Abstract
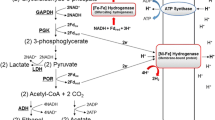
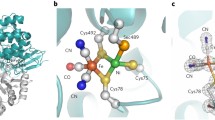
HydE, HydF, and HydG participate in the synthesis of the complex di-iron center of [FeFe] hydrogenases. The hydE, hydF, hydG, hydA, and hydB genes of Desulfovibrio vulgaris Hildenborough were cloned and His-tag pull-down assays were used to study the potential interaction between HydE, HydF, and HydG with the HydA and HydB protein subunits of the D. vulgaris [FeFe] hydrogenase. Interaction of HydE and HydG with HydA was demonstrated. HydF did not interact with HydA, and none of the accessory proteins appeared to interact with HydB. This suggests that specific protein–protein interactions may be required during [FeFe] cluster synthesis and/or insertion.



Similar content being viewed by others
References
Böck A, King PW, Blokesch M, Posewitz MC (2006) Maturation of hydrogenases. Adv Microb Physiol 51:1–71
Boyer ME, Stapleton JA, Kuchenreuther JM, Wang CW, Swartz JR (2008) Cell-free synthesis and maturation of [FeFe] hydrogenases. Biotechnol Bioeng 99(1):59–67
Brazzolotto X, Rubach JK, Gaillard J, Gambarelli S, Atta M, Fontecave M (2006) The [FeFe]-hydrogenase maturation protein HydF from Thermotoga maritime is a GTPase with an iron-sulfur cluster. J Biol Chem 281:769–774
Caffrey SM, Park H-S, Voordouw JK, He Z, Zhou J, Voordouw G (2007) Function of periplasmic hydrogenases in the sulfate reducing bacterium Desulfovibrio vulgaris Hildenborough. J Bacteriol 189:6159–6167
Girbal L, von Abendroth G, Winkler M, Benton PM, Meynial-Salles I, Croux C, Peters JW, Happe T, Soucaille P (2005) Homologous and heterologous overexpression in Clostridium acetobutylicum and characterization of purified clostridial and algal Fe- only hydrogenases with high specific activities. Appl Environ Microbiol 71(5):2777–2781
Huynh BH, Czechowski MH, Kruger HJ, DerVartanian DV, Peck Jr HD, LeGall J (1984) Desulfovibrio vulgaris hydrogenase: a nonheme iron enzyme lacking nickel that exhibits anomalous EPR and Mossbauer spectra. Proc Natl Acad Sci USA 81:3728:3732
King PW, Posewitz MC, Ghirardi MA, Seibert M (2006) Functional studies of [FeFe] hydrogenase maturation in an Escherichia coli biosynthetic system. J Bacteriol 188:2163–2172
McGlynn SE, Ruebush SS, Naumov A, Nagy LE, Dubini A, King PW, Broderick JB, Posewitz MC, Peters JW (2007) In vitro activation of [FeFe] hydrogenase: new insights into hydrogenase maturation. JBIC 12:443–447
Nicolet Y, Piras C, Legrand P, Hatchikan CE, Fontecilla-Camps JC (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7:13–23
Nicolet Y, Leomn BJ, Fontecilla-Camps JC, Peters JW (2000) A novel FeS cluster in Fe-only hydrogenases. TIBS 25:138–143
Nicolet Y, Rubach JK, Posewitz MC, Amara1 P, Mathevon C, Atta M, Fontecave M Fontecilla-Camps JC (2008) X-ray structure of the [FeFe]-hydrogenase maturase HydE from Thermotoga maritima. J Biol Chem doi:10.1074/jbc.M801161200
Pereira AS, Tavares P, Moura I, Moura JJG, Huynh BH (2001) Mossbauer characterization of the iron-sulfur clusters in Desulfovibrio vulgaris hydrogenase. J Am Chem Soc 123:2771–2782
Pereira PM, He Q, Valente FMA, Xavier AV, Zhou J, Pereira IAC, Louro RO (2008) Energy metabolism in Desulfovibrio vulgaris Hildenborough: insights from transcriptome analysis. Antonie van Leeuwenhoek 93:347–362
Pohorelic BKJ, Voordouw JK, Lojou E, Dolla A, Harder J, Voordouw G (2002) Effects of deletion of genes encoding Fe-only hydrogenase of Desulfovibrio vulgaris Hildenborough on hydrogen and lactate metabolism. J Bacteriol 184:679–686
Posewitz MC, King PW, Smolinski SL, Zhang L, Seibert M, Ghirardi MA (2004) Discovery of two novel radical S-adenosylmethionine proteins required for the assembly of an active [Fe] hydrogenase. J Biol Chem 279:25711–25720
Rubach JK, Brazzolotto X, Gaillard J, Fontecave M (2005) Biochemical characterization of the HydE and HydG iron-only hydrogenase maturation enzymes from Thermatoga maritima. FEBS Lett 579:5055–5060
Vignais PM, Billoud B (2007) Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev 107:4206–4272
Voordouw G, Brenner S (1985) Nucleotide sequence of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough). Eur J Biochem 148:515–520
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada and Natural Resources Canada under their joint Greenhouse Gas Mitigation program (GHGPJ 256813-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mansure, J.J., Hallenbeck, P.C. Desulfovibrio vulgaris Hildenborough HydE and HydG interact with the HydA subunit of the [FeFe] hydrogenase. Biotechnol Lett 30, 1765–1769 (2008). https://doi.org/10.1007/s10529-008-9755-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9755-9