grapow L, Bassi L, Brundu G et al (2016) Plant invasions on small Mediterranean islands: an overview. Plant Biosyst 150:1119–1133. https://doi.org/10.1080/11263504.2016.1218974
Chytrý M, Jarošík W, Pyšek P (2008) Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 89:1541–1553. https://doi.org/10.1890/07-0682.1
Corno L, Pilu R, Adani F (2014) Arundo donax L.: a non-food crop for bioenergy and bio-compound production. Biotechnol Adv 32:1535–1549. https://doi.org/10.1016/j.biotechadv.2014.10.006
Cramer W, Guiot J, Fader M et al (2018) Climate change and interconnected risks to sustainable development in the Mediterranean. Nat Clim Change 8:972–980. https://doi.org/10.1038/s41558-018-0299-2
Culhavi CD, Manea D (2011) Controlling Convolvulus arvensis L. in grain maize and winter wheat in Banat (Romania). Res J Agric Sci 43:21–27
Daehler CC, Denslow JS, Ansari S et al (2004) A risk assessment system for screening out invasive pest plants from Hawai’i and other Pacific Islands. Conserv Biol 18:360–368. https://doi.org/10.1111/j.1523-1739.2004.00066.x
Dawson W, Moser D, van Kleunen M et al (2017) Global hotspots and correlates of alien species richness across taxonomic groups. Nat Ecol Evol 1:0186. https://doi.org/10.1038/s41559-017-0186
Durand LZ, Goldstein G (2001) Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawai´i. Oecologia 126:345–354. https://doi.org/10.1007/s004420000535
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. https://doi.org/10.1146/annurev.arplant.40.1.503
Flexas J, Carriquí M (2020) Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: lessons for improving crop photosynthesis. Plant J 101:964–978. https://doi.org/10.1111/tpj.14651
Flexas J, Ribas-Carbó M, Díaz-Espejo A et al (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621. https://doi.org/10.1111/j.1365-3040.2007.01757.x
Flexas J, Niinemets Ü, Gallé A et al (2013) Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth Res 117:45–59. https://doi.org/10.1007/s11120-013-9844-z
Flexas J, Díaz-Espejo A, Gago J et al (2014) Photosynthetic limitations in Mediterranean plants: a review. Environ Exp Bot 103:12–23. https://doi.org/10.1016/j.envexpbot.2013.09.002
Funk JL (2013) The physiology of invasive plants in low-resource environments. Conserv Physiol 1(1):cot026. https://doi.org/10.1093/conphys/cot026
Funk JL, Vitousek PM (2007) Resource use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081. https://doi.org/10.1038/nature05719
Gago J, Carriquí M, Nadal M et al (2019) Photosynthesis optimized across land plant’s phylogeny. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2019.07.002
Galmés J, Ribas-Carbó M, Medrano H et al (2007) Response of leaf respiration to water stress in Mediterranean species with different growth forms. J Arid Envirom 68:206–222. https://doi.org/10.1016/j.jaridenv.2006.05.005
Garzón GA, Wrolstad RE (2009) Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem 114:44–49. https://doi.org/10.1016/j.foodchem.2008.09.013
Gaurav N, Kumar A, Tyagi M et al (2015) Morphology of Withania Somnifera (Distribution, morphology, phytosociology of Withania somnifera L. Dunal). Int J Curr Res 1:164–173
Genty B, Briantais JM, Baker NR et al (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subj 990:87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
Gimeno I, Vilà M, Hulme PE (2006) Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae in the western Mediterranean. J Biogeogr 33:1559–1565. https://doi.org/10.1111/j.1365-2699.2006.01525.x
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28:834–849. https://doi.org/10.1111/j.1365-3040.2005.01333.x
Guarino R, Chytrý M, Attorre F et al (2021) Alien plant invasions in Mediterranean habitats: an assessment for Sicily. Biol Invasions 23:3091–3107. https://doi.org/10.1007/s10530-021-02561-0
Harley PC, Loreto F, Di Marco G et al (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98:1429–1436. https://doi.org/10.1104/pp.98.4.1429
Heberling JM, Fridley JD (2013) Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol 200:523–533. https://doi.org/10.1111/nph.12388
Jagatheeswari D, Bharathi T, Ali HSJ (2013) Black night shade (Solanum nigrum L.)—an updated overview. Int J Pharm 4:288–295
Kalcsits LA, Buschhaus A, Guy RD (2014) Nitrogen isotope discrimination as an integrated measure of nitrogen fluxes, assimilation and allocation in plants. Physiol Plantarum 151:293–304. https://doi.org/10.1111/ppl.12167
Kunstler G, Falster D, Coomes D et al (2015) Plant functional traits have globally consistent effects on competition. Nature 529:204–207. https://doi.org/10.1038/nature16476
Le QV, Tennakoon KU, Metali F et al (2019) Photosynthesis in co-occurring invasive Acacia spp. and native Bornean heath forest trees at the post-establishment invasion stage. J Sustain for 38:230–243. https://doi.org/10.1080/10549811.2018.1530602
Martins SCV, Galmés J, Molins A et al (2013) Improving the estimation of mesophyll conductance to CO2: on the role of electron transport rate correction and respiration. J Exp Bot 64:3285–3298. https://doi.org/10.1093/jxb/ert168
Matzek V (2011) Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol Invasions 13:3005–3014. https://doi.org/10.1007/s10530-011-9985-y
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of rubus (Rosaceae). Am J Bot 89:1431–1438. https://doi.org/10.3732/ajb.89.9.1431
Meot-Duros L, Magné C (2009) Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem 47:37–41. https://doi.org/10.1016/j.plaphy.2008.09.006
Morais MC, Cabral JA, Gonçalves B (2021) Seasonal variation in the leaf physiology of co-occurring invasive (Hakea sericea) and native (Pinus pinaster) woody species in a Mediterranean-type ecosystem. For Ecol Manag 480:118662. https://doi.org/10.1016/j.foreco.2020.118662
Niinemets Ü (1999) Components of leaf dry mass per area—thickness and density—alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47. https://doi.org/10.1046/j.1469-8137.1999.00466.x
Niinemets Ü, Cescatti A, Rodeghiero M et al (2005) Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ 28:1552–1566. https://doi.org/10.1111/j.1365-3040.2005.01392.x
Oliveira MT, Matzek V, Medeiros CD et al (2014) Stress tolerance and ecophysiology ability of an invader and a native species in a seasonally dry tropical forest. PLoS ONE 9(8):e105514. https://doi.org/10.1371/journal.pone.0105514
Ollerton J, Watts S, Connerty S et al (2012) Pollination ecology of the invasive tree tobacco Nicotiana glauca: comparisons across native and non-native ranges. J Pollinat Ecol 9:85–95. https://doi.org/10.26786/1920-7603201212
Ordonez A, Olff H (2013) Do alien plant species profit more from high resource supply than natives? A trait-based analysis. Global Ecol Biogeogr 22:648–658. https://doi.org/10.1111/geb.12019
Pattison RR, Goldstein G, Ares A (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117:449–459. https://doi.org/10.1007/s004420050680
Peñuelas J, Sardans J, Llusià J et al (2010) Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob Change Biol 16:2171–2185. https://doi.org/10.1111/j.1365-2486.2009.02054.x
Petruzzellis F, Tordoni E, Tomasella M et al (2021) Functional differentiation of invasive and native plants along a leaf efficiency/safety trade-off. Environ Exp Bot 188:104518. https://doi.org/10.1016/j.envexpbot.2021.104518
Poorter H, Niinemets Ü, Poorter L et al (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Sharkey TD, Bernacchi CJ, Farquhar GD et al (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040. https://doi.org/10.1111/j.1365-3040.2007.01710.x
Soliman MI, Zaghloul MS, Heikal YM (2014) Genetic variation within and among three Egyptian Mesembryanthemum species using different genetic markers. Egypt J Basic Appl Sci 1:127–135. https://doi.org/10.1016/j.ejbas.2014.08.001
Terashima I, Hanba YT, Tholen D et al (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116. https://doi.org/10.1104/pp.110.165472
Thanos CA, Georghiou K, Skarou F (1989) Glaucium flavum seed germination—an ecophysiological approach. Ann Bot 63:121–130. https://doi.org/10.1093/oxfordjournals.aob.a087714
Thompson K, Davis MA (2011) Why research on traits of invasive plants tells us very little. Trends in Ecol Evol 26:155–156. https://doi.org/10.1016/j.tree.2011.01.007
Tomás M, Flexas J, Copolovici L et al (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281. https://doi.org/10.1093/jxb/ert086
Tordoni E, Petruzzellis F, Nardini A et al (2019) Make it simpler: alien species decrease functional diversity of coastal plant communities. J Veg Sci 30:498–509. https://doi.org/10.1111/jvs.12734
Tosens T, Nishida K, Gago J, et, (2016) The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytol 209:1576–1590. https://doi.org/10.1111/nph.13719
Traveset A, Richardson DM (2021) Plant invasions: the role of biotic interactions, CABI Invasives Series, 13
Valentini R, Epron D, Deangelis P et al (1995) In-situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q.cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ 18:631–640. https://doi.org/10.1007/s10661-020-08386-z
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Veromann-Jürgenson LL, Tosens T, Laanisto L et al (2017) Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living! J Exp Bot 68:1639–1653. https://doi.org/10.1093/jxb/erx045
Vilà M, Hulme PE (2017) Impact of Biological Invasions on Ecosystem Services. Springer. https://doi.org/10.1007/978-3-319-45121-3
Vorontsova MS, Knapp SA (2012) new species of Solanum (Solanaceae) from South Africa related to the cultivated eggplant. PhytoKeys 8:1–11. https://doi.org/10.3897/phytokeys.8.2462
Vymazal J (2013) Emergent plants used in free water surface constructed wetlands: a review. Ecol Eng 61:582–592. https://doi.org/10.1016/j.ecoleng.2013.06.023
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Zunzunegui M, Ruiz-Valdepeñas E, Sert MA et al (2020) Field comparision of ecophysiological traits between an invader and a native species in a Mediterranean coastal dune. Plant Physiol Biochem 146:278–286. https://doi.org/10.1016/j.plaphy.2019.11.032
Acknowledgements
We are grateful to Dr. Pablo Rodríguez-Lozano for his valuable help with the statistical analyses, and Dr. Miquel Ribas-Carbó for deep language revision of our manuscript. This work was supported by the project PGC2018-093824-B-C41 (Ministerio de Economía y Competitividad; MINECO, Spain) and the European Regional Development Fund (ERDF).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
M.A. and J.F. conceived the study. M.A. collected the data and prepared the datasets. M.A., C.D., and J.F. performed the statistical analyses. M.A. prepared figures and led the writing with significant contributions from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10530_2022_2797_MOESM1_ESM.jpg
Supplemental figure with the light saturation curves which represents the net CO2 assimilation (A) to the In-Chamber quantum sensor (PARi) for native (in yellow) and non-native (in green) plant species studied
Supplementary file1 (JPG 3155 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdallah, M., Douthe, C. & Flexas, J. Leaf morpho-physiological comparison between native and non-native plant species in a Mediterranean island. Biol Invasions 24, 2597–2612 (2022). https://doi.org/10.1007/s10530-022-02797-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02797-4