Abstract
Ageing is an inevitable aspect of life and thus successful ageing is an important focus of recent scientific efforts. The biological process of ageing is mediated through the interaction of genes with environmental factors, increasing the body’s susceptibility to insults. Elucidating this process will increase our ability to prevent and treat age-related disease and consequently extend life expectancy. Notably, centenarians offer a unique perspective on the phenomenon of ageing. Current research highlights several age-associated alterations on the genetic, epigenetic and proteomic level. Consequently, nutrient sensing and mitochondrial function are altered, resulting in inflammation and exhaustion of regenerative ability.
Oral health, an important contributor to overall health, remains underexplored in the context of extreme longevity. Good masticatory function ensures sufficient nutrient uptake, reducing morbidity and mortality in old age. The relationship between periodontal disease and systemic inflammatory pathologies is well established. Diabetes, rheumatoid arthritis and cardiovascular disease are among the most significant disease burdens influenced by inflammatory oral health conditions. Evidence suggests that the interaction is bi-directional, impacting progression, severity and mortality. Current models of ageing and longevity neglect an important factor in overall health and well-being, a gap that this review intends to illustrate and inspire avenues for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The attainment of very old age and accumulation of life experiences has always been fascinating to humankind. Throughout history humans have revered longevity. Elevated status of elders and ancestors in the late neolithic period or the pronounced role in culture (Xiao 2023), religion (James and Version 2004) or society (P C 2003) illustrate that point.
Research continues to examine the question: How do people achieve exceptional longevity and age successfully? Although many questions remain to be answered, metabolic dysregulation (Paolisso et al. 2000), inflammation (Arai et al. 2015; Franceschi et al. 2007), altered immune function (Salvioli et al. 2013; Wikby et al. 2005), endocrine changes (Paolisso et al. 1997; Suzuki et al. 2001) and increased oxidative stress (Belenguer-Varea et al. 2020) all seem to play a significant role in ageing. Social relationships (Holt-Lunstad et al. 2010), genetic background (Terry et al. 2004), posttranslational modifications (Ben-Avraham et al. 2017) and the interaction between our genes and the environment(Ebert et al. 2022; Christensen et al. 2006; Herskind et al. 1996a) also appear to have a large impact. Previous research has partially uncovered some influential factors, however the entire complexity of attaining extreme longevity remains elusive.
Furthermore, the factors that influence extreme longevity have received different amounts of attention, with some influential factors having been omitted. One of the neglected areas could be the importance of oral health in achieving very old age. The following narrative review will illustrate the current research in the oldest old and discuss a missing piece of the puzzle, that might help to gain a more comprehensive and deeper understanding.
How to characterize successful ageing?
Ageing is a complex process, in order to discover causative factors, a clear definition of “successful ageing” is vital. This would need to encompass the various pathways leading to exceptionally old age. In the current literature a multitude of different definitions exist, depending on the cultural, social or biological context of the examined hypothesis (Herskind et al. 1996b; Estebsari et al. 2020).
A recent review by Pignolo et. al. proposed two overarching criteria for attaining exceptional longevity: (1) biological age < chronological age, (2) slowed or delayed decline in functional status (López-Otín and Kroemer 2021). Considering the work of Rowe and Kahn, the functional aspect can be further divided into 3 subcategories: minimize risk of disease and disability, maintain physical and cognitive function and continuous engagement in life (Pignolo 2019). These assumptions seem to be the best approximation, considering they are common clinical features in centenarians. They offer potential endpoints for further research. For example, molecular tests like methylation clocks could provide additional insight and a more precise definition, as they already have in the definition of biological age (Rowe and Kahn 1997).
The ability to sustain health for longer, appears to be a core feature of exceptionally long-lived individuals. Supporting this idea, Fries et al. described a compression of morbidity, hypothesizing that the onset of chronic morbidity at a later stage in life, is greater than their gain in life expectancy, resulting in a smaller fraction of life spent with illness (Gutman et al. 2020). Recent evidence in a cohort aged 65 and over showed a reduction in disability by 2% a year (Fries 1980), and a reduced need for help with activities of daily living (ADLs), compared to the same age cohort 10 years prior (Fries 2005; Schoeni et al. 2001; Waidmann and Liu 2000; Cutler 2001; Freedman et al. 2006). These results are based on data between 1982 and 2005 (Fries 2005; Schoeni et al. 2001; Waidmann and Liu 2000; Cutler 2001; Freedman et al. 2006) and supports the notion of compressed. Further evidence suggests a slowing of this trend in recent years (Schoeni et al. 2008; Cai and Lubitz 2007; Fuller-Thomson et al. 2009).
The compression of morbidity is accentuated in increased age and therefore greatest in centenarians (Martin and Schoeni 2010), although the underlying mechanisms may differ (Andersen et al. 2012).
Lessons learned from centenarians
Due to the perceived significance of reaching 100 years, there are many non-verifiable claims of individuals reaching exceptional lifespans. The most reliable reports suggest that the oldest recorded individuals were Jeanne Calment as the longest living women, at 122 years and 165 days (Terry et al. 2008) and Jiroemon Kimura as the longest living man, at 116 years and 54 days (Robine and Allard 1998). Previously it has been estimated, that the likelihood of reaching 100 years of age for people born at the turn of the last century in North America was 7 in 1000 (0,7%). For birth cohorts born at the turn of the current millennia, it has been projected that 1 in 2 will reach the remarkable age of 100 (Gondo et al. 2017). With the increasing number of centenarians, it can be expected that the prevalence of the very rare supercentenarians (age > 110 years), currently estimated at 1 in 5 million, will increase as well (Christensen et al. 2009).
One of the major differences between centenarians and the rest of the ageing population is a pronounced compression of morbidity. Andersen et al. were able to illustrate this phenomenon in centenarians (> 100), semi-super centenarians (> 105) and supercentenarians (> 110), showing that the greatest delay in the onset of disease appeared in the oldest sub-cohorts. In this study, researchers postulated that supercentenarians sustain a health span that approximates their life span (Martin and Schoeni 2010).
People who have attained exceptional longevity may be classified by different morbidity profiles. Evert et al. described these profiles as: survivors, delayers and escapers. The cohort of survivors were those who had a diagnosis of an age-related disease before the age of 80 and survived into their hundreds. Delayers were diagnosed with an age-related disease between the ages of 80 and 100 and escapers weren´t diagnosed with an age-associated illness until they were over 100 years old (Young et al. 2010). It appears that the percentage of escapers rises with the age of the studied cohort. While 50% of centenarians in the New England Centenarian Study (NECS) were described as delayers, approximately 70% of the supercentenarians fitted the criteria for escapers (Martin and Schoeni 2010). This shows that there might be distinct differences in survival mechanisms within the oldest old.
In contrast, there are remarkable similarities between centenarians. A clustering of centenarians in certain areas of the world may be observed, these are referred to as blue zones (Evert et al. 2003).
Whilst the habitants in a blue zone may share a common genetic background, they also exhibit similarities in behavioral patterns that are considered to further longevity. These include: eating in moderation, mostly plants, exercise as part of a daily routine, purposeful living, maintaining a supportive social circle, spirituality and keeping a healthy BMI throughout their lifetime (López-Otín and Kroemer 2021; Evert et al. 2003). The overlap between nutrition and oral health is especially interesting and research has focused on analyzing the diet of the oldest old. The diet of a well renowned cluster of centenarians in Okinawa (Japan) is characterized by low energy density and is nutrient-rich, with a high content of antioxidants consisting mainly of sweet potatoes, vegetables and legumes. Additionally, estimations that this cohort managed to live with an approximately 11% caloric restriction over their lifetime exist, helping to maintain a low BMI and potentially furthering longevity (Buettner and Skemp 2016; Willcox et al. 2007; Willcox et al. 2014; Willcox et al. 2009; Willcox et al. 2017; Willcox and Willcox 2014). In contrast, recent lifestyle changes reversed this trend in younger generations, leading to increased appearance of diabetes and obesity (Willcox et al. 2009; Longo and Anderson 2022).
General hypotheses for successful ageing
As described previously, ageing is a dynamic process with a multitude of influential factors. One of the most important modulators of ageing appears to be inflammation. Arai et al. showed that low-level inflammation is the best predictor of all-cause mortality, capability and cognition in the very old and (semi-) supercentenarians (Arai et al. 2015). This landmark study confirmed the findings from younger and smaller cohorts (Willcox et al. 2012; Jenny et al. 2012; Akbaraly et al. 2013; Schnabel et al. 2013), illustrating the central role of inflammation in the ageing process. Interestingly, they managed to show that centenarians, who escaped morbidity and mortality longer, show higher levels of systemic inflammation markers. The authors theorize that whilst centenarians may be able to avoid low-grade inflammation for the majority of their life, they may still exhibit a rise in proinflammatory processes towards the end of their lifespan similar to the general population (Arai et al. 2015). Other hypotheses postulate that high levels of anti-inflammatory molecules might counteract the detrimental effect of high systemic inflammation in centenarians, therefore negating the mortality risk (Salvioli et al. 2013).
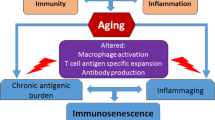
Another factor contributing to the rise of inflammatory markers in advanced age seems to be a dysregulation of the immune system and immunosenescence. In addition to the increased percentage of late T effector cells, memory T cells (Varadhan et al. 2014) myeloid cells and NK cells (Franceschi et al. 2000), the decrease in naïve lymphocytes (Varadhan et al. 2014) and B cells (Franceschi et al. 2000) can largely explain the reduced response to new antigens in old people (Salvioli et al. 2013). This results in an overall increased mortality risk (Wikby et al. 2005). Large cohort studies of elderly people in Scandinavia described an Immune Risk Phenotype (IRP), constituting an inversion of CD4/CD8 ratio, poor T cell proliferation response and persistent CMV infection, associated with increased risk of mortality (Wikby et al. 2005). Centenarians appear to be free of the IRP, implicating an impact of immundysregulation on the process of ageing (Larbi et al. 2008).
As already noted, maintaining a normal BMI seems to be a common denominator in the oldest old (Paolisso et al. 2000; Strindhall et al. 2013). Adipose tissue is known to contribute to the inflammatory load, partly due to an infiltration by macrophages, but also as a result of a metabolic reaction to food abundance (Paolisso et al. 1995). Hallmarks of this metabolically induced inflammation are: (1) orchestration by metabolic cells in response to excess energy and nutrients, (2) low grade inflammation, (3) chronic, proinflammatory milieu (Paolisso et al. 1995). This ‘metaflammation’ correlates with the onset of metabolic syndrome and Type 2 Diabetes (T2DM) (Gregor and Hotamisligil 2011). In contrast, insulin mediated glucose uptake appears to be preserved in healthy centenarians (Hotamisligil 2006), illustrating the importance of maintaining a healthy weight and body composition into old age. However, according to the obesity paradox, moderately elevated BMI is associated with a lower mortality in certain disease states e.g., cancer, end stage kidney disease and cardiovascular disease (Paolisso et al. 1996; Lennon et al. 2016; Park et al. 2014). This idea has been criticized because BMI is a relatively crude measure and fails to sufficiently incorporate the influence of body composition and metabolic health (Paolisso et al. 1996; Lavie et al. 2016; Bosello and Vanzo 2021; Elagizi et al. 2018; Caan et al. 2018).
Globally women have a far greater probability of surviving into old age (Bowman et al. 2017; Austad and Bartke 2015), although female centenarians are on average less healthier than their counterparts (Martin and Schoeni 2010; Austad and Fischer 2016). It is thought that women have the ability to deal more successfully with age-related morbidity and functional decline, that often leads to mortality in men at a younger age (Franceschi et al. 2000; Perls 1995). Additionally, it has been postulated that the relatively smaller build of a woman results in a lower GH secretion, which is positively associated with longevity (Perls and Fretts 1998; Bartke 2008; Bartke et al. 2013). Studies from nematodes (Caenorhabditis elegans), flies (Drosophila melanogaster) and mice (Mus musculus) demonstrate that a disruption of the IGF1 signaling pathway results in an increased lifespan among several species (Spoel et al. 2016; Kimura et al. 1997; Kenyon 2001). This further supports the key role of the GH-IGF1 axis in ageing. Studies of patients with Laron Syndrome (congenital IGF 1 deficiency) suggest a high degree of protection against cancer, T2DM and pro-ageing signaling (Clancy et al. 2001).
There are several genes implicated in the attainment of extreme longevity, however twin studies attribute only 25% of lifespan variance to genetic factors (Ebert et al. 2022; Christensen et al. 2006; Herskind et al. 1996a). It therefore follows that a much larger proportion must be derived through other mechanisms. In a landmark paper from López-Otín et al. from 2013, nine hallmarks of ageing have been described. These hallmarks mediate the influence that the environment has on the ageing process within the cell (Guevara-Aguirre et al. 2011).
Whilst these hallmarks partly explain the ageing process by predicting the cumulative effect of age related morbidity (López-Otín et al. 2013), other researchers have described them as insufficient (Fraser et al. 2022). At the Copenhagen ageing meeting in March 2022 these criticisms were addressed through expansion of the original hallmarks and the addition of 5 further hallmarks (Gems and Magalhães 2021).
Hallmarks of ageing
The nine original hallmarks of ageing are genomic instability, telomere attrition, epigenetic alteration, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication (Guevara-Aguirre et al. 2011). The recently added hallmarks include compromised autophagy, dysregulation of RNA splicing, microbiome disturbance, altered mechanical properties and inflammation (Gems and Magalhães 2021). The underlying assumption is that the general cause of age-related phenomena is the accretion of cellular damage in a time dependent manner (Schmauck-Medina et al. 2022; Gems and Partridge 2013; Kirkwood 2005). The hallmarks can be described as different manifestations of this lifelong attrition.
Genomic instability results from the cumulative insults to the genetic material (Vijg and Campisi 2008). It can be caused by exogenous factor e.g. physical agents or endogenous processes e.g. DNA replication errors (Moskalev et al. 2012). The damage can effect nuclear DNA, mitochondrial DNA or the integrity of the nuclear architecture itself and thereby causing age-associated pathologies (Guevara-Aguirre et al. 2011).
The notion of instability also relates to cell architecture, altering the mechanical properties of cells and tissues. Impairments of the cytoskeleton disrupt cell motility and intercellular communication (Gems and Magalhães 2021). Similarly, the age-related alterations of the extracellular matrix caused for example by glycation cross-links, have been shown to substantially alter cell behavior (Hoeijmakers 2009) and are linked to age-associated diseases (Jain 2018).
Telomeres are thought to be especially susceptible to age-related impairment. Damage present in these regions is notoriously hard to repair due to the presence of shelterins, preventing access of repair enzymes (Bahour et al. 2022). Additionally, the length of telomeres has been found to shorten with advancing age, imposing a proliferative limit (Fumagalli et al. 2012), influencing the regenerative ability of tissues (Hayflick and Moorhead 1961) and promoting senescence (Bahour et al. 2022).
Epigenetic alterations are associated with ageing (Armanios and Blackburn 2013; Fraga and Esteller 2007) Histone modification and the resulting expression of certain genes has been linked to prolonged lifespan. Sirtuins have become one of the major focus of research in this area, influencing for example genomic stability or glucose metabolism (Han and Brunet 2012; Kanfi et al. 2012, 2010). Furthermore, alteration in DNA methylation, chromatin remodeling and transcriptional alterations have been identified as targets for ageing research (Guevara-Aguirre et al. 2011).
An additional layer of dysregulation arises in RNA processing, (Zhong et al. 2010) further disrupting the control of gene expression. Alterations of mRNA influence the ageing process (Gems and Magalhães 2021) and have been implicated in cancer growth (Holly 2013) and cell senescence (Yuan et al. 2019; Shen et al. 2019).
The loss of proteostasis also appears to be a feature of ageing (Latorre et al. 2017; Powers et al. 2009). Key systems for protein stability, the chaperone system and disposal, the autophagy-lysosomal system and ubiquitin–proteasome system display a reducing activity with advancing age (Koga et al. 2011; Calderwood et al. 2009; Tomaru et al. 2012). Their reactivation has been identified as a potential target for pharmacological intervention (Rubinsztein et al. 2011).
In a recent publication by Schmauck-Medina et al. compromised autophagy is considered a hallmark in its own right (Gems and Magalhães 2021). It acts as a central component of several age-related conditions for example immunosenescence and neurodegenerative disease (Johnson et al. 2015; Wong et al. 2020). Autophagy stands at the intersection between hallmarks, influencing the regulation of a selection of cellular functions, including DNA repair, cellular stress-response and glucose and lipid metabolism (Aman et al. 2021).
Deregulated nutrient-sensing manifests itself in the aforementioned Insulin and IGF1 signaling system, which plays a role in glucose sensing, as well as other important systems that mediate the ageing process. Among these the mTOR pathway is responsible for sensing high amino acid concentrations, indicative of a fed state. In contrast, the AMPK pathway is activated by high AMP levels and sirtuins by high NAD+ levels, both of which are surrogate parameters for low energy states (Kaushik 2018). These are some of the most promising targets for research with possible dietary (Houtkooper et al. 2010; Fontana et al. 2010; Weindruch and Walford 1982; Colman et al. 2009) and pharmacological interventions (Rubinsztein et al. 2011; Yin and Klionsky 2022; Johnson et al. 2013).
Mitochondrial dysfunction appears to be a characteristic of ageing, implicating diminishing ATP production and electron leakage, due to increasing inefficiency of the respiratory chain (Walters et al. 2016). This potentially increases reactive oxygen species (ROS) to detrimental levels (Green et al. 2011) and defective mitochondrial biogenesis (Hekimi et al. 2011; Wang and Klionsky 2011). Additionally, mitochondrial homeostasis seems to be intimately intertwined with the nutrient sensing system through SIRT1 (Sahin and DePinho 2012; Rodgers et al. 2005), SIRT3 (Lee et al. 2008; Lombard et al. 2007; Giralt and Villarroya 2012) and AMPK (Qiu et al. 2010). Altered mitochondrial function represents a vital intersection between several mechanisms of ageing and may further stem cell depletion (Hawley et al. 2010; Fang et al. 2014; Scheibye-Knudsen et al. 2015).
Cellular senescence is the permanent arrest of the cell cycle with stereotypic phenotypic alterations (Lou et al. 2020; Campisi 2007; Collado et al. 2007). Telomere shortening, DNA damage outside the telomere region and increased mitogenic signaling due to for example the de-repression of the INK4/ARF locus are known to be a causative factor of cellular senescence (Campisi 2007; Kuilman et al. 2010). Senescence can be seen as a compensatory mechanism to arrest damaged cells and replace them. With increasing age, the balance of arrest and replacement is disturbed, leading to accumulated senescent cells. Due to their prolific pro-inflammatory secretory ability, they may contribute to further ageing. This phenomenon has been described as the “senescence-associated secretory phenotype” (Collado et al. 2007; Gorgoulis and Halazonetis 2010).
Stem cell exhaustion leading to reduced regenerative ability of tissue, appears to be another characteristic of the ageing process. It is thought to be the consequence of the accumulation of different insults e.g. DNA damage (Rodier and Campisi 2011) over a lifetime, (Guevara-Aguirre et al. 2011) leading to a reduced proliferation (Rodier and Campisi 2011) or inadequate hyperproliferation further depleting the stem cell reserve (Rossi et al. 2007).
Altered intercellular communication is an integrative part of ageing. Alterations appear on the neuronal, neuroendocrine and endocrine level (Rera et al. 2011; Zhang et al. 2013; Russell and Kahn 2007; Rando and Chang 2012). One of the major manifestations and drivers is inflammation (Laplante and Sabatini 2012). Other forms of cell–cell communications are known to further drive the ageing process e.g. over gap junction mediated cell–cell contact with ROS (Salminen et al. 2012) and exosome regulated communication (Nelson et al. 2012; Duggan et al. 2022; Xu and Tahara 2013). Altering intercellular communications will be an important target for potential interventions (Wang et al. 2021a; Piper et al. 2011; Sanchez-Roman et al. 2012; Loffredo et al. 2013).
In the 2022 publication by Schmauck-Medina et al. inflammation is considered a separate hallmark (Gems and Magalhães 2021). High levels of proinflammatory markers like IL-1, IL-6, C-reactive protein correlate with ageing (Arai et al. 2015; Claesson et al. 2012). Furthermore, age-dependent chronic inflammation, also known as inflammaging, has been implicated in a wide range of diseases (Franceschi et al. 2007, 2017; Laplante and Sabatini 2012; Ferrucci and Fabbri 2018; Leonardi et al. 2018).
Microbiome disturbances are thought to be associated with inflammation, this is more pronounced with age-associated structural impairment of barriers e.g. blood brain barrier (Gems and Magalhães 2021; Teissier et al. 2022). Recent advances in sequencing technology resulted in the discovery of age related changes in the gut microbiome, notably a shift of microbial populations and a reduction of diversity (Zhu et al. 2020).
Oral conditions in medicine
According to the FDI World Dental Federation: ‘Oral health is multifaceted and includes the ability to speak, smile, smell, taste, touch, chew, swallow, and convey a range of emotions through facial expressions with confidence and without pain, discomfort, and disease of the craniofacial complex.’ (Wilmanski et al. 2021) A range of disease states impact oral health, most notably dental caries, tooth loss, periodontal disease, oral and dental trauma, oral cancer, noma and birth defects (Glick et al. 2017). Oral health has been recognized as playing a substantial role in overall health, wellbeing and quality of life (Wilmanski et al. 2021; Health and [https:, , www.who.int, health-topics, oral-health#tab=tab_1; Petersen and Kwan 2009). In 2019 the Global Burden of Disease was estimated at 3.5 billion affected individuals for oral disease, one of the most common non-communicable diseases (NCDs) worldwide (Peres et al. 2019). Unfortunately, medical training often neglects oral and dental medicine. This leaves doctors with very little knowledge regarding an important influencing factor of their patients´ general health (Dörfer et al. 2017; GBD 2019).
In contrast, dentistry is moving away from a disease-based treatment model, towards individualized prevention, beginning to emphasize the systemic implications of oral disease (Doshi et al. 2019; Ahluwalia et al. 2016).
In addition, research regarding the relationship between periodontal disease and systemic conditions has moved into the focus of dental research, with links to as many as 57 systemic diseases currently under investigation (Schmalz et al. 2020). In general, periodontal disease can be described as a dynamic process leading to a dysbiosis resulting in chronic inflammation and destruction of the tooth supporting tissue (gingiva, periodontal ligament, alveolar bone) (Schmalz and Ziebolz 2020). (Fig. 1) During this process pathogens and inflammatory molecules may leak into the blood stream and potentially aggravate other present systemic diseases (Fig. 2). The bi-directional association between T2DM and periodontal disease is widely accepted (Loos 2016). In non-diabetic individuals the presence of periodontitis is associated with higher HbA1c and a higher risk of developing pre-diabetes and diabetes (Meyle and Chapple 2000). If diabetes is present, periodontitis is associated with poorer glycemic control and a higher risk of complications, including diabetic retinopathy, cardiovascular disease, ischemic stroke, neuropathic foot ulcers and chronic kidney disease (Lalla and Papapanou 2011; Graziani et al. 2018; Sanz et al. 2018, 2020; Borgnakke and Poudel 2021; Song et al. 2021; Dyke et al. 2021; Borgnakke et al. 2015; Nguyen et al. 2020; Zhao et al. 2020). Poor diabetic control is linked to accelerated attachment loss (Lalla and Papapanou 2011; Alvarenga et al. 2020). Obesity and metabolic syndrome have also been implicated with periodontal disease, possibly through the creation of a pro-inflammatory state (Genco and Borgnakke 2000; Demmer et al. 2012). The association between rheumatoid arthritis and periodontal disease is well documented (Arana et al. 2017; Dursun et al. 2016; Potempa et al. 2017). A common denominator is the presence of IL-1beta and tumor necrosis factor-alpha in both rheumatoid arthritis and periodontal disease (Potempa et al. 2017; Cheng et al. 2017), which also has been described as a feature of immunosenescence (Kaur et al. 2013; Mirrielees et al. 2010).
Current model for host bacteria interaction in the pathogenesis of Periodontitis. Extensive biomass drives a proportionate immune response resulting in gingivitis. Without the removal of the biofilm an increase in pathogenicity occurs, resulting in tissue destruction and periodontitis. PMNs polymorphonuclear neutrophils, AMPs antimicrobial peptides, fMLP N-formylmethionyl-leucyl-phenylalanin, LPS lipopolysaccharide, GCF gingival crevicular fluid, DAMPs damage-associated molecular patterns, MMPs matrix metalloproteinases (Schmalz and Ziebolz 2020)
Periodontitis and systemic implications. Periodontitis occurs as a consequence of the imbalance between the subgingival microbiota and the host immune response (Gondo et al. 2006; Arnold et al. 2010; Hajishengallis 2021). The commensal bacteria population shifts into a pathogenic dysbiotic state (Dyke et al. 2020), stimulating an inflammatory response. This results in a local accumulation of leucocytes, specifically neutrophiles (Gondo et al. 2006). They contribute to the tissue destruction through the secretion of Matrix-Metalloproteinases (MMPs) and reactive oxygen species (ROS) (Lamont et al. 2018; Curtis et al. 2000). The failure to control the dysbiotic environment leads to tissue penetration of bacteria. Through the interaction with macrophages and dendritic cells an increased production of cytokines, e.g. Interleukin 1 (IL1), Interleukin 6 (IL6) and tumor necrosis factor alpha (TNF ⍺) occurs. Furthermore, proinflammatory cytokines promote chemotaxis, increasing immune cell recruitment and aggravating the inflammatory process (Curtis et al. 2000; Hajishengallis and Hajishengallis 2014). 1) In periodontitis the risk of bacteraemia and systematic dissemination of bacteria is increased (Hajishengallis 2014). a In addition, further dissemination occurs through survival in phagocytic host cells. Consequently, periodontal bacteria can be found in atherosclerotic lesions, possibly aggravating the process (Pelletier et al. 2010). 2) The local inflammatory response consisting of IL 1, IL6 and TNF ⍺ can enter the circulation and possibly induces changes in the b liver. Thus, leading to metabolic dysfunction expressed as insulin resistance (Gondo et al. 2006; Schenkein et al. 2000) and potentially triglyceride accumulation (Carrion 2012). This process aggravates atherogenesis. Additionally, circulation cytokines may induce an acute phase reaction in the liver, which in turn is marked by elevated levels of C reactive Protein, Fibrinogen and Serum Amyloid A, furthering the atherosclerotic processes. 3) The swallowing of large quantities of bacteria present in periodontitis, is known to influence the gut microbiome, c leading to dysbiosis (Gondo et al. 2006; Jepsen et al. 2000). Altered gut microbiota has been linked to increased gut permeability and endotoxaemia due to an induced downregulation of tight junction proteins (Arimatsu et al. 2014; Yamazaki et al. 2021; Cani et al. 2008 Kashiwagi et al. 2021), further promoting a systemic inflammatory state (Jepsen et al. 2000). Dysbiosis has been related to changes in metabolites produced by the microbiota, which may increase insulin resistance independent of the effect of proinflammatory cytokines induced by the endotoxaemia (Arimatsu et al. 2014)
Proinflammatory states in patients with periodontitis have been associated with a stronger inflammatory response when challenged with bacteria or lipopolysaccharides (LPS) compared to healthy individuals (Fagiolo et al. 1993). Some research has suggested that this increased response could be the result of hyperreactive myeloid cells. This may be induced by epigenetic rewiring, following changes in cell metabolism, caused by bacteremia and systemic inflammation present in periodontitis (Zanni et al. 2003; Ling et al. 2015; Barutta et al. 2022; Netea et al. 2020). This process causes a prolonged hyperactivation of the innate immune response, recently described as “trained immunity” (Ling et al. 2015) and potentially increases inflammation and metabolic degradation present in periodontitis. Furthering immune cell activation and tissue destruction, reactive oxygen species (ROS) have been identified as playing a crucial role in periodontal inflammation (Saeed et al. 2014).
This offers a glimpse into the possible role of inflammation in periodontal disease and the ageing process as a potential point of interconnection.
With age being a major influence on oral conditions and tooth loss (Bekkering et al. 2018; Sczepanik et al. 2000; Kassebaum et al. 2014; Frazão et al. 2003; Barbato and Peres 2015; Silva et al. 2009), remarkably little research has been done in the cohort ageing most successfully.
Oral conditions in centenarians: current state of knowledge
Oral medicine for the elderly encompasses a particular set of challenges. Influential factors include comorbidities, polypharmacy, age-associated cognitive impairment and a lower priority in an institutionalized setting (Haugejorden et al. 2003; Fure 2003; Schmalz et al. 2021). The edentulous rate in the elderly varies between 22 and 64% (Wong et al. 2019; Rantzow et al. 2018; Ziebolz et al. 2017; Fitzpatrick 2000; Hopcraft et al. 2012; Simunković et al. 2005; Rabiei and Kasemnezhad 2010) and is an important risk factor for malnutrition (Haugejorden et al. 2003; Wong et al. 2019; Northridge et al. 2012; Montal et al. 2006). Additionally, for those with remaining teeth, a high burden of periodontal disease and increased need for general dental treatment has been reported (Lamy et al. 1999; Cousson et al. 2012). Advancing age may also reduce the capacity to perform sufficient oral hygiene (Rantzow et al. 2018).
Very few publications on the oral health of centenarians exist. A systematic review from 2020 revealed only two papers fitting the search criteria (Jordan 2016). Since then, the same workgroup has published some of the first studies investigating the oral conditions of centenarians. They managed to show that a surprisingly large percentage of centenarians still had teeth (64%) with the mean number of teeth being 9.1 SD 7.1. Furthermore, most centenarians showed no or only moderate periodontal disease, with a minority of 19% having severe periodontitis (Micheelis 2011; Frese et al. 2020). Other recent studies in a different cohort found higher rates of edentulousness, suggesting that the cultural background may play a pivotal role in interpreting this data (Sekundo et al. 2020a). Although these findings are important, further research is needed to establish the complex interaction between systemic inflammation, nutrition and oral health.
Albani A et al. examined two studies from England (New Castle 85 + Study) and Japan (TOOTH) to illuminate the connection between oral and overall health. In the Newcastle cohort difficulty eating food due to dental problems, was associated with higher odds of frailty, mobility limitations, slower gait speed and weaker grip strength. For the TOOTH cohort: frailty, mobility limitations and slow gait speed showed a similar association. The risk of frailty was increased by two-thirds in the subcohort with no natural teeth remaining (Sekundo et al. 2020b). Although having a cross-sectional study design and distinct differences existing in the two studied cohorts e.g. edentulous rate, this publication still demonstrates an association between oral health and overall function (Sekundo et al. 2020b).
It must be noted that these studies were performed in people under 100 years and therefore the results may not necessarily apply to centenarians or (semi-)supercentenarians. More research is needed to understand the dental and periodontal health of the oldest people in our societies. Currently oral health and illness aren´t integrated into the complex construct of successful ageing.
Synthesis of oral health and successful ageing
To attain extreme longevity, maintenance of homeostasis appears to be a prerequisite. The ageing process can be approached as web of complex interactions between several factors (see Fig. 3). These might be attenuated by life-style choices, genetic predispositions and social support (see Fig. 3) The factors may either have a direct effect on the deterioration of the organism or influence ageing potentially through one or several processes described in the hallmarks of ageing. In the research by López-Otín et al. primary hallmarks include genomic instability, telomere attrition, epigenetic alterations and loss of proteostasis. They result from insults to the body leading to negative consequences. Antagonistic hallmarks can be seen as protective compensatory mechanisms responding to damage. These initially protective processes become detrimental to cellular function. Deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence are included in this category. Finally, altered intercellular communication and stem cell exhaustion are integrative hallmarks. They are the result of an accumulation of primary damage and insufficient compensatory mechanisms, exhausting the organisms’ regenerative abilities (Guevara-Aguirre et al. 2011). Inflammation and altered mechanical properties of the cellular structure are two newly proposed hallmarks. They are derived from alterations in intercellular communication and can therefore be classified as integrative hallmarks. Similarly, autophagy is responsible for regulating various hallmarks and hence is considered within the same category (Gems and Magalhães 2021).
Interconnections between ageing related processes and the potential role of oral health. 1) In ageing, multiple factors within a variety of systems are in a state of dynamic change. These links have been established between inflammation, immune function, endocrine changes, genetics/epigenetics and metabolic changes. 2) Beneficial behavioral patterns e.g. frequent exercise have multiple positive effects. In the case of exercise; reduced inflammation, improved immune function, enhanced hormonal health, engaged epigenetic mechanisms improving memory function and better insulin sensitivity have been reported. Similar links exist for several other behavioral patterns e.g. social support, diet, purposeful living and spirituality. Oral health has an important role in preventing age related decline. 3) Oral disease is associated with reduced masticatory function, leading to an impaired nutrient intake, especially protein. Periodontal disease increases the risk of T2DM and prediabetes in non-diabetics and has been linked to poorer glycemic control and a higher complication rate in diabetic patients. The presence of a proinflammatory state, characterized by interleukine-1beta and tumor necrosis factor-alpha, potentially influences autoimmune disease e.g. rheumatoid arthritis and immune senescence. Bacterial antigens and cytokines trigger changes in the cell metabolism of myeloid cells, leading to epigenetic changes, which ultimately enhance the inflammatory response. Recent research suggests that ageing is not only expressed on the macroscopic scale but also on the molecular level, providing possible targets for pro longevity interventions
Considering the strong link between inflammatory processes (Potempa et al. 2017; Cheng et al. 2017) and dental pathologies resulting in difficulties eating food and functionality in advanced age (Sekundo et al. 2020b; Beker et al. 2019; Albani et al. 2021), oral health is important in the complex web of interactions.(see Fig. 3) It is vital in order to meet the nutritional requirements to sustain a healthy body (Sekundo et al. 2020b). For example, protein appears to be a key nutrient in maintaining muscle mass and reducing frailty (Hakeem et al. 2019; Tôrres et al. 2015; Wolfe 2012). Poor masticatory function leads to a reduction in protein intake, which could have a significant effect on morbidity and mortality (Wolfe et al. 2008; Coelho-Júnior et al. 2020; Motokawa et al. 2021).
Furthermore, periodontal disease is common in the elderly (Petersen and Kwan 2010). The pathological process in periodontitis is characterized by inflammation. It is conceivable that an improved ability to deal with low grade inflammation also results in lower levels of periodontal disease. The periodontal environment offers a unique portal of interaction between the immune system and bacterial flora. Oral health or a low grade of disease could be a potential surrogate for the bodies capabilities to deal with inflammatory processes in general. It is unclear at this point whether periodontitis is purely a feature of ageing.
An inflammatory state is central to many non-communicable diseases and may be the common denominator in these pathologies (Okamoto et al. 2019).
Attaining extreme longevity could be the result of an extraordinary capacity to deal with inflammation, preventing the establishment of systemic inflammation until very late in life.
Ageing is a dynamic process with multiple factors influencing a system striving for balance. (Fig. 4) The human body aims to re-establish homeostasis when unbalanced by stressors.
Ageing: balance and imbalance. The human body seeks a homeostatic state. 1) Stressors (symbolized by weights) influence the biological state. Normally the organism has the capability to maintain balance. 2) Insults e.g., disease, injuries, famine, etc. force the system out of its equilibrium. The impact of the stressing event has been symbolized by the amplitude A1. In the young resilient body, a conservation of a relative state of balance is often possible, offering the opportunity to regain an equilibrium e.g., fight disease, heal injuries, refeeding. 3) Ageing increases the length of the lever, thus reducing resilience and leaving the organism more susceptible to insults. *Genetics, good dietary habits, exercise, purposeful living and social support can act as pillars of support, counteracting the increased vulnerability and enhancing the organisms´ compensatory capacity. 4) Due to the increased susceptibility, the same insult results in a much larger negative impact (amplitude A2 > A1). This leads to a state of imbalance, exceeding the capacity of compensatory mechanisms. This results in incomplete recovery, chronic disease, frailty or death
The older an individual becomes, the more difficult it is for the body to deal with insults, hence the greater the impact on the organism.
Certain behavioral patterns, like regular exercise, diet or purposeful living act as support to give the system more resilience. They act as pillars supporting the system. Retaining good oral health may be a supporting pillar enabling successful ageing.
Implications for future research
As the communication between medical and dental research develops, connections between the fields will increase and ultimately contribute to a better understanding of the human body. Within centenarian research only a handful of studies take oral health into consideration (Micheelis 2011; Frese et al. 2020; Sekundo et al. 2020a; Nomura et al. 2020; Fabbri and Rabe 2007; Kaufman et al. 2014). The relationship between oral health and its potential influence on other modifying factors of ageing remains understudied.
Oral health could be an aggravating/complicating factor unbalancing the system. The masticatory system stands at the crossroads between nutrient uptake, inflammation and metabolic processes. Factors like IL-1beta are linked to periodontitis, systemic inflammation and immunosenescence (Potempa et al. 2017; Cheng et al. 2017; Kaur et al. 2013; Mirrielees et al. 2010). The role of oral health within this complex interaction needs to be investigated in centenarians.
There is a bi-directional interaction between systemic and oral inflammation. Inflammation acts as a potential accelerator, surrogate and end stage in the ageing process and therefore offers great potential for further exploration. Possible routes to consider are the implications of the molecular mechanisms of ageing in relation to periodontitis. Research has already begun to explore some of these avenues.
Links have been established between genomic instability and periodontal disease (Qiu et al. 2000; Xu et al. 2021). Epigenetic alterations and their role in periodontal disease are a focus in recent publications (Geng et al. 2020; Borba et al. 2019; Zhang et al. 2021; Jiang et al. 2022; Wang et al. 2021b). The implications of deregulated nutrient sensing and cellular senescence have also been explored (Coêlho et al. 2020; Azevedo et al. 2020; Zheng et al. 2019; Yang et al. 2021; Chen et al. 2021).
Another interesting avenue to examine the links between periodontitis and the molecular mechanisms of ageing is the aged mouse model. Research by Liang et al. suggests that aged mice offer a sufficient and low-cost model to research chronic periodontitis, (Kuang et al. 2020) whilst offering the opportunity to study mechanistical influences and interventions (Kuang et al. 2020; Zayed et al. 2020). Remarkable findings using this model demonstrated that a short-term inhibition of mTORC1 through rapamycin could reverse periodontal inflammation, periodontal bone loss and pathogenic microbial alterations (Zayed et al. 2020). Most aged mice models aim for a survival rate of 85–90%, (Liang 2010; An et al. 2020) providing the use-case in geriatric dentistry. However, this doesn´t necessarily represent the very exclusive cohort of centenarians, highlighting the need for focused dental research in this unique cohort.
Centenarians as models of successful ageing display low levels of inflammation over their lifespan. This suggests that they are better able to keep their system balanced despite insults. Considering the lack of data regarding probable differences in inflammatory markers, healthy centenarians are an intriguing cohort to study the relationship of inflammation and periodontal disease. Considering the role inflammation plays in the ageing process, investigating the prerequisite processes (hallmarks of ageing) could provide a better understanding of the relationship between oral disease and ageing.
Centenarians are a very exclusive cohort and the main challenge lies in the recruitment process. In previous studies the recruitment rate ranged between 13 and 97% (Frese et al. 2020; Nomura et al. 2020; Ono et al. 2021; Flurkey and J M., and Harrison, D E, 2007; Arai et al. 2010; Feng et al. 2022; Rong et al. 2019; Hartvigsen and Christensen 1976; Frisoni 2001). Notably, sampling method, recruitment strategy, invasiveness/effort of examinations and cultural background, differed between the studies, potentially accounting for the wide range of successful recruitment. Therefore, the study design should provide a minimal threshold, ideally visitations at home with a maximum time demand of 2–3 h. Considering the challenges of the recruitment process, it seems prudent to try to address as many scientifical questions as possible. This could be achieved by combing different diagnostical tools e.g., dental examination and measurement of inflammatory molecules in the blood during the same visitation.
Conclusion
This review illustrates that oral health plays a significant role in reaching exceptional longevity, especially considering its influence on nutrient uptake (Wolfe et al. 2008; Coelho-Júnior et al. 2020; Motokawa et al. 2021). Ageing research has greatly advanced following the landmark paper describing the hallmarks of ageing by establishing a common language and concept of the ageing process (Guevara-Aguirre et al. 2011; Gems and Magalhães 2021) Aspects of the ageing process have been examined in centenarians, most notably the impact of chronic inflammation (Arai et al. 2015). Inflammation plays a key role in the pathogenesis of periodontal disease and its’ relation to other systemic comorbidities, (Zanni et al. 2003; Gondo et al. 2006) however the link between oral health and successful ageing remains underexplored. More research is needed to get a deeper understanding of the relationship between oral health and other processes known to play an important role in ageing. Translating and synthesizing this knowledge will be a challenge, but must include dental research in order to see the full picture. Similar to the Greek on the rosetta stone, which acted as the key in deciphering the hieroglyphs, oral health could be a translational piece to understand the process of ageing.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADLs:
-
Activities of daily living
- AMP:
-
Adenosine monophosphate
- AMPK:
-
5’AMP-activated protein kinase
- ATP:
-
Adenosine triphosphate
- BMI:
-
Body mass index
- CD:
-
Cluster of differentiation
- CMV:
-
Cytomegalovirus
- DNA:
-
Desoxyribonucleic acid
- FDI:
-
Fédération Dentaire Internationale
- GH:
-
Growth hormone
- HbA1c:
-
Hemoglobin A1c
- IGF1:
-
Insulin-like growth factor 1
- IL:
-
Interleukine
- INK4:
-
Inhibitors of CDK4
- IRP:
-
Immune risk phenotype
- LPS:
-
Lipopolysaccharides
- mRNA:
-
Messenger ribonucleic acid
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- NAD:
-
Nicotinamide adenine dinucleotide
- NCDs:
-
Non-communicable diseases
- NECS:
-
New England Centenarian study
- NK cell:
-
Natural killer cell
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- SIRT:
-
Sirtuin
- SD:
-
Standard deviation
- T2DM:
-
Type 2 Diabetes mellitus
- TOOTH:
-
The Tokyo Oldest Old Survey on Total Health
References
Ahluwalia A, Crossman T, Smith H (2016) Current training provision and training needs in oral health for UK general practice trainees: survey of general practitioner training programme directors. BMC Med Educ 16:142
Akbaraly TN, Hamer M, Ferrie JE, Lowe G, Batty GD, Hagger-Johnson G, Singh-Manoux A, Shipley MJ, Kivimäki M (2013) Chronic inflammation as a determinant of future aging phenotypes. CMAJ 185(16):E763-770
Albani V, Nishio K, Ito T, Kotronia E, Moynihan P, Robinson L, Hanratty B, Kingston A, Abe Y, Takayama M et al (2021) Associations of poor oral health with frailty and physical functioning in the oldest old: results from two studies in England and Japan. BMC Geriatr 21(1):187
Alvarenga MOP, Miranda GHN, Ferreira RO, Saito MT, Fagundes NCF, Maia LC, Lima RR (2020) Association between diabetic retinopathy and periodontitis-a systematic review. Front Public Health 8:550614
Aman Y, Schmauck-Medina TAO, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras KAO, Simonsen A, Johansen T, Tavernarakis NAO et al (2021) Autophagy in healthy aging and disease. Nat Aging 1(8):634–650
An JAO, Kerns KAO, Ouellette A, Robinson L, Morris HAO, Kaczorowski C, Park SI, Mekvanich T, Kang A, McLean JAO et al (2020) Rapamycin rejuvenates oral health in aging mice. Elife. https://doi.org/10.7554/eLife.54318
Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT (2012) Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci 67(4):395–405
Arai Y, Iinuma T, Takayama M, Abe Y, Fukuda R, Ando J, Ohta K, Hanabusa H, Asakura K, Nishiwaki Y et al (2010) The Tokyo oldest old survey on total health (TOOTH): a longitudinal cohort study of multidimensional components of health and well-being. BMC Geriatr. https://doi.org/10.1186/1471-2318-10-35
Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, Suematsu M, Hirose N, von Zglinicki T (2015) Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2(10):1549–1558
Arana C, Moreno-Fernández AM, Gómez-Moreno G, Morales-Portillo C, Serrano-Olmedo I, de la Cuesta Mayor MC, Martín Hernández T (2017) Increased salivary oxidative stress parameters in patients with type 2 diabetes: relation with periodontal disease. Endocrinol Diabetes Nutr 64(5):258–264
Arimatsu K, Yamada H, Miyazawa H, Minagawa T, Nakajima M, Ryder MI, Gotoh K, Motooka D, Nakamura S, Iida T, Yamazaki K (2014) Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 4:4828. https://doi.org/10.1038/srep04828
Armanios M, Blackburn EH (2013) The telomere syndromes. Nat Rev Genet. https://doi.org/10.1038/nrg3436
Arnold J, Dai J, Nahapetyan L, Arte A, Ma J, Hausman D, Wl R, Hensley R, Martin P, Macdonald Davey A et al (2005) Predicting successful aging in a population-based sample of georgia centenarians. Curr Gerontol Geriatr Res. https://doi.org/10.1155/2010/989315
Austad SN, Bartke A (2015) Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology 62(1):40–46
Austad SN, Fischer KE (2016) Sex differences in lifespan. Cell Metab 23(6):1022–1033
Azevedo AM, Carvalho Rocha LP, de Faria Amormino SA, Cavalieri Gomes C, Ornelas Dutra W, Santiago Gomez R, da Costa JE, Rocha Moreira P (2020) DNA methylation profile of genes related to immune response in generalized periodontitis. J Periodontal Res 55(3):426–431
Bahour N, Cortez B, Pan H, Shah H, Doria A, Aguayo-Mazzucato CAO (2022) Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience 44(1):415–427
Barbato PR, Peres KG (2015) Contextual socioeconomic determinants of tooth loss in adults and elderly: a systematic review. Rev Bras Epidemiol 18(2):357–371
Bartke A (2008) Growth hormone and aging: a challenging controversy. Clin Interv Aging 3(4):659–665
Bartke A, Sun LY, Longo V (2013) Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev 93(2):571–598
Barutta F, Bellini S, Durazzo M, Gruden G (2022) Novel insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines. https://doi.org/10.3390/biomedicines10010178
Beker N, van der Maarel-Wierink CD, de Baat C, Holstege H (2019) Self-reported oral health in the Dutch 100-plus Study of cognitively healthy centenarians: an observational cohort study. BMC Geriatr 19(1):355
Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden C, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT et al (2018) Metabolic induction of trained immunity through the mevalonate pathway. Cell 172(1–2):135-146.e139
Belenguer-Varea Á, Tarazona-Santabalbina FJ, Avellana-Zaragoza JA, Martínez-Reig M, Mas-Bargues C, Inglés M (2020) Oxidative stress and exceptional human longevity: Systematic review. Free Radic Biol Med 149:51–63
Ben-Avraham D, Govindaraju DR, Budagov T, Fradin D, Durda P, Liu B, Ott S, Gutman D, Sharvit L, Kaplan R et al (2017) The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci Adv 3(6):e1602025
Borba TT, Molz P, Schlickmann DS, Santos C, Oliveira CF, Prá D, Neto LK, Franke SIR (2019) Periodontitis: Genomic instability implications and associated risk factors. Mutat Res Genet Toxicol Environ Mutagen 840:20–23
Borgnakke WS, Anderson PF, Shannon C, Jivanescu A (2015) Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep 15(11):93
Borgnakke WS, Poudel P (2021) Diabetes and oral health: summary of current scientific evidence for why transdisciplinary collaboration is needed. Front Dental Med. https://doi.org/10.3389/fdmed.2021.709831
Bosello O, Vanzo A (2021) Obesity paradox and aging. Eat Weight Disord 26(1):27–35
Bowman K, Atkins JL, Delgado J, Kos K, Kuchel GA, Ble A, Ferrucci L, Melzer D (2017) Central adiposity and the overweight risk paradox in aging: follow-up of 130,473 UK Biobank participants. Am J Clin Nutr 106(1):130–135
Britannica, The Editors of Encyclopaedia. "xiao". Encyclopedia Britannica, 18 Feb. 2023, https://www.britannica.com/topic/xiao-Confucianism. Accessed 28 June 2023
Buettner D, Skemp S (2016) Blue zones: lessons from the world’s longest lived. Am J Lifestyle Med 10(5):318–321
Caan BJ, Cespedes Feliciano EM, Kroenke CH (2018) The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res 78(8):1906–1912
Cai L, Lubitz J (2007) Was there compression of disability for older Americans from 1992 to 2003? Demography 44(3):479–495
Calderwood SK, Murshid A, Prince T (2009) The shock of aging: molecular chaperones and the heat shock response in longevity and aging–a mini-review. Gerontology. https://doi.org/10.1159/000225957
Campisi J (2007) d’Adda di Fagagna F: cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. https://doi.org/10.2337/db07-1403
Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, Cutler CW (2012) Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol 189(6):3178–3187
Chen J, Zhang Y, Gao J, Li T, Gan X, Yu H (2021) Sirtuin 3 deficiency exacerbates age-related periodontal disease. J Periodontal Res 56(6):1163–1173
Cheng Z, Meade J, Mankia K, Emery P, Devine DA (2017) Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Pract Res Clin Rheumatol 31(1):19–30
Christensen K, Johnson TE, Vaupel JW (2006) The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet 7(6):436–448
Christensen K, Doblhammer G, Rau R, Vaupel JW (2009) Ageing populations: the challenges ahead. Lancet 374(9696):1196–1208
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O et al (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488(7410):178–184
Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L (2001) Extension of life-span by loss of CHICO, a drosophila insulin receptor substrate protein. Science 292(5514):104–106
Coêlho MC, Queiroz IC, Viana Filho JMC, Aquino SG, Persuhn DC, Oliveira NFP (2020) miR-9-1 gene methylation and DNMT3B (rs2424913) polymorphism may contribute to periodontitis. J Appl Oral Sci 28:e20190583
Coelho-Júnior HJ, Calvani R, Picca A, Gonçalves IO, Landi F, Bernabei R, Cesari M, Uchida MC, Marzetti E (2020) Protein-related dietary parameters and frailty status in older community-dwellers across different frailty instruments. Nutrients 12(2):508
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130(2):223–233
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325(5937):201–204
Cousson PY, Bessadet M, Nicolas E, Veyrune JL, Lesourd B, Lassauzay C (2012) Nutritional status, dietary intake and oral quality of life in elderly complete denture wearers. Gerodontology 29(2):e685-692
Curtis MA, Diaz PI, Van Dyke TE (2020) The role of the microbiota in periodontal disease. Periodontol 2000 83(1):14–25
Cutler DM (2001) Declining disability among the elderly. Health Aff (millwood) 20(6):11–27
Demmer RT, Holtfreter B, Desvarieux M, Jacobs DR Jr, Kerner W, Nauck M, Völzke H, Kocher T (2012) The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the study of health in pomerania (SHIP). Diabetes Care 35(10):2036–2042
Dörfer C, Benz C, Aida J, Campard G (2017) The relationship of oral health with general health and NCDs: a brief review. Int Dent J 67(Suppl 2):14–18
Doshi M, Weeraman M, Mann J (2019) A survey of the knowledge of junior doctors in managing oral conditions in adult inpatients. Br Dent J 227(5):393–398
Duggan MR, Lu A, Foster TC, Wimmer M, Parikh V (2022) Exosomes in age-related cognitive decline: mechanistic insights and improving outcomes. Front Aging Neurosci 14:834775
Dursun E, Akalin FA, Genc T, Cinar N, Erel O, Yildiz BO (2016) Oxidative stress and periodontal disease in obesity. Medicine 95(12):e3136
Ebert T, Tran N, Schurgers L, Stenvinkel P, Shiels PG (2022) Ageing - oxidative stress, PTMs and disease. Mol Aspects Med 86:101099
Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV (2018) An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis 61(2):142–150
Estebsari F, Dastoorpoor M, Khalifehkandi ZR, Nouri A, Mostafaei D, Hosseini M, Esmaeili R, Aghababaeian H (2020) The concept of successful aging: a review article. Curr Aging Sci. https://doi.org/10.2174/1874609812666191023130117
Evert J, Lawler E, Bogan H, Perls T (2003) Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci 58(3):232–237
Fabbri LM, Rabe KF (2007) From COPD to chronic systemic inflammatory syndrome? Lancet 370(9589):797–799
Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R (1993) Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 23(9):2375–2378
Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA (2014) Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157(4):882–896
Feng L, Chu Z, Quan X, Zhang Y, Yuan W, Yao Y, Zhao Y, Fu S (2022) Malnutrition is positively associated with cognitive decline in centenarians and oldest-old adults: a cross-sectional study. EClinicalMedicine. https://doi.org/10.1016/j.eclinm.2022.101336
Ferrucci L, Fabbri E (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15(9):505–522
Fitzpatrick J (2000) Oral health care needs of dependent older people: responsibilities of nurses and care staff. J Adv Nurs 32(6):1325–1332
Flurkey KC, Harrison DE (2007) Mouse models in aging research. In: Fox JGBS, Davisson MT, Newcomer CE, Quimby FW, Smith AL (eds) The-mouse-in-biomedical-research normative biology husbandry and models. Elsevier, Amsterdam, pp 637–672
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span–from yeast to humans. Science 328(5976):321–326
Fraga MF, Esteller M (2007) Epigenetics and aging: the targets and the marks. Trends Genet 8:413–418
Franceschi C (2000) Bonafè M Fau - Valensin S, Valensin S: Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. https://doi.org/10.1016/S0264-410X(99)00513-7
Franceschi C, Motta L, Valensin S, Rapisarda R, Franzone A, Berardelli M, Motta M, Monti D, Bonafè M, Ferrucci L et al (2000) Do men and women follow different trajectories to reach extreme longevity? Italian multicenter study on centenarians (IMUSCE). Aging (milano) 12(2):77–84
Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S (2017) Inflammaging and “Garb-aging.” Trends Endocrinol Metab 28(3):199–212
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M et al (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128(1):92–105
Fraser HAOX, Kuan V, Johnen R, Zwierzyna M, Hingorani AD, Beyer AAO, Partridge LAO (2022) Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell. https://doi.org/10.1111/acel.13524
Frazão P, Antunes JLF, Narvai PC (2003) Perda dentária precoce em adultos de 35 a 44 anos de idade: Estado de São Paulo, Brasil, 1998. Rev Bras Epidemiol 6(1):49–57
Freedman VA, Agree EM, Martin LG, Cornman JC (2006) Trends in the use of assistive technology and personal care for late-life disability, 1992–2001. Gerontologist 46(1):124–127
Frese C, Zenthöfer A, Aurin K, Schoilew K, Wohlrab T, Sekundo C (2020) Oral health of centenarians and supercentenarians. J Oral Sci 62(1):9–12
Fries JF (1980) Aging, natural death, and the compression of morbidity. N Engl J Med 303(3):130–135
Fries JF (2005) Frailty, heart disease, and stroke: the compression of Morbidity paradigm. Am J Prev Med 29(5 Suppl 1):164–168
Frisoni GB, Louhija J, Geroldi C, Trabucchi M (2001) Longevity and the epsilon2 allele of apolipoprotein E: the finnish centenarians study. J Gerontol A Biol Sci Med Sci 56(2):M75-78
Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M (2009) Basic ADL disability and functional limitation rates among older AMERICANS from 2000–2005: the end of the decline? J Gerontol A Biol Sci Med Sci 64(12):1333–1336
Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14(4):355–365
Fure S (2003) Ten-year incidence of tooth loss and dental caries in elderly Swedish individuals. Caries Res 37(6):462–469
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Reference Life Table. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME), 2021. In.
Gems D, Partridge L (2013) Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75:621–644
Gems D, de Magalhães JP (2021) The hoverfly and the wasp: a critique of the hallmarks of aging as a paradigm. Ageing Res Rev. https://doi.org/10.1016/j.arr.2021.101407
Genco RJ, Borgnakke WS (2020) Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000 83(1):40–45
Geng F, Zhang Y, Lu Z, Zhang S, Pan Y (2020) Fusobacterium nucleatum caused dna damage and promoted cell proliferation by the Ku70/p53 pathway in oral cancer cells. DNA Cell Biol 39(1):144–151
Giralt A, Villarroya F (2012) SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J 444(1):1–10
Glick M, Williams DM, Kleinman DV, Vujicic M, Watt RG, Weyant RJ (2017) A new definition for oral health developed by the FDI World Dental Federation opens the door to a universal definition of oral health. J Public Health Dent. https://doi.org/10.1016/j.jdent.2016.12.005
Gondo Y, Hirose N, Arai Y, Inagaki H, Masui Y, Yamamura K, Shimizu K, Takayama M, Ebihara Y, Nakazawa S, Kitagawa K et al (2006) Functional status of centenarians in Tokyo, Japan: developing better phenotypes of exceptional longevity. J Gerontol A Biol Sci Med Sci 61(3):305–310
Gondo Y, Hirose N, Yasumoto S, Arai Y, Saito Y (2017) Age verification of the longest lived man in the world. Exp Gerontol 99:7–17
Gorgoulis VG, Halazonetis TD (2010) Oncogene-induced senescence: the bright and dark side of the response. Curr Opin Cell Biol 22(6):816–827
Graziani F, Gennai S, Solini A, Petrini M (2018) A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J Clin Periodontol 45(2):167–187
Green DR, Galluzzi L, Kroemer G (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333(6046):1109–1112
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S et al (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3(70):70ra13
Gutman D, Rivkin E, Fadida A, Sharvit L, Hermush V, Rubin E, Kirshner D, Sabin I, Dwolatzky T, Atzmon G (2020) Exceptionally long-lived individuals (ELLI) demonstrate slower aging rate calculated by DNA methylation clocks as possible modulators for healthy longevity. Int J Mol Sci. https://doi.org/10.3390/ijms21020615
Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35(1):3–11
Hajishengallis GAO, Chavakis TAO (2021) Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 21(7):426–440
Hajishengallis E, Hajishengallis G (2014) Neutrophil homeostasis and periodontal health in children and adults. J Dent Res 93(3):231–237
Hakeem FF, Bernabé E, Sabbah W (2019) Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology 36(3):205–215
Han S, Brunet A (2012) Histone methylation makes its mark on longevity. Trends Cell Biol 1:42–49
Hartvigsen J, Christensen K (2008) Pain in the back and neck are with us until the end: a nationwide interview-based survey of Danish 100-year-olds. Spine. https://doi.org/10.1097/BRS.0b013e31816b45f1
Haugejorden O, Klock KS, Trovik TA (2003) Incidence and predictors of self-reported tooth loss in a representative sample of Norwegian adults. Commun Dent Oral Epidemiol 31(4):261–268
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11(6):554–565
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hekimi S, Lapointe J, Wen Y (2011) Taking a “good” look at free radicals in the aging process. Trends Cell Biol 21(10):569–576
Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW (1996a) The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet 97(3):319–323
Herskind AM, McGue M, Iachine IA, Holm N, Sørensen TI, Harvald B, Vaupel JW (1996b) Untangling genetic influences on smoking, body mass index and longevity: a multivariate study of 2464 Danish twins followed for 28 years. Hum Genet 98(4):467–475
Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361(15):1475–1485
Holly AC, Melzer D, Pilling LC, Fellows AC, Tanaka T, Ferrucci L, Harries LW (2013) Changes in splicing factor expression are associated with advancing age in man. Mech Ageing Dev 134(9):356–366
Holt-lunstad J, Smith TB, Layton JB, Layton JB (2010) Social relationships and mortality risk: a meta-analytic review. PLoS Med. https://doi.org/10.1371/journal.pmed.1000316
Hopcraft MS, Morgan MV, Satur JG, Wright FA (2012) Edentulism and dental caries in victorian nursing homes. Gerodontology 29(2):e512-519
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Houtkooper RH, Williams RW, Auwerx J (2010) Metabolic networks of longevity. Cell 142(1):9–14
Jain N (2018) Vogel VA-O: Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater 17(12):1134–1144
The Bible Authorized King James Version: Cambridge 2004.
Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE et al (2012) Long-term assessment of inflammation and healthy aging in late life: the cardiovascular health study all stars. J Gerontol A Biol Sci Med Sci 67(9):970–976
Jepsen S, Suvan J, Deschner J (2020) The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000 83(1):125–153
Jiang Y, Yang P, Li C, Lu Y, Kou Y, Liu H, Guo J, Li M (2022) Periostin regulates LPS-induced apoptosis via Nrf2/HO-1 pathway in periodontal ligament fibroblasts. Oral Dis. https://doi.org/10.1111/odi.14189
Johnson SC, Rabinovitch PS, Kaeberlein M (2013) mTOR is a key modulator of ageing and age-related disease. Nature 493(7432):338–345
Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS (2015) Modulating mTOR in aging and health. Interdiscip Top Gerontol 40:107–127
Jordan AR, Micheelis W (2016) Fünfte Deutsche Mundgesundheitsstudie-(DMS IV), vol. 35: Deutscher Zahnärzte Verlag DÄV Köln
Kanfi Y, Peshti V, Gil R, Naiman S, Nahum L, Levin E, Kronfeld-Schor N, Cohen HY (2010) SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 9(2):162–173
Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature 483(7388):218–221
Kashiwagi Y, Aburaya S, Sugiyama N, Narukawa Y, Sakamoto Y, Takahashi M, Uemura H, Yamashita R, Tominaga S, Hayashi S et al (2021) Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci Rep. https://doi.org/10.1038/s41598-021-97868-2
Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2014) Global burden of severe tooth loss: a systematic review and meta-analysis. J Dent Res 93(7 Suppl):20s–28s
Kaufman LB, Setiono TK, Doros G, Andersen S, Silliman RA, Friedman PK, Perls TT (2014) An oral health study of centenarians and children of centenarians. J Am Geriatr Soc 62(6):1168–1173
Kaur S, White S, Bartold P (2013) Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res 92(5):399–408
Kaushik S (2018) Cuervo AA-OX: the coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19(6):365–381
Kenyon C (2001) A conserved regulatory system for aging. Cell 105(2):165–168
Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277(5328):942–946
Kirkwood TB (2005) Understanding the odd science of aging. Cell 120(4):437–447
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10(2):205–215
Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M, Li J, Song J (2020) Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 21(1):13–27
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24(22):2463–2479
Lalla E, Papapanou PN (2011) Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7(12):738–748
Lamont RAO, Koo H, Hajishengallis GAO (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16(12):745–759
Lamy M, Mojon P, Kalykakis G, Legrand R, Butz-Jorgensen E (1999) Oral status and nutrition in the institutionalized elderly. J Dent 27(6):443–448
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293
Larbi A, Franceschi C, Fau - mazzatti D, Mazzatti D, Fau - Solana R, Solana R, FauWikby A, Wikby A, Fau - Pawelec G, Pawelec G (2008) Aging of the immune system as a prognostic factor for human longevity. Physiology (bethesda). https://doi.org/10.1152/physiol.00040.2007
Latorre E, Birar VC, Sheerin AN, Jeynes JCC, Hooper A, Dawe HR, Melzer D, Cox LS, Faragher RGA, Ostler EL et al (2017) Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biol 18(1):31
Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV (2016) Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog Cardiovasc Dis 58(5):537–547
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379
Lennon H, Sperrin M, Badrick E, Renehan AG (2016) The obesity paradox in cancer: a review. Curr Oncol Rep 18(9):56
Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M (2018) Ageing: from inflammation to cancer. Immun Ageing. https://doi.org/10.1186/s12979-017-0112-5
Liang S, Hosur KB, Domon H, Hajishengallis G (2010) Periodontal inflammation and bone loss in aged mice. J Periodontal Res 45(4):574–578
Ling MR, Chapple IL, Matthews JB (2015) Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun 21(7):714–725
Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL et al (2013) Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153(4):828–839
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A et al (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27(24):8807–8814
Longo VD, Anderson RM (2022) Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell 185:1455–1470
Loos BG (2016) Periodontal medicine: work in progress! J Clin Periodontol 43(6):470–471
López-Otín C, Kroemer G (2021) Hallmarks of health. Cell 184(1):33–63
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, Fang EF (2020) Mitophagy and neuroprotection. Trends Mol Med 26(1):8–20
Martin LG, Schoeni RF, Andreski PM (2010) Trends in health of older adults in the United States: past, present, future. Demography 47(Suppl 1):S17-40
Meyle J, Chapple I (2015) Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000 69(1):7–17
Micheelis W (2011) Oral health in Germany: an oral epidemiological outline. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54(9):1022–1026
Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR 3rd, Ebersole JL, Miller CS (2010) Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol 37(12):1068–1074
Montal S, Tramini P, Triay JA, Valcarcel J (2006) Oral hygiene and the need for treatment of the dependent institutionalised elderly. Gerodontology 23(2):67–72
Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE (2012) Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev 11(1):51–66
Motokawa K, Mikami Y, Shirobe M, Edahiro A, Ohara Y, Iwasaki M, Watanabe Y, Kawai H, Kera T, Obuchi S et al (2021) Relationship between chewing ability and Nutritional Status in Japanese older adults: a cross-sectional study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18031216
Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T (2012) A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11(2):345–349
Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM et al (2020) Defining trained immunity and its role in health and disease. Nat Rev Immunol 20(6):375–388
Nguyen ATM, Akhter R, Garde S, Scott C, Twigg SM, Colagiuri S, Ajwani S, Eberhard J (2020) The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res Clin Pract 165:108244
Nomura Y, Kakuta E, Okada A, Otsuka R, Shimada M, Tomizawa Y, Taguchi C, Arikawa K, Daikoku H, Sato T et al (2020) Effects of self-assessed chewing ability, tooth loss and serum albumin on mortality in 80-year-old individuals: a 20-year follow-up study. BMC Oral Health 20(1):122
Northridge ME, Ue FV, Borrell LN, De La Cruz LD, Chakraborty B, Bodnar S, Marshall S, Lamster IB (2012) Tooth loss and dental caries in community-dwelling older adults in northern Manhattan. Gerodontology 29(2):e464-473
Ohtsu AAO, Takeuchi Y, Katagiri S, Suda W, Maekawa S, Shiba T, Komazaki R, Udagawa S, Sasaki N, Hattori M et al (2021) Influence of Porphyromonas gingivalis in gut microbiota of streptozotocin-induced diabetic mice. Oral Dis 25(3):868–880
Okamoto N, Amano N, Nakamura T, Yanagi M (2019) Relationship between tooth loss, low masticatory ability, and nutritional indices in the elderly: a cross-sectional study. BMC Oral Health 19(1):110
Ono R, Abe M, Koike N, Inokawa H, Tsuchiya Y, Umemura Y, Sasawaki Y, Yamamoto T, Wakisaka S, Kanamura N et al (2021) Quantitative morphometric analysis of molar teeth and alveolar bone using micro-computed tomography in aged mice. J Oral Biosci 63(3):265–270
P C: The Spartans: An Epic History: Pan; 2003.
Oral Health [https://www.who.int/health-topics/oral-health#tab=tab_1
Paolisso G, Gambardella A, Balbi V, Ammendola S, D’Amore A, Varricchio M (1995) Body composition, body fat distribution, and resting metabolic rate in healthy centenarians. Am J Clin Nutr 62(4):746–750
Paolisso G, Gambardella A, Ammendola S, D’Amore A, Balbi V, Varricchio M, D’Onofrio F (1996) Glucose tolerance and insulin action in healthy centenarians. Am J Physiol 270(5 Pt 1):E890-894
Paolisso G, Ammendola S, Del Buono A, Gambardella A, Riondino M, Tagliamonte MR, Rizzo MR, Carella C, Varricchio M (1997) Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. J Clin Endocrinol Metab 82(7):2204–2209
Paolisso G, Barbieri M, Bonafè M, Franceschi C (2000) Metabolic age modelling: the lesson from centenarians. Eur J Clin Invest 30(10):888–894
Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP, Kalantar-Zadeh K (2014) Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56(4):415–425
Pelletier M, Maggi L, Micheletti A, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Romagnani S, Cassatella MA et al (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115(2):335–343
Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C et al (2019) Oral diseases: a global public health challenge. Lancet 394(10194):249–260
Perls TT (1995) The oldest old. Sci Am 272(1):70–75
Perls TT, Fretts RC (1998) Why Women Live Longer than Men-What gives women the extra years? Sci Am 2:100–103
Petersen PE, Kwan SYL (2010) The 7th WHO Global Conference on Health Promotion - towards integration of oral health, Nairobi, Kenya. Commun Dental Health 27:129–136
Pignolo RJ (2019) Exceptional human longevity. Mayo Clin Proc 94(1):110–124
Piper MD, Partridge L, Raubenheimer D, Simpson SJ (2011) Dietary restriction and aging: a unifying perspective. Cell Metab 14(2):154–160
Potempa J, Mydel P, Koziel J (2017) The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol 13(10):606–620
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Qiu H, Liu Y, Bai S, Li C, Feng M (2000) Oral mucosal conditions and some related factors in 140 Uygur centenarians. Chin Med J 113(4):358–360
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12(6):662–667
Rabiei M, Kasemnezhad E, Masoudi Rad H, Shakiba M, Pourkay H (2010) Prevalence of oral and dental disorders in institutionalised elderly people in Rasht Iran. Gerodontology 27(3):174–177
Rando TA, Chang HY (2012) Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 148(1–2):46–57
Rantzow VAO, Andersson P, Lindmark U (2018) Occurrence of oral health problems and planned measures in dependent older people in nursing care. J Clin Nurs. https://doi.org/10.1111/jocn.14584
Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr, Jones DL, Walker DW (2011) Modulation of longevity and tissue homeostasis by the DROSOPHILA PGC-1 homolog. Cell Metab 14(5):623–634
Robine JM, Allard M (1998) The oldest human. Science 279(5358):1834–1835
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434(7029):113–118
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–556
Rong C, Shen SH, Xiao LW, Huang Q, Lu HT, Wang HX, Li ZX, Wang XM (2019) A comparative study on the health status and behavioral lifestyle of centenarians and non-centenarians in Zhejiang province China-A Cross-Sectional Study. Front Public Health. https://doi.org/10.3389/fpubh.2019.00344
Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447(7145):725–729
Rowe JW, Kahn RL (1997) Successful aging. Gerontologist. https://doi.org/10.1093/geront/37.4.433
Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell 146(5):682–695
Russell SJ, Kahn CR (2007) Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8(9):681–691
Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA et al (2014) Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345(6204):1251086
Sahin E, DePinho RA (2012) Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol 13(6):397–404
Salminen A, Kaarniranta K, Kauppinen A (2012) Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging 4(3):166–175
Salvioli S, Monti D, Lanzarini C, Conte M, Pirazzini C, Bacalini MG, Garagnani P, Giuliani C, Fontanesi E, Ostan R et al (2013) Immune system, cell senescence, aging and longevity–inflamm-aging reappraised. Curr Pharm Des 19(9):1675–1679
Sanchez-Roman I, Gómez A, Pérez I, Sanchez C, Suarez H, Naudí A, Jové M, Lopez-Torres M, Pamplona R, Barja G (2012) Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology 13(4):399–411
Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P et al (2018) Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes federation and the European federation of periodontology. J Clin Periodontol 45(2):138–149
Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F et al (2020) Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol 47(3):268–288
Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM 3rd, Bohr VA (2015) Protecting the mitochondrial powerhouse. Trends Cell Biol 25(3):158–170
Schenkein HA, Papapanou PN, Genco R, Sanz M (2020) Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 83(1):90–106
Schmalz G, Ziebolz D (2020) Changing the focus to the whole patient instead of one oral disease: the concept of individualized prevention. Adv Prev Med 2020:6752342
Schmalz G, Krause F, Grzelkowski M, Merle C, Rotzoll D, Haak R, Ziebolz D (2020) Evaluation of an educational concept for risk-oriented prevention in undergraduate dental education. BMC Med Educ 20(1):298
Schmalz G, Denkler CR, Kottmann T, Rinke S, Ziebolz D (2021) Oral health-related quality of life, oral conditions, and risk of malnutrition in older german people in need of care-a cross-sectional study. J Clin Med. https://doi.org/10.3390/jcm10030426
SchmauckMedina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, Cao S, Soendenbroe C, Mansell E, Vestergaard MB et al (2022) New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging. https://doi.org/10.18632/aging.204248
Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF Jr, Wang TJ et al (2013) Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol 33(7):1728–1733
Schoeni RF, Freedman VA, Wallace RB (2001) Persistent, consistent, widespread, and robust? another look at recent trends in old-age disability. J Gerontol B Psychol Sci Soc Sci 56(4):S206-218
Schoeni RF, Freedman VA, Martin LG (2008) Why is late-life disability declining? Milbank Q 86(1):47–89
Sczepanik FSC, Grossi ML, Casati M, Goldberg M, Glogauer M, Fine N, Tenenbaum HC (2020) Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol 2000 84(1):45–68
Sekundo C, Langowski E, Kilian S, Frese C (2020a) Periodontal and peri-implant diseases in centenarians. J Clin Periodontol 47(10):1170–1179
Sekundo C, Langowski E, Kilian S, Frese C (2020b) Oral health and functional capacity of centenarians. Sci Rep 10(1):22215
Shen T, Li H, Song Y, Li L, Lin J, Wei G, Ni T (2019) Alternative polyadenylation dependent function of splicing factor SRSF3 contributes to cellular senescence. Aging 11(5):1356–1388
Silva DD, Rihs LB, Sousa Mda L (2009) Factors associated with maintenance of teeth in adults in the State of São Paulo, Brazil. Cad Saude Publica 25(11):2407–2418
Simunković SK, Boras VV, Pandurić J, Zilić IA (2005) Oral health among institutionalised elderly in Zagreb. Croatia Gerodontology 22(4):238–241
Song TJ, Jeon J, Kim J (2021) Cardiovascular risks of periodontitis and oral hygiene indicators in patients with diabetes mellitus. Diabetes Metab 47(6):101252
Strindhall J, Skog M, Ernerudh J, Bengner M, Löfgren S, Matussek A, Nilsson BO, Wikby A (2013) The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (dordr) 35(3):985–991
Suzuki M, Wilcox BJ, Wilcox CD (2001) Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr 10(2):165–171
Teissier TAO, Boulanger E, Cox LAOX (2022) Interconnections between inflammageing and Immunosenescence during Ageing. Cells. https://doi.org/10.3390/cells11030359
Terry DF, Wilcox MA, McCormick MA, Pennington JY, Schoenhofen EA, Andersen SL, Perls TT (2004) Lower all-cause, cardiovascular, and cancer mortality in centenarians’ offspring. J Am Geriatr Soc 52(12):2074–2076
Terry DF, Sebastiani P, Andersen SL, Perls TT (2008) Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med 168(3):277–283
Tomaru U, Takahashi S, Ishizu A, Miyatake Y, Gohda A, Suzuki S, Ono A, Ohara J, Baba T, Murata S et al (2012) Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am J Pathol 180(3):963–972
Tôrres LHDN, Tellez M, Hilgert JB, Hugo FN (2015) de Sousa MdLR, Ismail AI: frailty, frailty components, and oral health: a systematic review. J Am Geriatr Soc 63(12):2555–2562
van der Spoel E, Jansen SW, Akintola AA, Ballieux BE, Cobbaert CM, Slagboom PE, Blauw GJ, Westendorp RGJ, Pijl H, Roelfsema F et al (2016) Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell 15(6):1126–1131
Van Dyke TE, Bartold PM, Reynolds EC (2020) The Nexus Between Periodontal Inflammation and Dysbiosis. Front Immunol. https://doi.org/10.3389/fimmu.2020.00511
Van Dyke TE, Kholy KE, Ishai A, Takx RAP, Mezue K, Abohashem SM, Ali A, Yuan N, Hsue P, Osborne MT et al (2021) Inflammation of the periodontium associates with risk of future cardiovascular events. J Periodontol 92(3):348–358
Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N et al (2014) Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 69(2):165–173
Vijg J, Campisi J (2008) Puzzles, promises and a cure for ageing. Nature 454(7208):1065–1071
Waidmann TA, Liu K (2000) Disability trends among elderly persons and implications for the future. J Gerontol B Psychol Sci Soc Sci 55(5):S298-307
Walters HE, Deneka-Hannemann S, Cox LS (2016) Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging. https://doi.org/10.18632/aging.100872
Wang K, Klionsky DJ (2011) Mitochondria removal by autophagy. Autophagy 7(3):297–300
Wang X, Fu CH, Zhu X, Liu J, Gong X, Pan Q, Ma X (2021a) Exosomes and exosomal microRNAs in age-associated stroke. Curr Vasc Pharmacol 19(6):587–600
Wang P, Wang B, Zhang Z, Wang Z (2021b) Identification of inflammation-related DNA methylation biomarkers in periodontitis patients based on weighted co-expression analysis. Aging (albany NY) 13(15):19678–19695
Weindruch R, Walford RL (1982) Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215(4538):1415–1418
Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Löfgren S, Nilsson B-O, Ernerudh J, Pawelec G, Johansson B (2005) An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol 60(5):556–565
Willcox BJ (2007) Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. https://doi.org/10.1196/annals.1396.037
Willcox BJ, Willcox DC (2014) Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care. https://doi.org/10.1097/MCO.0000000000000019
Willcox DC, Willcox BJ, Todoriki H, Suzuki M (2009) The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. https://doi.org/10.1080/07315724.2009.10718117
Willcox DC et al (2012) Gender gap in health span and life expectancy in Okinawa: health behaviours. Asian J Gerontol Geriatr 7:49–58
Willcox DC, Scapagnini G, Willcox BJ (2014) Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev. https://doi.org/10.1016/j.mad.2014.01.002
Willcox BJ, Willcox DC, Suzuki M (2017) Demographic, phenotypic, and genetic characteristics of centenarians in Okinawa and Japan: Part 1-centenarians in Okinawa. Mech Ageing Dev. https://doi.org/10.1016/j.mad.2016.11.001
Wilmanski TAOX, Diener C, Rappaport NAO, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman GAO, Robinson MAOX et al (2021) Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab 3(2):274–286
Wolfe RR (2012) The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr 108(Suppl 2):S88-93
Wolfe RR, Miller SL, Miller KB (2008) Optimal protein intake in the elderly. Clin Nutr 27(5):675–684
Wong FM, Ng YT, Leung WK (2019) Oral health and its associated factors among older institutionalized residents—a systematic review. Int J Environ Res Public Health 16(21):4132
Wong SQ, Kumar AV, Mills J (2020) Lapierre LA-OX: Autophagy in aging and longevity. Hum Genet 139(3):277–290
Xu D, Tahara H (2013) The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev 65(3):368–375
Xu X, Zhao Y, Gu D, Pei Y, Wu B (2021) Health behaviors and self-reported oral health among centenarians in Nanjing, China: a cross-sectional study. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18147285
Yamazaki K, Kato T, Tsuboi Y, Miyauchi E, Suda W, Sato K, Nakajima M, Yokoji-Takeuchi M, Yamada-Hara M, Tsuzuno T et al (2021) Oral Pathobiont-induced changes in gut microbiota aggravate the pathology of nonalcoholic fatty liver disease in mice. Front Immunol 12:508
Yang CN, Lin SK, Kok SH, Wang HW, Lee YL, Shun CT, Chi CW, Yang H, Hong CY (2021) The possible role of sirtuin 5 in the pathogenesis of apical periodontitis. Oral Dis 27(7):1766–1774
Yin Z, Klionsky DJ (2022) Intermittent time-restricted feeding promotes longevity through circadian autophagy. Autophagy 18(3):471–472
Young RD, Desjardins B, McLaughlin K, Poulain M, Perls TT (2010) Typologies of extreme longevity myths. Curr Gerontol Geriatr Res 2010:423087
Yuan F, Hankey W, Wagner EJ, Li W, Wang Q (2019) Alternative polyadenylation of mRNA and its role in cancer. Genes Dis 8(1):61–72
Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M et al (2003) Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol 38(9):981–987
Zayed M, Iohara K, Watanabe H, Nakashima M (2020) CCR3 antagonist protects against induced cellular senescence and promotes rejuvenation in periodontal ligament cells for stimulating pulp regeneration in the aged dog. Sci Rep 10(1):8631
Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D (2013) Hypothalamic programming of systemic ageing involving IKK-β NF-Κb and GnRH. Nature 497(7448):211–216
Zhang X, Zhang S, Yan X, Shan Y, Liu L, Zhou J, Kuang Q, Li M, Long H, Lai W (2021) m6A regulator-mediated RNA methylation modification patterns are involved in immune microenvironment regulation of periodontitis. J Cell Mol Med 25(7):3634–3645
Zhao D, Khawaja AT, Jin L, Chan KW, Tonetti M, Tang SCW, Pelekos G (2020) Effect of non-surgical periodontal therapy on renal function in chronic kidney disease patients with periodontitis: a systematic review and meta-analysis of interventional studies. Clin Oral Investig 24(4):1607–1618
Zheng YAOX, Shi B, Ma M, Wu X, Lin X (2019) The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J Cell Physiol 234(5):5488–5495
Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T et al (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140(2):280–293
Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation. https://doi.org/10.1186/s12974-020-1705-z
Ziebolz D, Werner C, Schmalz G, Nitschke I, Haak R, Mausberg RF, Chenot JF (2017) Oral Health and nutritional status in nursing home residents-results of an explorative cross-sectional pilot study. BMC Geriatr 17(1):39
Acknowledgements
The authors acknowledge support from Leipzig University for Open Access Publishing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The hypothesis has been created by MP and GS. The main article has been written by MP. GS, KEAS, TE and DZ were instrumental in the initial review and refining of ideas.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poser, M., Sing, K.E.A., Ebert, T. et al. The rosetta stone of successful ageing: does oral health have a role?. Biogerontology 24, 867–888 (2023). https://doi.org/10.1007/s10522-023-10047-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-023-10047-w