Abstract
Honey bee (Apis mellifera) grooming behavior is an important mechanism of resistance against the parasitic mite Varroa destructor. This research was conducted to study associations between grooming behavior and the expression of selected immune, neural, detoxification, developmental and health-related genes. Individual bees tested in a laboratory assay for various levels of grooming behavior in response to V. destructor were also analyzed for gene expression. Intense groomers (IG) were most efficient in that they needed significantly less time to start grooming and fewer grooming attempts to successfully remove mites from their bodies than did light groomers (LG). In addition, the relative abundance of the neurexin-1 mRNA, was significantly higher in IG than in LG, no groomers (NG) or control (bees without mite). The abundance of poly U binding factor kd 68 and cytochrome p450 mRNAs were significantly higher in IG than in control bees. The abundance of hymenoptaecin mRNA was significantly higher in IG than in NG, but it was not different from that of control bees. The abundance of vitellogenin mRNA was not changed by grooming activity. However, the abundance of blue cheese mRNA was significantly reduced in IG compared to LG or NG, but not to control bees. Efficient removal of mites by IG correlated with different gene expression patterns in bees. These results suggest that the level of grooming behavior may be related to the expression pattern of vital honey bee genes. Neurexin-1, in particular, might be useful as a bio-marker for behavioral traits in bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The parasitic mite Varroa destructor has caused the loss of millions of honey bee (Apis mellifera) colonies and thus is considered the number one health problem of honey bees worldwide (Stankus 2008; Guzman-Novoa et al. 2010; Le Conte et al. 2010). Varroa mites weaken bees by feeding on their haemolymph after wounding their cuticle, which may result in the invasion of secondary pathogens, leading to their early death (De Jong et al. 1982). Varroa mites also suppress bee immunity (Yang and Cox-Foster 2005; Navajas et al. 2008; Nazzi et al. 2012) and act as vectors of several honey bee viruses (Kevan et al. 2006; Emsen et al. 2015; Hamiduzzaman et al. 2015; Anguiano-Baez et al. 2016). On the behavioral level, Varroa hampers non-associative learning (Kralj et al. 2007), and reduces the proportion of foragers that return to the hive (Kralj and Fuchs 2006). Control of Varroa infestations in honey bee colonies has become a daunting task for beekeepers and scientists. Most beekeepers use synthetic miticides to control the parasites, but the continuous use of pesticides leads to the development of resistance in the mites (Milani 1999). Furthermore, the use of pesticides increases the risk of contamination of honey and other hive products (Wallner 1999). Other ways of controlling this mite are thus needed. One potential approach to controlling V. destructor would be the development of honey bee strains resistant to the parasite. This could theoretically be achieved by natural selection (bees not treated against the mite) or by breeding bees expressing traits associated to mite resistance or tolerance (Rinderer et al. 2010; Arechavaleta-Velasco et al. 2012; Guzman-Novoa et al. 2012; Hunt et al. 2016).
The original host of V. destructor, the Asiatic bee Apis cerana, naturally resists infestations by Varroa through multiple mechanisms. The most important mechanism of A. cerana resistance appears to be through grooming behavior (Peng et al. 1987). The western honey bee, A. mellifera, also expresses grooming behavior against Varroa, but to a lesser degree than its Asiatic counterpart (Buchler et al. 1992; Fries et al. 1996). Through grooming behavior, some adult bees physically remove mites from their bodies using their legs and mandibles (Ruttner and Hanel 1992; Fries et al. 1996; Boecking and Spivak 1999; Bahreini and Currie 2015). Grooming behavior is also a defense mechanism against tracheal mites (Pettis and Pankiw 1998; Danka and Villa 2003, 2005).
Bees groom themselves at various levels of intensity. Guzman-Novoa et al. (2012) reported that bees that groom at high intensity remove significantly more mites from their bodies than bees that do it lightly, suggesting that grooming intensity is an important factor for resistance to Varroa. Not much is known about the genetic mechanisms regulating grooming behavior but it appears to be a quantitative trait with a genetic component (Moretto et al. 1993; Page and Guzman-Novoa 1997; Arechavaleta-Velasco et al. 2012). Grooming behavior is also influenced by environmental effects (Currie and Tahmasbi 2008). The degree to which grooming behavior is influenced by genes is unknown but, if there is significant genetic variability for this trait, bees could be bred for high grooming expression and intensity to develop resistant stock to V. destructor (Hunt et al. 2016).
A number of studies have shown that V. destructor parasitism alters the expression pattern of immune-related (Yang and Cox-Foster 2005; Navajas et al. 2008; Hamiduzzaman et al. 2012) and behavioral-related genes in honey bees (Le Conte et al. 2011). However, there are no studies of gene expression in bees that exhibit intense grooming behavior. To learn more about genes that may be involved in bee behavioral mechanisms of resistance against mites, we explored the association of different degrees of grooming behavior with mRNA abundance of some candidate genes for which expression information exists for other traits, and from some genes tested for the first time. We chose genes that have reduced expression in response to V. destructor parasitism such as the immune related gene, hymenoptaecin (Hym), the putative cell proliferation regulator, poly U binding factor kd 68 (pUf68), and a gene related to longevity, development and general health, vitellogenin (Vg). We also tested a gene for the autophagy-linked FYVE protein, blue cheese (BlCh), whose expression is changed by V. destructor parasitism (Yang and Cox-Foster 2005; Navajas et al. 2008; Dainat et al. 2012; Hamiduzzaman et al. 2012). Honey bees like other insects rely on detoxification genes such as the cytochrome p450 gene, CYP9Q3, which has shown altered expression patterns when insects are exposed to different types of chemicals (Mao et al. 2011), or when performing physical activities such as hygienic behavior (Boutin et al. 2015). But the expression of CYP9Q3 has not been assessed for bees that are exposed to mites or as a response to other behavioral activities such as grooming behavior. Expression of the neural gene neurexin-1 (AmNrx1) occurs primarily in the central nervous system and in the mushroom body of the brain, which is an important organ for higher-order processing and learning in the bee (Heisenberg 1998; Szyska et al. 2008) and AmNrx1 is among a small number of candidate genes for honey bee grooming behavior identified in a quantitative trait locus for honey bee grooming behavior (Arechavaleta-Velasco et al. 2012). AmNrx1 is also known to be related to autism disorder in humans, a syndrome that is associated with repetitive movements or ataxias (Feng et al. 2006; Sudhof 2008; Reichelt et al. 2012) and in self-grooming behavior in mice (Etherton et al. 2009). Therefore this gene could potentially affect grooming behavior, but has not been studied in relation to this trait in bees.
The objectives of this study were (1) to correlate the effect of two levels of grooming behavior (light and intense) with the time required to start grooming and with the number of attempts needed by individual bees exposed to Varroa mites to successfully remove the parasite from their bodies, and (2) to analyze the association between these levels of grooming behavior and the expression of selected genes in tested bees.
Materials and Methods
Collection of V. destructor Mites
Grooming experiments were conducted at the Honey Bee Research Centre of the University of Guelph, in Guelph, Ontario, Canada between April and August, 2013. Adult foundress Varroa mites from heavily infested honey bee colonies were harvested from brood cells containing white-eyed pupae using a fine paint brush. The harvested mites were held in Petri dishes lined with moist filter paper and containing two white-eyed bee pupae collected from a non-infested colony; the pupae served as a food source for the mites. The mites were kept at room temperature (26 ± 2 °C) and used within 2 h from the time of collection.
Grooming Behavior in Individual Bees
Grooming behavior at the individual level was performed in the laboratory using a modified version of the method described by Aumeier (2000). All worker bees were sampled from five local, randomly selected colonies, presumably representing a broad sample of genotypes because queens of the colonies were open mated to approximately 12–20 haploid drones. Worker bees for all treatments were collected from the brood nest of the source colonies using a bee vacuum (Gary and Lorenzen 1990). Individual Petri dishes (9 cm diameter) were prepared in advance of the assays by lining their bottom with a circular piece of white filter paper to provide contrast for observation of bees and mites. Petri dishes were covered with plastic wrap. The plastic wrap was perforated 20–30 times with a nail (50 × 3 mm) in order to allow air to pass through. One worker bee was introduced into each dish and was then given 2–3 min to become accustomed to the Petri dish. The plastic wrap was then lifted slightly in order to place a single mite on the bee’s thorax using a fine brush (except for control bees that were only touched with the brush on the thorax). A stopwatch was started immediately upon application of the mite and the bee was observed for up to 3 min. Grooming instances exhibited by the bee were recorded, specifically describing the time elapsed until she started to groom, the number of grooming attempts, whether or not she removed the mite and the intensity with which she groomed. The following variables were recorded: time (s) elapsed from the moment a mite was placed on the bee thorax until she started to groom, time to mite removal, and the number of grooming attempts a bee required to successfully remove a mite. A grooming attempt was defined as an uninterrupted period of time during which grooming was observed, and that ended when the bee paused (a bee could have several of these grooming instances within 3 min). In the event that a bee successfully removed the mite within 3 min, the trial ended and the time of removal was recorded. Bees that could not remove the mite within 3 min were only classified by the intensity with which they groomed. “Light grooming” (LG) consisted of slow swipes of one or occasionally two legs across the thorax or abdomen. “Intense grooming” (IG) consisted of vigorous wiping and shaking and always involved the use of more than two legs. Whether the grooming was recorded as “light” or “intense” and how many grooming attempts were performed by each bee was left to the observer’s judgement. However, there was only one observer, and therefore all incidences were judged by the same person as described by Guzman-Novoa et al. (2012). Some bees did not groom and were recorded as “no grooming” (NG). Since control bees were not exposed to the irritation caused by Varroa mites and were not assessed for mite removal, they were only evaluated for whether or not they groomed, and for those that groomed, the time to start grooming and grooming attempts within 3 min were recorded. Grooming trials were performed with a total of 240 bees. Samples of 12–16 individuals for each IG, LG, NG and control bees were randomly collected at the end of trials and frozen at −70 °C for further analysis of gene expression.
RNA Extraction and cDNA Synthesis
Total RNA was extracted by homogenizing each adult bee sample in extraction buffer as per Chen et al. (2000). The homogenates were extracted twice with chloroform and the RNA was precipitated using LiCl as described by Sambrook et al. (1989). The amount of total RNA extracted was determined with a spectrophotometer (Nanovue GE Healthcare, Cambridge, UK). RNA samples were stored at−70 °C. For cDNA synthesis, 2 µg of total RNA was reverse-transcribed using Oligo (dT)18 and M-MuLV RT with the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas Life Sciences, Burlington, ON, Canada), following the instructions of the manufacturer. The cDNA was stored at −20 °C.
Primers
The primers used to amplify the genes evaluated are shown in Table 1. To design some of the primers, the complete sequences of the genes were obtained from the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). The sequences were aligned using CLUSTALX and the primers were designed using the Gene Runner (Version 3.05, Hastings Software, Inc., NY). The oligo nucleotides were ordered from Laboratory Services of the University of Guelph (Guelph, ON, Canada).
PCR Amplifications
Each of the target genes (except CYP9Q3) was co-amplified together with the honey bee ribosomal protein RpS5 gene (Thompson et al. 2007) in the same tube and reaction as a constitutive control. The glyceraldehyde 3-phosphate dehydrogenase2 (GAPD2) gene (Thompson et al. 2007) was used as another standard control to co-amplify with CYP9Q3. All PCR reactions were done with a Mastercycler (Eppendorf, Mississauga, ON, Canada). Each 15 µL of reaction contained 1.5 µL of 10× PCR buffer (New England BioLabs, Pickering, ON, Canada), 0.5 µL 10 mM of dNTPs (Bio Basic Inc., Markham, ON, Canada), 1 µL of 10 µM for each primer of target and housekeeping genes (Laboratory Services, University of Guelph, Guelph, ON, Canada), 0.2 µL 5 U/µL of Taq polymerase (New England BioLabs, Pickering, ON, Canada), 2 µL of the cDNA sample, and 6.8 µL of dd H2O. To amplify AmNrx1, CYP9Q3, Hym and Vg, the thermocycler was programmed to run at 94 °C for 3 min, followed by 35 cycles of 30 s at 94 °C, 60 s at 58 °C and 60 s at 72 °C, and a final extension step at 72 °C for 10 min. To amplify pUf68 and BlCh, the annealing temperature was 55 °C while the other conditions described above remained the same.
Separation and Semi-Quantification of PCR Products
PCR products were separated on 1% TAE agarose gels and stained with ethidium bromide. A 100 bp DNA ladder (Bio Basic Inc., Markham, ON, Canada) was included in each gel. Images of the gels were captured using a digital camera with a Benchtop UV Transilluminator (BioDoc-ItM Imaging System, Upland, CA). The intensity of the amplified bands was measured in pixels using the Scion Image Program (Scion Corporation, Frederick, MD) as per Dean et al. (2002). The ratio of band intensity between the target gene and the housekeeping gene was calculated to determine the relative expression units (REU) of each gene. To determine whether quantification at 35 amplification cycles was not affected by signal saturation of the band intensities, randomly selected samples with high, medium and low REUs were also quantified in the same manner with fewer amplification cycles, and the pattern of expression based on the REU values were not significantly different when 25, 30 and 35 amplification cycles were used (F2,15 = 0.30, p = 0.75). We analyzed results at 35 cycles because in most cases the relationship between the number of cycles and molecules is relatively linear at 35 cycles when semi-quantitative RT-PCR is used, which provides high amplification efficiency.
Quantitative Real-Time-PCR Methods
To confirm the correlation between AmNrx1 mRNA abundance and grooming behavior obtained with the semi-quantification method (this gene was the gene that most consistently correlated with grooming behavior), target-specific qRT-PCR primers (Table 1) corresponding to the Neurexin1A gene were designed using the Primer Express 3.0 software (ABI, Applied Biosystems, Foster City, CA). The qRT-PCR was performed using the Light Cycler 480 II Real Time PCR System (Roche, Indianapolis, IN) using the SYBR Green dye-based detection system. All reactions were performed in a final volume of 10 µL, consisting of 5 µL of SensiFAST SYBR no-ROX master mix (Bioline, Taunton, MA), gene-specific primers at a final concentration of 0.2 µM each, and 20 ng of cDNA template. No-template and no-reverse transcriptase samples were included in each PCR plate as negative controls. Along with the target-gene, the qRT-PCR plate also included AmRPL8 (60S ribosomal protein L8) as an internal reference housekeeping gene to verify equal amounts of target cDNA in all samples. All reactions were set up in triplicate for each of the biological replicates. PCR conditions were as follows: 95 °C for 5 min, 45 cycles of 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 30 s. To determine the specificity of the reaction a melt curve analysis was carried out following PCR, confirming amplification of a single product. Quantification of gene expression, displayed as Relative Expression Value (REV) was calculated using the Relative Standard Curve Method (User Bulletin 2: ABI PRISM 7700 Sequence Detection System) as described in Subramanyam et al. (2006).
Statistical Analysis
Data on time to start grooming, number of grooming attempts, time to successful mite removal and gene expression were subjected to analysis of variance (ANOVA), excluding non-groomers and negative control values from the analyses because they represented 0 values. A correlation analysis was performed with AmNrx1expression data from the semi-quantification method and from the qRT-PCR to validate results. To obtain descriptive statistics and perform ANOVAS, the package IBM-SPSS v. 23 (SPSS Inc., Chicago, IL) was used. Significant differences among means were separated with Fisher’s protected LSD or Tamhane’s T2 tests (α = 0.05).
Results
IG bees started to groom themselves significantly faster than LG and control bees. LG bees also initiated grooming activity significantly faster than control bees (F3, 206 = 220.83, p < 0.0001), whereas NG did not groom at all within the 3 min lapse of the trial (Fig. 1). To achieve mite removal success, IG bees required significantly less time and fewer grooming attempts than LG bees (F2, 177 = 76.50, p < 0.0001 and F2, 207 = 50.65, p < 0.0001 for time and removal attempts, respectively), whereas NG bees did not groom or remove mites (Fig. 2a, b), indicating that IG bees are more efficient at removing mites than other bees.
Mean time to start grooming ± SE (s) within 3 min in individual worker bees either not exposed to V. destructor (control bees, only touched with a fine brush on the thorax), or exposed to a mite (by placing a mite on their bodies). Exposed bees responded by not grooming (excluded from the analysis due to 0 values), or by grooming at light pace (LG) or at vigorous pace (IG). Different letters indicate significant differences of means based on analysis of variance and Tamhane’s T2 tests (p < 0.01; n = 240)
Mite removal success of worker bees exposed to V. destructor for 3 min in the laboratory. a Mean time spent for mite removal ± SE (s) and b mean number of attempts until successful mite removal for individual bees exposed to V. destructor by placing a mite on their bodies. Only bees that responded by grooming at light pace (LG) or at vigorous pace (IG) were included in the analysis. Different letters indicate significant differences of means based on analysis of variance and Tamhane’s T2 tests (p < 0.01; n = 210)
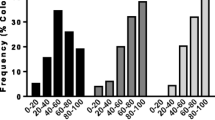
The expression of AmNrx1 was significantly higher in IG than in LG, NG and control bees. There were no significant differences in the level of expression of this gene among LG, NG and control bees, indicating that only intense grooming was associated with a high expression level of AmNrx1 (F3, 48 = 12.20, p < 0.0001, Fig. 3a).
Relative RT-PCR quantification units of AmNrx1 (a), pUf68 (b), CYP9Q3 (c), BlCh (d), Hym (e) and Vg (f), relative to house-keeping genes (RpS5 or GAPD2) of individual worker bees not exposed to V. destructor (control bees, only touched with a fine brush on the thorax) or exposed to it (by placing a mite on their bodies). Exposed bees responded by not grooming (NG), or by grooming at light pace (LG) or at vigorous pace (IG). Different letters indicate significant differences of means based on analysis of variance and Fisher’s protected LSD tests (p < 0.05; n = 64 for all genes, except for AmNrx1 with n = 52 and Hym with n = 40)
The expression of pUf68 increased significantly in both IG and LG bees relative to NG and control bees with no differences between IG and LG bees. However, the level of gene expression in NG was higher than in control bees (F3, 60 = 20.94, p < 0.0001, Fig. 3b).
The expression of CYP9Q3 was significantly higher in IG than in control bees, but not different from that of NG and LG bees (F3, 60 = 5.04, p < 0.01, Fig. 3c). Conversely to the above results, the expression of BlCh was significantly higher in LG and NG than in IG bees, while there were no significant differences in expression of BlCh gene between control bees and bees of the rest of the treatments (F3, 60 = 5.45, p < 0.05, Fig. 3d). Hym was significantly upregulated in IG compared to NG bees, but there were no significant differences in gene expression levels among NG, LG and control bees (F3, 36 = 4.12, p < 0.05, Fig. 3e). Finally, expression of Vg was not associated to grooming behavior or the presence of Varroa, since no differences in expression for this gene were observed among all treatments (F3, 60 = 0.125, p > 0.05, Fig. 3f).
The results from qRT-PCR of AmNrx1 supported those obtained with the semi-quantitative method. IG bees had higher AmNrx1 mRNA abundance than did LG and NG bees (F2, 22 = 3.768, p < 0.05). Additionally, expression data from the semi-quantification method and from the qRT-PCR for the same bees were significantly correlated (r = 0.65, p < 0.001).
Discussion
Bees that performed instances of intense grooming were significantly faster to start grooming and required fewer grooming attempts and less time to remove Varroa mites from their bodies than bees performing light grooming. These results indicated that IG bees were very sensitive to the mite presence on their bodies and were efficient at removing them. Guzman-Novoa et al. (2012) compared different presumably Varroa-susceptible and resistant genotypes of honey bees for grooming ability, and found that a significantly higher number of mites were dislodged from the bees’ bodies by intense grooming than by light grooming regardless of genotype, which agrees with the findings here reported.
Grooming behavior allows insects to clean their body surface and sensory organs (Zhukovskaya et al. 2013). Therefore, this behavior is linked with the ability of the insect to perceive stimuli from its environment. Parasitic mites provide mechanical and chemosensory stimuli, which may result in the initiation of grooming behavior by the affected bee. Thus, sensory recognition of the parasite could lead to behavioral and immune responses such as grooming behavior (Roode and Lefevre 2012). More efficient grooming bees may rely on quick recognition of Varroa presence by tactile or chemosensory sensors. This in turn would activate defense mechanisms, including reacting through physical activities such as grooming behavior, to successfully remove the mites from their bodies. The age and reproductive status of mites could also be a factor that influences the sensitivity of honey bees to perform grooming behavior. Kirrane et al. (2012) evaluated in laboratory cages the grooming response of honey bees to V. destructor, and concluded that the grooming success of bees was affected by the age and reproductive status of the mites. The highest mite drop was for daughter mites and the lowest for foundress mites, which suggests that the former age group stimulated bees to remove mites from their bodies more frequently than when parasitized with foundresses. We used foundress mites in our study, so, perhaps had we used only daughter mites we would have seen a higher frequency of mite removal and probably higher levels of gene expression. This hypothesis however, remains to be tested.
Supporting the above potential explanations, Biswas et al. (2010) reported that the expression of the neural gene AmNrx1 was affected by sensory experience in honey bees, which may play a role in the development of synaptic connections that could influence learning and the expression of behavioral traits. Also, Arechavaleta-Velasco et al. (2012) demonstrated that some candidate genes, including AmNrx1, were associated with grooming behavior. Similarly, successful mite removal by IG bees in this study suggested that these bees may have a higher sensitivity to Varroa, resulting in increased expression of neuron-related genes, such as AmNrx1. The significantly higher expression level of AmNrx1 in IG than in LG, NG and control bees supported results from the above studies and the notion that this gene is associated with grooming behavior and/or physical activity. Further study is needed to distinguish between AmNrx1 effects on grooming or activity states.
The putative cell proliferation regulator protein, pUf68, also known as half pint, plays important regulatory roles in controlling the production of complex diverse proteins in a wide range of organisms (Bourgeois et al. 2004). pUf68 is particularly known for its role in pre-mRNA splicing, which could possibly be related to physical activity in the insect. It might be that products of pUf68 are linked to functions of the peripheral nervous system (PNS) of bees. Physical activity such as grooming behavior in bees might have an impact on the splicing of pUf68 and transcript proliferation in cells through the PNS. The significantly higher expression of pUf68 in both IG and LG than in NG and control bees suggested that it could be affected by grooming activity or vice versa. Contrary to our results, the expression of pUf68 was found to be suppressed by Varroa parasitism in adult bees (Yang and Cox Foster 2005; Navajas et al. 2008) and brood (Dainat et al. 2012; Hamiduzzaman et al. 2012). Perhaps the difference between our results and those of the above studies is related to time of exposure to the mite. In our study, bees were exposed to Varroa <3 min and so, presumably the mite did not have time to inoculate immune-suppressive effectors through its saliva while feeding on the bees’ haemolymph (Yang and Cox-Foster 2007; Richards et al. 2011). Therefore, the mite may have been unable to suppress the expression of this immune related gene in the bees. Probably the high physical activity of grooming bees, leads to physiological changes resulting in higher expression of pUf68. It is also possible that the expression of this gene unchains higher physical activity through neural mechanisms stimulated by the presence of a mite. Regardless of why the expression of this gene is affected, this is the first report of a relationship between pUf68 mRNA abundance and grooming behavior in bees. Further studies will be needed to clarify the mechanisms through which grooming activity and the expression of this gene in honey bees are related.
Expression of the detoxification gene, CYP9Q3, in IG bees was significantly higher than in control bees, but similar to that of LG and NG bees. These results suggested an effect on gene expression related to the presence of Varroa on the bee’s body (since control bees were treated identically but not challenged with a mite) but not necessarily associated with the physical activity of grooming behavior. It may be that the short exposure to the mite unchains a physiological reaction leading to a higher expression of this gene only in bees exposed to the mite regardless of their physical activity. Perhaps expression of CYP9Q3 can respond to a non-chemical stress, such as the attachment of a Varroa mite (Mao et al. 2011; Boncristiani et al. 2012). Supporting the hypothesis that CYP9Q3 is not related to physical activity, Boutin et al. (2015) found that cytochrome p450 genes were over-expressed in non-hygienic bees compared to hygienic bees, and hypothesized that the products of these genes degrade the odorant pheromones and chemicals that signal the presence of diseased brood and thus resulted in these bees being less efficient in detecting killed brood. Although no studies have been conducted to demonstrate a relationship between mite odors and grooming behavior, it is possible that the increased expression of CYP9Q3 in our study had been influenced by scents of the mite. Odorant substances such as pheromones may influence gene expression in the honey bee. For example, Grozinger et al. (2003) reported that queen mandibular pheromone (QMP) affects gene expression in the bee brain, which showed correlation with behavioral responses (i.e. brood care, nursing) in adult worker bees.
Navajas et al. (2008) reported that the expression of the autophagy-linked gene BlCh, was up-regulated in bees presumed to be Varroa-tolerant, while the expression of Dlic2 and Atg18 genes, which influence neural reactions, was down-regulated. Interestingly, in another study, the expression of BlCh was negatively correlated with Dlic2 and Atg18 in Varroa-parasitized bees (Simonsen et al. 2007). These findings agree with our results of increased BlCh expression in NG and LG bees and of decreased expression of this gene in IG bees. Intense physical activity during grooming could be related to the nervous system being stimulated by the products of Dlic2 and Atg18 genes, which would also result in suppression of BlCh in IG bees. Future experiments however, are required to confirm whether this explanation is plausible.
The expression of Hym in IG bees was similar to that of LG and control bees, but it was lower in NG bees. This result is difficult to explain but perhaps it is related to differences in activity between the groups of bees. Control bees as well as LG and IG bees all groomed (and thus were active), whereas NG bees showed reduced activity. It also seems that mite parasitism had no effect on Hym expression since control bees were not exposed to mites but did not differ from LG and IG bees that were parasitized by a mite. Another possibility is that mRNA abundance of genes such as Hym, CYP9Q3 and AmNrx1 are all increased by stress, which in turn increases the tendency for intense grooming. Genotypic variation between bees of different sources could also differentially influence gene expression in Varroa-parasitized and not parasitized bees (Navajas et al. 2008). However, these and other potential explanations of our results, require further experimentation.
There was no significant difference in the expression of the developmental and general health related gene, Vg, among bees of the different treatments, indicating that neither physical activity nor short exposure to Varroa affects the expression of this gene and that this gene does not seem to be related to grooming behavior.
Because Varroa poses a serious threat to bee health, researchers have been trying to find mite-resistance traits in bees. Several studies have indicated that grooming behavior may be a very important trait in conferring resistance to bees against the mite at the colony and individual levels (Moretto et al. 1993; Arechavaleta-Velasco and Guzman-Novoa 2001; Andino and Hunt 2011; Hunt et al. 2016; Invernizzi et al. 2016). These and a previous study (Guzman-Novoa et al. 2012) demonstrate and confirm the importance of efficient grooming for successful mite removal in honey bees. At the molecular level, Arechavaleta-Velasco et al. (2012) searched for genes influencing grooming behavior by analyzing the DNA of bee genotypes in backcross workers derived from high- and low-grooming parents. These workers varied in tendency to initiate grooming instances after being challenged with Varroa mites on their bodies. These researchers identified a single chromosomal region containing a set of candidate genes, which includes AmNrx1, using quantitative-trait-locus (QTL) interval mapping. Consistent with this finding, of all the genes tested in this study, AmNrx1 was most highly and consistently related to intense grooming and thus, warrants further investigation.
One limitation of this study is the small number of genes selected to study in the context of grooming behavior. Analyzing more genes based on their specific function might have been more informative in evaluating their expression pattern during grooming instances. Despite this limitation, some of the selected genes showed association with IG, indicating that probably multiple genes rather than a single gene might be involved in regulating grooming behavior. However, whether the genes are influencing the behavior or vice versa still needs to be confirmed. Therefore, more studies need to be conducted to understand the involvement of some of these and other genes that are related to neural sensitivity as they respond to the irritation caused by ectoparasitic mites on the bees. Finding candidate genes that influence the intensity with which bees groom themselves in response to parasitic mites is critical for developing marker assisted selection assays to breed for mite resistance in honey bees.
References
Andino GK, Hunt GJ (2011) A scientific note on a new assay to measure honeybee mite grooming behavior. Apidologie 42:481–484
Anguiano-Baez R, Guzman-Novoa E, Hamiduzzaman MM, Espinosa-Montaño LG, Correa-Benítez A (2016) Varroa destructor (Mesostigmata: Varroidae) parasitism and climate differentially influence the prevalence, levels and overt infections of deformed wing virus in honey bees (Hymenoptera: Apidae). J Insect Sci 16:44
Arechavaleta-Velasco ME, Guzmán-Novoa E (2001) Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 32:157–174
Arechavaleta-Velasco ME, Alcala-Escamilla K, Robles-Rios C, Tsuruda JM, Hunt GJ (2012) Fine-scale lingkage mapping reveals a small set of candidate genes influencing honey bee grooming behavior in response to varroa mites. PLoS One 7:e47269
Aumeier P (2000) Grooming as a tolerance factor against Varroa jacobsoni: a critical assessment on Africanized bees. Apidologie 31:633–634
Bahreini R, Currie RW (2015) The effect of queen pheromone status on Varroa mite removal from honey bee colonies with different grooming ability. Exp Appl Acarol 66:383–397
Biswas S, Reinhard J, Oakeshott J, Russel R, Srinivasan MV, Claudianos C (2010) Sensory regulation of Neuroligins and Neurexin 1 in the honeybee brain. PLoS ONE 5:e9133
Boecking O, Spivak M (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30:141–158
Boncristiani H, Underwood R, Schwarz R, Evans JD, Pettis J, vanEngelsdorp D (2012) Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J Insect Physiol 58:613–620
Bourgeois CF, Lejeune F, Stévenin J (2004) Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog Nucleic Acid Res Mol Biol 78:37–88
Boutin S, Alburaki M, Mercier PL, Giovenazzo P, Derome N (2015) Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genom 16:500
Buchler R, Drescher W, Tornier I (1992) Grooming behaviour of Apis ceranae, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Exp Appl Acarol 16:313–319
Chen CYJ, Jin S, Goodwin PH (2000) An improved method for the isolation of total RNA from Malva pusilla tissues infected with Colletotrichum gloeosporioides. J Phytopathol 148:57–60
Currie RW, Tahmasbi GH (2008) The ability of high- and low-grooming lines of honey bees to remove the parasitic mite Varroa destructor is affected by environmental conditions. Can J Zool 86:1059–1067
Dainat B, Evans JD, Chen YP, Gauthier L, Neumann P (2012) Predictive markers of honey bee colony collapse. PLoS One 7:e32151
Danka RG, Villa JD (2003) Autogrooming by resistant honey bees challenged with individual tracheal mites. Apidologie 34:591–596
Danka RG, Villa JD (2005) An association in honey bees between autogrooming and the presence of migrating tracheal mites. Apidologie 36:331–333
De Jong D, de Jong PH, Gonçalves LS (1982) Weight loss and other damage to developing worker honey bees (Apis mellifera) due to infestation with Varroa jacobsoni. J Apic Res 21:165–167
Dean JD, Goodwin PH, Hsiang T (2002) Comparison of relative RT-PCR and Northern blot analyses to measure expression of ß-1,3-glucanase in Nicotiana benthamiana infected with Colletotrichum destructivum. Plant Mol Biol Rep 20:347–356
Emsen B, Hamiduzzaman MM, Goodwin PH, Guzman-Novoa E (2015) Lower virus infections in Varroa destructor-infested and uninfested brood and adult honey bees (Apis mellifera) of a low mite population growth colony compared to a high mite population growth colony. PLoS One 10:e0118885
Etherton MR, Blaiss CA, Powell CM, Südhof TC (2009) Mouse neurexin-1a deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA 106:17998–18003
Evans JD (2006) Beepath: an ordered quantitative-PCR array for exploring honey bee immunity and disease. J Invertebr Pathol 93:135–139
Feng J, Schroer R, Yan J, Song W, Yang C et al (2006) High frequency of neurexin1 beta signal peptide structural variants in patients with autism. Neurosci Lett 409:10–13
Fries I, Huazhen W, Wei S, Jin CS (1996) Grooming behavior and damaged mites (Varroa jacobsoni) in Apis cerana cerana and Apis mellifera ligustica. Apidologie 27:3–11
Gary NE, Lorenzen K (1990) Vacuum devices for capturing and partitioning commingled subpopulations of honey bees (Hymenoptera: Apidae). Ann Entomol Soc Am 83:1152–1154
Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE (2003) Pheromone-mediated gene expression in the honey bee brain. Proc Natl Acad Sci USA 100:14519–14525
Guzman-Novoa E, Eccles L, Calvete Y, McGowan J, Kelly PG, Correa-Benítez A (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41:443–450
Guzman-Novoa E, Emsen B, Unger P, Espinosa-Montaño LG, Petukhova T (2012) Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J Invertebr Pathol 110:314–320
Hamiduzzaman MM, Sinia A, Guzman-Novoa E, Goodwin PH (2012) Entomopathogenic fungi as potential biocontrol agents of the ecto-parasitic mite, Varroa destructor, and their effect on the immune response of honey bees (Apis mellifera L.). J Invertebr Pathol 111:237–243
Hamiduzzaman MM, Guzman-Novoa E, Goodwin PH, Reyes-Quintana M, Koleoglu G, Correa-Benítez A, Petukhova T (2015) Differential responses of Africanized and European honey bees (Apis mellifera) to viral replication following mechanical transmission or Varroa destructor parasitism. J Invertebr Pathol 126:12–20
Heisenberg M (1998) What do the mushroom bodies do for insects? Learn Mem 5:1–10
Hunt G, Given JK, Tsuruda JM, Andino GK (2016) Breeding mite-biting bees to control Varroa. Bee Culture 8:41–47
Invernizzi C, Zefferino I, Santos E, Sánchez L, Mendoza Y (2016) Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J Apic Res. doi:10.1080/00218839.2016.1159055
Kevan PG, Hannan MA, Ostiguy N, Guzmán-Novoa E (2006) A summary of the varroa-virus disease complex in honey bees. Am Bee J 146:694–697
Kirrane MJ, de Guzman LI, Rinderer TE, Frake AM, Wagnitz J, Whelan PM (2012) Age and reproductive status of adult Varroa mites affect grooming success of honey bees. Exp Appl Acarol 58:423–430
Kralj J, Fuchs S (2006) Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 37:577–587
Kralj J, Brockmann A, Fuchs S, Tautz J (2007) The parasite mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J Comp Physiol A 193:363–370
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41:353–363. doi:10.1051/apido/2010017
Le Conte Y, Alaux C, Martin JF, Harbo JR, Harris JW, Dantec C, Séverac D, Cros-Arteil S, Navajas M (2011) Social immunity in honeybees (Apis mellifera): transcriptome analysis of varroa-hygienic behaviour. Insect Mol Biol 20:399–408
Mao W, Schuler MA, Berenbaum MR (2011) CYP9Q-mediated detoxification of acaricides in the honey bee (Apis mellifera). Proc Natl Acad Sci USA 108:12657–12662
Milani N (1999) The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 30:229–234
Moretto G, Gonçalves LS, De Jong D (1993) Heritability of Africanized and European honey bee defensive behavior against the mite Varroa jacobsoni. Rev Brasil Genet 16:71–77
Navajas M, Migeon A, Alaux C, Martin-Magniette ML, Robinson GE, Evans JD, Cros-Arteil S, Crauser D, Le Conte Y (2008) Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom 9:301
Nazzi F, Brown SP, Annoscia D, Del Piccolo F, Di Prisco G et al (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog 8:e1002735
Page RE Jr, Guzmán-Novoa E (1997) The genetic basis of disease resistance. In: Morse RA, Flottum K (eds), Honey bee pests, predators, and diseases, 3rd edn. A I Root Co, Medina, pp 469–492
Peng YS, Fang Y, Xu S, Ge L (1987) The resistance mechanism of the Asian honey bee, Apis cerana Fabr. to an ectoparasitic mite Varroa jacobsoni Oudemanns. J Invertebr Pathol 49:54–60
Pettis JS, Pankiw T (1998) Grooming behaviour by Apis mellifera L. in the presence of Acarapis woodi (Rennie) (Acari: Tarsonemidae). Apidologie 29:241–253
Reichelt AC, Rodgers RJ, Clapcote SJ (2012) The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology 62:1519–1526
Richards EH, Jones B, Bowman A (2011) Salivary secretions from the honeybee mite, Varroa destructor: effects on insect haemocytes and preliminary biochemical characterization. Parasitology 138:602–608
Rinderer TE, Harria JW, Hunt GJ, de Guzman LI (2010) Breeding for resistance to Varroa destructor in North America. Apidologie 41:409–424
Roode JC, Lefévre T (2012) Behavioral immunity in insects. Insects 3:789–820
Ruttner F, Hänel H (1992) Active defence against Varroa mites in a Carniolan strain of honeybee (Apis mellifera carnica Pollmann). Apidologie 23:173–187
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Simonsen A, Cumming RC, Lindmo K, Galaviz V, Cheng S, Rusten TE, Finley KD (2007) Genetic modifiers of the Drosophila blue cheese gene link defects in lysosomal transport with decreased life span and altered ubiquitinated-protein profiles. Genetics 176:1283–1297
Stankus T (2008) A review and bibliography of the literature of honey bee Colony Collapse Disorder: a poorly understood epidemic that clearly threatens the successful pollination of billions of dollars of crops in America. J Agr Food Inform 9:115–143
Subramanyam S, Sardesai N, Puthoff DP, Meyer JM, Nemacheck JA, Gonzalo M, Williams CE (2006) Expression of two wheat defense-response genes, Hfr-1 and Wci-1, under biotic and abiotic stress. Plant Sci 170:90–103
Sudhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911
Szyska P, Galkin A, Menzel R (2008) Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body. Front Syst Neurosci 2:3
Thompson GJ, Yockey H, Lim J, Oldroyd BP (2007) Experimental manipulation of ovary activation and gene expression in honey bee (Apis mellifera) queens and workers: testing hypotheses of reproductive regulation. J Exp Zool 307A:600–610
Wallner K (1999) Varroacides and their residues in bee products. Apidologie 30:235–248
Yang X, Cox-Foster DL (2005) Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci USA 102:7470–7475
Yang X, Cox-Foster D (2007) Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 134:405–412
Zhukovskaya M, Yanagawa A, Forschler BT (2013) Grooming behavior as a mechanism of insect disease defense. Insects 4:609–630
Acknowledgements
We thank Paul Kelly for managing the colonies and for supplying the brood and mites used in these experiments. This study was partially funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) discovery grant to EG (Grant 400571) and by a grant from the United States Department of Agriculture (USDA) Grant 2008-35302-18803 to GJH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies were in accordance with the ethical standards.
Human and animal rights and Informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Edited by Yoon-Mi Hur.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hamiduzzaman, M.M., Emsen, B., Hunt, G.J. et al. Differential Gene Expression Associated with Honey Bee Grooming Behavior in Response to Varroa Mites. Behav Genet 47, 335–344 (2017). https://doi.org/10.1007/s10519-017-9834-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-017-9834-6