Abstract
Family history of hypertension, smoking, diabetes and alcohol consumption and atherosclerotic plaque were identified as common risk factors in IS. We aimed at investigating the relationship between Thymidylate Synthase (TS) gene polymorphisms and ischemic stroke (IS).This case–control research selected and genotyped three single nucleotide polymorphisms (SNPs)of TS( rs699517, rs2790, and rs151264360) with Sanger sequencing in Chinese Han population. We also adopted logistic regression analysis in genetic models for calculating odds ratios and 95% confidence intervals. Genotype-Tissue Expression(GTEx) database analyzed the tissue-specific expression and TS polymorphisms. The ischemic stroke patients showed higher low-density lipoprotein cholesterol and total homocysteine (tHcy). It was found that patients with the TT genotype of rs699517 and GG genotype of rs2790 had larger degrees of tHcy than those with CC + CT genotypes and AA + AG genotypes, respectively. The genotype distribution of the three SNPs did not deviate from Hardy–Weinberg equilibrium (HWE). Haplotype analysis showed that T-G-del was the major haplotype in IS, and C-A-ins was the major haplotype in controls. GTEx database indicated that the rs699517 and rs2790 increased the expression of TS in healthy human and associated with TS expression level in a single tissue. In conclusion: This study has shown that TS rs699517 and rs2790 were significantly related to ischemic stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral stroke, also known as cerebrovascular accident, is a complex neurological disorder and the second leading cause of death worldwide (Katan and Luft 2018), with the features such as high level of morbidity, disability rate, mortality rate, recurrence rate, and a lot of complications, bringing serious harm to human health (Gorelick 2019). Stroke is an acute cerebrovascular disease including hemorrhagic stroke and ischemic stroke (Khaku and Tadi 2023), which result from the sudden vascular rupture in the brain or the vascular occlusion preventing blood flow to the brain, causing damage to brain tissue (Paul and Candelario-Jalil 2021). In China, stroke has become the first cause of death and the leading cause of adult disability (Xu et al. 2021). Ischemic stroke(IS) accounts for about 70% of stroke cases, and its incidence varies widely geographically with higher incidence in the northern China than in southern China (Wu et al. 2013).
There are a lot of risk factors for stroke, such as age, race, diabetes, gender, hypertension, obesity, atherosclerosis, and dyslipidemia (Bhat et al. 2008; Khoury et al. 2013; Kleindorfer et al. 2010; Tirschwell et al. 2004). In addition, genetic factors are considered to be significant for stroke (Boehme et al. 2017). It has been identified that polymorphisms of some genes are related to the growth of stroke, such as DIAPH1 (Ren et al. 2020), ABO (Ling et al. 2016), MTHFR (Chauhan and Debette 2016), Kalirin (Li et al. 2017), and Lp-PLA2 G994T (Ni et al. 2017).
Ischemic stroke is a disease caused by multiple genes and factors, such as environmental, dietary habits and genetic factors (Liu et al. 2021). Stroke prevention follows the strategy of tertiary prevention (Manosalva et al. 2018). Identification of genetic variants which related to ischemic stroke risk could clearly illustrate the pathogenesis of stroke and provide a approach to prevent and treat this complicated disease. Folic acid metabolism plays an indirect or direct role in the function, division, and differentiation of cells (Hiraoka and Kagawa 2017). Insufficient or excessive folic acid will impair hematopoiesis, impact cell cycle progression and accelerate DNA damage (Henry et al. 2017). The reductases controlling the metabolism of folic acid and homocysteine are MTHFR, MTR, CBS, TS and so on (Hiraoka and Kagawa 2017; Moulik et al. 2017). The normal action of various enzymes is directly related to the normal operation of folic acid and homocysteine metabolism (Hiraoka and Kagawa 2017).
Thymidyate Synthase (TS) is an enzyme which involved in folic acid metabolism (Liu et al. 2016). To be specific, TS, the key enzyme in de novo synthesis of deoxythymine monophosphates (dTMP), catalyzes the conversion of deoxyuridine monophosphate (dUMP) to dTMP, a nucleotide necessary for DNA synthesis and recovery as a substrate (Hiraoka and Kagawa 2017; Kawakami et al. 1999; Choi and Mason 2000; Zhou et al. 2012). Hence, abnormality in the genes that encode these components can result in various issues such as MTHFR reduction and TS protein increase, even lead to vascular disease by accumulating tHcy, folate deficiency or both (Ahn et al. 2018; Choi et al. 2016). The MTHFR (677) C > T gene polymorphism also affects circulating tHcy levels and plays an important role in predicting the risk of ischemic stroke (Huang et al. 2022).TS genetic variants have been reported in cancer therapeutics because of the participation of TS in DNA repair mechanisms (Ahn et al. 2018; Jeon et al. 2021; Silva et al. 2020; Han et al. 2018; Donner et al. 2019), then we supposed that the single-nucleotide polymorphisms (SNPs) of TS is associated with the attack of stroke closely. Jung Oh Kim’s study displayed that TS was associated with Hypertensive, Diabetic, tHcy and Folate susceptibility to stroke, and TS polymorphism are related to ischemic stroke in the Korean population Kim et al. 2021.
In Chinese Han population, the relationship between TS genetic variants and ischemic stroke susceptibility is still unclear. This study aimed to confirm the relationships between ischemic stroke and TS gene polymorphisms in the northern Han Chinese population, providing available data for preventing and managing ischemic stroke.
Materials and Methods
Study Subjects
All patients were from the Liaocheng People’s Hospital, including 259 ischemic stroke patients and 240 control members. All patients were informed of the purpose of sample collection and signed the written informed consent. The inclusive criteria: the patients who suffering from ischemic stroke from firstly diagnosed depending on clinical features and neuroimaging criteria. Anterior circulation stroke is the inclusion standards of patients. Besides, the exclusive criteria: brain injuries, cerebral tumors, not stroke for the first diagnosis, autoimmune diseases, osteoarthrosis and psychological illness. Control subjects without history of stroke or cerebrovascular diseases were selected from health check-up center of our hospital and received a physical examination. Several healthy controls suffered from vascular risk factors including diabetes, hypertension, drinking, smoking, and hypercholesterolemia. The color Doppler ultrasonography (Model: Philips EPIQ7C) was made to investigate the carotid artery (Abreu et al. 2015). The ethics was approved from Committee of Liaocheng People’s Hospital (ethical code: 2,020,016). All tests were conducted under the guidance of the Declaration of Helsinki (Snaedal 2014).
Single-Nucleotide Polymorphism Selection and Genotyping
Detailed data for TS SNPs (rs699517, rs2790, and rs151264360) were acquired from the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/). The minor allele frequency of these SNPs was 5% greater than in Chinese Han population (Dong et al. 2021). The blood genomic DNA purification kit (GoldMag) was used to extract the genomic DNA of peripheral blood samples in all subjects according to the manufacturer’s protocol. Detected by NanoDrop 2000C spectrophotometer (Thermo Scientific), the purity and concentration of genomic DNA were kept at − 20 °C before further analysis. The Agena Bioscience Assay Design Suite V2.0 software was adopted to design primers for PCR amplification. Primer information was listed in Table 1. Sanger sequencing was adopted to identify SNPs genotyping.
Bioinformatics Data Analysis
We selected GTEx database (Version 8) (https://gtexportal.org/home/)to analyze the tissue-specific expression and TS polymorphisms (Expression and (GTEx), 2013; Stanfill and Cao 2021).
Statistical Analysis
Student’s t-test was adopted to analyze the diversities of demographic features in case and control individuals for constant variables. Pearson’s χ2 tests were used for classified variables. A chi-square goodness-of-fit test was used to test Hardy–Weinberg Equilibrium (HWE) for each genotype. The relationships between TS SNPs and ischemic stroke risk were assessed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) applying logistic regression analysis. The SHEsis analysis platform was adopted to make the linkage disequilibrium index (D-prime and r2) with the deductive haplotypes of these three SNPs. SPSS v24.0 (SPSS) was adopted to calculate all statistical analyses, and a two-tailed P < 0.05 indicated a statistically significant difference.
Results
Characteristics of Individuals
The relationship of Thymidyate Synthase single-nucleotide polymorphisms with ischemic stroke risk was determined by enrolling 259 ischemic stroke patients and 240 control members. The general clinical data were displayed in Table 2. No obvious significance was found in body mass index, mean age, high-density lipoprotein cholesterol, serum total cholesterol or overall triglyceride degrees between the patients and controls. According to the risk factor profile, hypertension, smoking, diabetes, alcohol consumption, family history and atherosclerotic plaque were ordinary risk factors among the patients. The ischemic stroke patients showed higher low-density lipoprotein cholesterol and tHcy.
TS SNPs and Ischemic Stroke Risk Assessment
The genotype distribution of the three TS SNPs (rs699517, rs2790, and rs151264360) did not deviate from HWE P value. The correlation results of TS SNPs genotype and allele frequency in patients with ischemic stroke and control group are shown in Table 3. No great diversities were shown in the TS rs151264360 genotypes or allele allocation between ischemic stroke patients and controls (P all > 0.05). On the contrary, the distributions of rs699517 genotypes (TT vs CC AOR = 3.128, 95% CI = 1.724–5.677; CT vs CC AOR = 1.933,95% CI = 1.080–3.462; CT + TT vs CC AOR = 2.404, 95% CI = 1.378–4.195; allele T vs C AOR = 1.684, 95% CI = 1.298–2.185) and rs2790 genotypes (GG vs AA AOR = 3.997, 95% CI = 2.009–7.953; AG vs AA AOR = 1.831, 95% CI = 1.259–2.664; AG + GG vs AA AOR = 2.076, 95% CI = 1.448–2.977; allele G vs A: AOR = 1.424, 95% CI = 1.091–1.859) were greatly different between ischemic patients and control members.
Haplotype Analysis
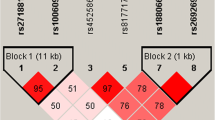
LD measurement and haplotype analysis were made by SHEsis. These three SNPs were in linkage disequilibrium (Fig. 1). Of 8 possible haplotypes, only 4 had frequencies of > 0.03 were included in our haplotype analysis (Table 4). It was found that T-G-del was the major haplotype in IS, and C-A-ins was the major haplotype in controls (40.0% and 42.5%). Besides, it was found that the T-G-del haplotype may be related to an increasing risk of IS (OR = 1.832, 95% CI = 1.401–2.395), while the C-A-ins haplotype may be associated with a decreasing risk of IS.
Multiple Logistic Regression Analysis
Logistic regression analysis was adopted to analyze IS risk factors in Table 5. The risk factors contained hypertension (OR = 3.982; 95% CI = 1.915–8.282), diabetes (OR = 4.250; 95% CI = 1.595–11.324), smoking (OR = 2.462; 95% CI = 1.240–4.885), alcohol consumption (OR = 3.057; 95% CI = 1.487–6.284), family history (OR = 3.855; 95% CI = 1.446–10.274), atherosclerotic plaque (OR = 1.946; 95% CI = 1.013–3.741), LDL-C (OR = 3.129; 95% CI = 1.802–5.435) and tHcy (OR = 1.441; 95% CI = 1.347–1.543). Nevertheless, after being corrected through comparison, TG and HDL-C showed no statistical significance (Table 2). Therefore, further studies need to determine our outcomes in larger sample size.
Association Between TS rs699517, rs2790 and Serum tHcy Levels
The relationship between serum degrees of tHcy and IS was explored. The tHcy degrees among IS patients were greatly increased compared with the controls (Fig. 2A). Even logistic regression was adopted for ordinary risks, including the hypertension, diabetes, smoking, alcohol consumption, family history and atherosclerotic plaque, tHcy levels were still associated with a growing risk of IS (Table 5). It was found that patients with the TT genotype of rs699517 and GG genotype of rs2790 had larger degrees of tHcy than those with CC + CT genotypes and AA + AG genotypes, respectively (Fig. 2B, D). However, individuals with the TT genotype of rs699517 and GG genotype of rs2790 in the control group showed no great diversity in tHcy levels compared with the CC + CT and AG + GG of control group, respectively (Fig. 2C, E).
An increased level of tHcy in IS patients compared with controls (A). ***, P < 0.001. In IS patients, rs699517 TT genotype and rs2790 GG genotype had higher levels of tHcy than those with CC + CT genotypes and AA + AG genotypes, respectively (B, D). ***, P < 0.001. The levels of tHcy showed no significant differences among different genotype in controls (C, E).
Bioinformatics Data Analysis
GTEx researched autopsy samples from healthy human donors. Genotype and allele frequencies distribution of rs699517 and rs2790 in control and IS patients (Fig. 3A-D). The TT genotype of rs699517 and GG genotype of rs2790 increased the expression of TS in healthy human (Fig. 3E, F). The expressed quantitative trait locus (eQTL) indicated that the TS rs699517 and rs2790 were associated with TS expression level in a single tissue (Fig. 4).
Genotype and allele frequencies distribution of rs699517 and rs2790 in control and IS patients (A–D). **, P < 0.01. There were significant differences in genotype and allele frequencies of rs699517 and rs2790 between two groups. The rs699517 TT genotype and rs2790 GG genotype were associated with increased levels of TS compared with CC (E) and AA (F) respectively. **, P < 0.01
Discussion
This research examined potential relationships between three SNPs of the TS and ischemic stroke in Chinese Han population. It was observed that rs699517 and rs2790 were related to IS. The T allele of rs699517 and G allele of rs2790 were risk factors for IS. However, the rs151264360 genotypes or allele distributions have no great diversities between ischemic stroke patients and controls. This is the first research to show that these SNPs were related to the attack of ischemic stroke in Chinese population.
Relationships between the occurrence of IS and folate-associated genes have been recognized in numerous research (Fekih-Mrissa et al. 2013; Holmes et al. 2011). Most previous research indicated that MTHFR (677) C > T was related to an increasing risk of stroke (Qin et al. 2020; Chang et al. 2019; Kim et al. 2013). TS was located on chromosome 18p11.32 and was mutated in various types of diseases (Kim et al. 2021; Gusella and Padrini 2007). TS is the most important protein taking part in tHcy and folate metabolism, and its polymorphism may exert a significant effect on the disease susceptibility of everybody (Ho et al. 2013). One of the most commonly researched polymorphisms is in the the 3′-untranslated region (3′-UTR) of TS mRNA, associated with a lessening of mRNA stability and translation, which lead to the low expression of protein (Ulrich et al. 2000). The TS 3′-UTR polymorphic allele (del) doubled the risk of cytological abnormalities, possibly due to TS reduction catalyzed by TS enzyme. The activity of this enzyme is reduced by TS 3′-UTR polymorphism, which is critical for cancer development (Ulrich et al. 2000; Mandola et al. 2004). Hyper homocysteine is one of the risk factors for IS, and insufficient MTHFR activity can lead to elevated plasma tHcy (Kim et al. 2017).
Alteration in folate metabolism can occur because of modified activity/availability of folate pathway enzymes, which relies on the polymorphisms in their coding genes in turn (Hiraoka and Kagawa 2017). These polymorphisms lead to reducing folate availability in the place of reaction, resulting in hyperhomocysteinaemia through epigenetic impacts including DNA methylation, uracil misincorporation and modified purine synthesis (Moulik et al. 2017). The toxicity of antifolic acid drugs used in cancer therapy is also affected by the modification of genes encoding folic acid pathway protein, that is, the reduction of protein vector, folic acid vector, and the alteration of enzyme activities such as TS and MTHFR (Petrone et al. 2021; Song et al. 2021). Nevertheless, the mechanisms regulating TS expression are not clear, and polymorphisms within the TS are seemingly significant determinants of the level of TS expression (Donner et al. 2019). TS binds to RNA and inhibits the mRNA translation, thereby regulating cell cycle progression (Choi and Mason 2000). Bioinformatics data analysis indicated that the rs699517 TT genotype and rs2790 GG genotype increased the expression of TS. Our results suggested that the rs699517 TT genotype and rs2790 GG genotype were related to increasing levels of TS comparing with CC and AA, respectively. Meanwhile, rs699517 TT genotype and rs2790 GG genotype increased the risk of IS. The GTEx resource has provided insights into the regulatory impact of genetic variation on gene expression across human tissues. The GTEx database showed that the rs699517 and rs2790 had differences in TS expression (Fig. 4). We observed that the rs699517 TT genotype frequency and rs2790 GG genotype frequency in IS were clearly higher than that in control (Fig. 3A, B). The GTEx database also showed that subjects carrying the rs699517 TT genotype frequency and rs2790 GG genotype frequency had higher levels of TS expression (Fig. 3E, F). Further mechanism experiments are needed to verify the connection between these 2 SNPs and TS expression. In a word, the important role of rs699517 and rs2790 in TS may be considered as a novel target for predicting the risk of IS.
Luca Zanoli et al. found that aortic stiffness was increased in patients with inflammation and dependent on disease duration and white blood cell count (Zanoli et al. 2017). Emerging evidence suggests that perturbations of folate/homocysteine metabolism can directly modify production of inflammatory mediators (Hammons et al. 2012). This research also examined the association between tHcy and IS risk. The tHcy serum levels of IS patients were higher significantly than the controls. The high expression of TS can upregulate tHcy levels and reduce folate degrees, causing stroke occurrence (Ho et al. 2013). There is an inverse correlation between plasma folate concentrations and tHcy levels (Brevik et al. 2005). So rs699517 and rs2790 may accelerate the inflammatory response of the body and increase the susceptibility to ischemic stroke by affecting the metabolism of folic acid. Past research illustrated vascular disease among patients who suffering from greatly increasing plasma tHcy levels (Park et al. 2013). It is believed that tHcy increases thrombotic risk by stimulating endothelial damage in blood vascular system (Joachim et al. 2013).
Nevertheless, this research is preliminary because of small sample size, shortage of measuring TS mRNA and protein expression and simultaneously only evaluated three SNPs in TS. Therefore, it is necessary to perform a replicative study with a larger sample size to confirm our findings, including genotyping, expression, and interpretation. Moreover, the functional mechanisms should be clearly illuminated for better understanding of the etiology of ischemic stroke.
Conclusion
Our research suggested two polymorphisms in TS, rs699517 C > T and rs2790 A > G, increasing the susceptibility to ischemic stroke in the north of Chinese Han population. Once the correlation between TS and ischemic stroke is confirmed by larger cohort of patients, TS SNPs could be potential markers of ischemic stroke, which would assist to prevent ischemic stroke in Chinese Han individuals.
Data Availability
The author will provide the raw data supporting the conclusions without reservation.
References
Abreu TQ, Ferreira EB, de Brito Filho SB, de Sales KP, Lopes FF, de Oliveira AE (2015) Prevalence of carotid artery calcifications detected on panoramic radiographs and confirmed by Doppler ultrasonography: their relationship with systemic conditions. Indian J Dental Res : Off Publ Indian Soc Dental Res 26(4):345–350. https://doi.org/10.4103/0970-9290.167644
Ahn TK, Kim JO, Kim HW, Park HS, Shim JH, Ropper AE et al (2018) 3’-UTR polymorphisms of MTHFR and TS associated with osteoporotic vertebral compression fracture susceptibility in postmenopausal women. Int J Mol Sci. https://doi.org/10.3390/ijms19030824
Bhat VM, Cole JW, Sorkin JD, Wozniak MA, Malarcher AM, Giles WH et al (2008) Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke 39(9):2439–43. https://doi.org/10.1161/strokeaha.107.510073
Boehme AK, Esenwa C, Elkind MS (2017) Stroke risk factors, genetics, and prevention. Circu Res 120(3):472–95. https://doi.org/10.1161/circresaha.116.308398
Brevik A, Vollset SE, Tell GS, Refsum H, Ueland PM, Loeken EB et al (2005) Plasma concentration of folate as a biomarker for the intake of fruit and vegetables: the hordaland homocysteine study. Am J Clin Nutr 81(2):434–439. https://doi.org/10.1093/ajcn.81.2.434
Chang G, Kuai Z, Wang J, Wu J, Xu K, Yuan Y et al (2019) The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr 19(1):331. https://doi.org/10.1186/s12877-019-1304-y
Chauhan G, Debette S (2016) Genetic risk factors for ischemic and hemorrhagic stroke. Curr Cardiol Rep 18(12):124. https://doi.org/10.1007/s11886-016-0804-z
Choi SW, Mason JB (2000) Folate and carcinogenesis: an integrated scheme. J Nutr 130(2):129–132. https://doi.org/10.1093/jn/130.2.129
Choi Y, Kim JO, Shim SH, Lee Y, Kim JH, Jeon YJ et al (2016) Genetic variation of methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TS) genes is associated with idiopathic recurrent implantation failure. PloS one 11(8):e0160884. https://doi.org/10.1371/journal.pone.0160884
Dong SQ, Wang TM, Zhang JB, He YQ, Xue WQ, Wu ZY et al (2021) Polymorphisms in TYMS for prediction of capecitabine-induced hand-foot syndrome in chinese patients with colorectal cancer. Cancer Res Treat 53(3):724–732. https://doi.org/10.4143/crt.2020.457
Donner DB, Nakakura EK, Venook AP, Lenz HJ, Zhang W, Hwang J et al (2019) High thymidylate synthase gene expression predicts poor outcome after resection of hepatocellular carcinoma. PloS one 14(7):e0219469. https://doi.org/10.1371/journal.pone.0219469
Fekih-Mrissa N, Mrad M, Klai S, Mansour M, Nsiri B, Gritli N et al (2013) Methylenetetrahydrofolate reductase (C677T and A1298C) polymorphisms, hyperhomocysteinemia, and ischemic stroke in Tunisian patients. J Stroke Cerebrovasc Dis : Off J Natl Stroke Assoc 22(4):465–469. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.03.011
Gorelick PB (2019) The global burden of stroke: persistent and disabling. Lancet Neurol 18(5):417–8. https://doi.org/10.1016/s1474-4422(19)30030-4
Gusella M, Padrini R (2007) G>C SNP of thymidylate synthase with respect to colorectal cancer. Pharmacogenomics 8(8):985–996. https://doi.org/10.2217/14622416.8.8.985
Hammons AL, Summers CM, Jochems J, Arora JS, Zhang S, Blair IA et al (2012) Pemetrexed alters folate phenotype and inflammatory profile in EA.hy 926 cells grown under low-folate conditions. Euro J Pharmacol 696(1–3):12–7. https://doi.org/10.1016/j.ejphar.2012.08.008
Han R, Wei J, Zhang H, Su X, Chu X, Chen Y et al (2018) Influence of TS (rs34743033) and RUNX1 (rs2014300) gene polymorphisms on survival outcomes of fluorouracil-based chemotherapy in Chinese advanced gastric cancer patients. Cancer Manag Res 10:1429–1437. https://doi.org/10.2147/CMAR.S158647
Henry CJ, Nemkov T, Casas-Selves M, Bilousova G, Zaberezhnyy V, Higa KC et al (2017) Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 102(12):1985–94. https://doi.org/10.3324/haematol.2017.171074
Hiraoka M, Kagawa Y (2017) Genetic polymorphisms and folate status. Congenit Anom 57(5):142–9. https://doi.org/10.1111/cga.12232
Ho V, Massey TE, King WD (2013) Effects of methionine synthase and methylenetetrahydrofolate reductase gene polymorphisms on markers of one-carbon metabolism. Genes Nutr 8(6):571–580. https://doi.org/10.1007/s12263-013-0358-2
Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, Cooper J et al (2011) Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet (london, England) 378(9791):584–594. https://doi.org/10.1016/s0140-6736(11)60872-6
Huang LW, Li LL, Li J, Chen XR, Yu M (2022) Association of the methylenetetrahydrofolate reductase (MTHFR) gene variant C677T with serum homocysteine levels and the severity of ischaemic stroke: a case-control study in the southwest of China. J Int Med Res 50(2):3000605221081632. https://doi.org/10.1177/03000605221081632
Jeon YJ, Cho SH, Kim EJ, Ryu CS, Park HS, Kim JW et al (2021) 3’-UTR polymorphisms in thymidylate synthase with colorectal cancer prevalence and prognosis. J Pers Med. https://doi.org/10.3390/jpm11060537
Joachim E, Goldenberg NA, Bernard TJ, Armstrong-Wells J, Stabler S, Manco-Johnson MJ (2013) The methylenetetrahydrofolate reductase polymorphism (MTHFR c.677C>T) and elevated plasma homocysteine levels in a U.S. pediatric population with incident thromboembolism. Thromb Res 132(2):170–4. https://doi.org/10.1016/j.thromres.2013.06.005
Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38(2):208–11. https://doi.org/10.1055/s-0038-1649503
Kawakami K, Omura K, Kanehira E, Watanabe Y (1999) Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res 19(4b):3249–3252
Khaku AS, Tadi P. 2023 Cerebrovascular Disease. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC
Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O et al (2013) Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke 44(6):1500–4. https://doi.org/10.1161/strokeaha.113.001318
Kim OJ, Hong SH, Jeon YJ, Oh SH, Kim HS, Park YS et al (2013) Gene-environment interactions between methylenetetrahydrofolate reductase (MTHFR) 677C>T and metabolic syndrome for the prevalence of ischemic stroke in Koreans. Neurosci Lett 533:11–16. https://doi.org/10.1016/j.neulet.2012.11.031
Kim JO, Park HS, Ryu CS, Shin JW, Kim J, Oh SH et al (2017) Interplay between 3’-UTR polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and the risk of ischemic stroke. Sci Rep 7(1):12464. https://doi.org/10.1038/s41598-017-12668-x
Kim JO, Park HS, Ko EJ, Sung JH, Kim J, Oh SH et al (2021) The 3’-UTR polymorphisms in the thymidylate synthase (TS) gene associated with the risk of ischemic stroke and silent brain infarction. J Pers Med. https://doi.org/10.3390/jpm11030200
Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML et al (2010) Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 41(7):1326–31. https://doi.org/10.1161/strokeaha.109.575043
Li H, Yu S, Wang R, Sun Z, Zhou X, Zheng L et al (2017) Genetic variant of kalirin gene is associated with ischemic stroke in a chinese han population. BioMed Res Int 2017:6594271. https://doi.org/10.1155/2017/6594271
Ling X, Zheng Y, Tao J, Zheng Z, Chen L (2016) Association study of polymorphisms in the ABO gene with ischemic stroke in the Chinese population. BMC Neurol 16(1):146. https://doi.org/10.1186/s12883-016-0671-7
Liu P, Zhang M, Xie X, Jin J, Holman CD (2016) Polymorphisms of 5,10-methylenetetrahydrofolate reductase and thymidylate synthase, dietary folate intake, and the risk of leukemia in adults. Tumour Biol : J Int Soc Oncodevelopmental Biol Med 37(3):3265–3275. https://doi.org/10.1007/s13277-015-4168-6
Liu H, Hou L, Xu S, Li H, Chen X, Gao J et al (2021) Discovering cerebral ischemic stroke associated genes based on network representation learning. Front Genet 12:728333. https://doi.org/10.3389/fgene.2021.728333
Mandola MV, Stoehlmacher J, Zhang W, Groshen S, Yu MC, Iqbal S et al (2004) A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics 14(5):319–327. https://doi.org/10.1097/00008571-200405000-00007
Manosalva HA, Pio F, Jeerakathil T, Saqqur M, Camicioli R, Suchowersky O (2018) Vascular parkinsonism in a tertiary care stroke prevention clinic and the development of a new screening strategy. J Stroke Cerebrovasc Dis : Off J Natl Stroke Assoc 27(1):153–61. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.020
Moulik NR, Kumar A, Agrawal S (2017) Folic acid, one-carbon metabolism & childhood cancer. Indian J Med Res. 146(2):163–74. https://doi.org/10.4103/ijmr.IJMR_275_15
Ni J, Gu H, Hu W, Zhou F, Zhu X, Wang K (2017) Association of Lp-PLA2 G994T gene polymorphism with risk of ischemic stroke in Chinese population. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.21999
Park SY, An SA, Lee HB, Kim Y, Kim NK, Kim SH et al (2012) Different impact of hyperhomocysteinemia on cerebral small vessel ischemia and cervico-cerebral atherosclerosis in non-stroke individuals. Thromb Res 131(1):e12-6. https://doi.org/10.1016/j.thromres.2012.11.011
Paul S, Candelario-Jalil E (2021) Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 335:113518. https://doi.org/10.1016/j.expneurol.2020.113518
Petrone I, Bernardo PS, Dos Santos EC, Abdelhay E (2021) MTHFR C677T and A1298C polymorphisms in breast cancer, gliomas and gastric cancer: a review. Genes. https://doi.org/10.3390/genes12040587
Qin X, Spence JD, Li J, Zhang Y, Li Y, Sun N et al (2020) Interaction of serum vitamin B(12) and folate with MTHFR genotypes on risk of ischemic stroke. Neurology 94(11):e1126–e1136. https://doi.org/10.1212/wnl.0000000000008932
Ren Z, Chen X, Tang W, Li J, Yang S, Chen Y et al (2020) Association of DIAPH1 gene polymorphisms with ischemic stroke. Aging 12(1):416–35. https://doi.org/10.18632/aging.102631
Silva NNT, Santos ACS, Nogueira VM, Carneiro CM, Lima AA (2020) 3’UTR polymorphism of thymidylate synthase gene increased the risk of persistence of pre-neoplastic cervical lesions. BMC Cancer 20(1):323. https://doi.org/10.1186/s12885-020-06811-7
Snaedal J (2014) The helsinki declaration. Laeknabladid 100(3):135. https://doi.org/10.17992/lbl.2014.03.533
Song S, Tian B, Zhang M, Gao X, Jie L, Liu P et al (2021) Diagnostic and prognostic value of thymidylate synthase expression in breast cancer. Clin Exp Pharmacol Physiol 48(2):279–287. https://doi.org/10.1111/1440-1681.13415
Stanfill AG, Cao X (2021) Enhancing research through the use of the genotype-tissue expression (GTEx) database. Biol Res Nurs 23(3):533–540. https://doi.org/10.1177/1099800421994186
The Genotype-Tissue Expression (GTEx) 2013 project. Nature genetics. 45(6): 580-5 doi https://doi.org/10.1038/ng.2653
Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT Jr, Psaty BM (2004) Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 63(10):1868–75. https://doi.org/10.1212/01.wnl.0000144282.42222.da
Ulrich CM, Bigler J, Velicer CM, Greene EA, Farin FM, Potter JD (2000) Searching expressed sequence tag databases: discovery and confirmation of a common polymorphism in the thymidylate synthase gene. Cancer Epidemiol, Biomark Prev : Publ Am Assoc Cancer Res, Cosponsored Am Soc Prev Oncol 9(12):1381–1385
Wu X, Zhu B, Fu L, Wang H, Zhou B, Zou S et al (2013) Prevalence, incidence, and mortality of stroke in the chinese island populations: a systematic review. PloS one 8(11):e78629. https://doi.org/10.1371/journal.pone.0078629
Xu X, Tan Z, Fan M, Ma M, Fang W, Liang J et al (2021) Comparative study of multi-delay pseudo-continuous arterial spin labeling perfusion MRI and CT perfusion in ischemic stroke disease. Front Neuroinformatics 15:719719. https://doi.org/10.3389/fninf.2021.719719
Zanoli L, Boutouyrie P, Fatuzzo P, Granata A, Lentini P, Oztürk K et al (2017) Inflammation and aortic stiffness: an individual participant data meta-analysis in patients with inflammatory bowel disease. J Am Heart Assoc. https://doi.org/10.1161/jaha.117.007003
Zhou JY, Shi R, Yu HL, Zeng Y, Zheng WL, Ma WL (2012) The association between two polymorphisms in the TS gene and risk of cancer: a systematic review and pooled analysis. Int J Cancer 131(9):2103–2116. https://doi.org/10.1002/ijc.27465
Acknowledgements
We would like to thank all of our patients and healthy controls who generously agreed to participate in this study.
Funding
This study was founded by National Natural Science Foundation of China [#81701159],Taishan Scholar Project of Shandong Province of China [# tsqn202103200], Natural Science Foundation project of Shandong Province (# ZR2021MH303), the Key Research Project of Shandong Province of China[#2018GSF118046] and Tianjin Key Research and Development Plan, Key Project of Science and Technology Support (# 20YFZCSY00010).
Author information
Authors and Affiliations
Contributions
FY, ZW, and YX devised the idea and designed the study for this article. FY, LS, QW, XX, ZL, LH, ZZ, ZW, and YX contributed the acquisition of the data and conducted the data analyses. FY wrote the first draft of the manuscript. All authors made the critical revision of the paper and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
The studies involving human participants were approved by Ethics Committee of Liaocheng People’s Hospital (ethical code: 2020016). The participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, F., Shi, L., Wang, Q. et al. The Association Between Thymidylate Synthase Gene Polymorphisms and the Risk of Ischemic Stroke in Chinese Han Population. Biochem Genet 62, 468–484 (2024). https://doi.org/10.1007/s10528-023-10431-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10431-8