Abstract
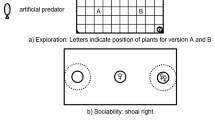
Quantitative genetic studies frequently utilize inbred strains of animals as tools for partitioning the direct and indirect effects of genes from environmental effects in generating an observed phenotype, however, this approach is rarely applied to behavioral studies. Guppies, Poecilia reticulata, perform a set of anti-predator behaviors that may provide an ideal system to study how complex behavioral traits are generated. To assess the utility of ornamental guppies in quantitative genetics studies of behavior, we assayed five morphologically distinct strains of ornamental guppies for response to predator cues and for variation in response among strains. Despite individual variation, all five strains responded to predator cues and differences among strains were found for all assayed behaviors, including measures of boldness and predator avoidance.






Similar content being viewed by others
References
Agrawal, A. F., Brodie, E. D., and Brown, J. (2001). Parent-offspring coadaptation and the dual genetic control of maternal care. Science 292:1710–1712
Anholt, R. R .H., and Mackay, T. F. C. (2001). The genetic architecture of odor-guided behavior in Drosophila melanogaster. Behav. Genet. 31:17–27
Baum, A. E., Solberg, L. C., Kopp, P., Ahmadiyeh, N., Churchill, G., Takahashi, J. S., Jameson, J. L., and Redei, E. E. (2005). Quantitative trait loci associated with elevated thyroid- stimulating hormone in the Wistar-Kyoto rat. Endocrinology 146:870–878
Biesiadecki, B. J., Brand, P. H., Koch, L. G., Metting, P. J., and Britton, S. L. (1999). Phenotypic variation in sensorimotor performance among eleven inbred rat strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 276:R1383-R1389
Blizard, D. A., Wada, Y., Onuki, Y., Kato, K., Mori, T., Taniuchi, T., Hosokawa, H., Otobe, T., Takahashi, A., Shisa, H., Hiai H., and Makino, J. (2005). Use of a standard strain for external calibration in behavioral phenotyping. Behav. Genet. 35:323–332
Bothe, G. W. M ., Bolivar, V. J., Vedder, M. J., and Geistfeld, J. G. (2004). Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 3:149–157
Brodie, E. D. (1989). Genetic correlations between morphology and anti-predator behavior in natural populations of the garter snake Thamnophis ordinoides. Nature 342:542–543
Brodie, E. D. (1992). Correlational selection for color pattern and antipredator behavior in the Garter snake Thamnophis ordinoides. Evolution 46:1284–1298
Brooks, R., and Endler, J. A. (2001). Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 55:1002–1015
Crow, J. F. (1983). Genetics Notes. Minneapolis, MN: Burgess Publishing
Dugatkin, L. A. (1988). Do guppies play tit for tat during predator inspection visits. Behav. Ecol. Sociobiol. 23:395–399
Dugatkin, L. A., and Alfieri, M. (1992). Interpopulational differences in the use of the tit-for-tat strategy during predator inspection in the guppy, Poecilia reticulata. Evol. Ecol. 6:519–526
Flint, J. (2003). Analysis of quantitative trait loci that influence animal behavior. J. Neurobiol. 54:46–77
Ghalambor, C. K., Walker, J. A., and Reznick, D. N. (2002). Multi-trait selection, adaptation, and constraints on the evolution of performance: an empirical example using Trinidadian guppies. Integr. Comp. Biol. 42:1234–1234
Godin, J. G. J., and Dugatkin, L. A. (1996). Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl. Acad. Sci. USA 93:10262–10267
Guo, S. (2004). Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 3:63–74
Ha, J. C. (1990). Eve-Row. Seattle, WA
Hager, R., and Johnstone, R. A. (2003). The genetic basis of family conflict resolution in mice. Nature 421:533–535
Houde, A. E. (1997). Sex, Color and Mate Choice in Guppies. Princeton, NJ: Princeton University Press
Isles, A. R., Humby, T., Walters, E., and Wilkinson, L. S. (2004). Common genetic effects on variation in impulsivity and activity in mice. J. Neurosci. 24:6733–6740
JMP, Version 5.0.1a. SAS Institute Inc., Cary, NC, 1989–2005
Kelley, J.L., and Magurran, A.E. (2003). Learned predator recognition and antipredator responses in fishes. Fish Fish. 4:216–226
Kölliker, M., Brinkhof, M. W. G., Heeb, P., Fitze, P. S., and Richner, H. (2000). The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. Roy. Soc. Lond. Ser. B Biol. Sci. 267:2127–2132
Lindholm, A. K., Breden, F., Alexander, H. J., Chan, W. K., Thakurta, S. G., and Brooks, R. (2005). Invasion success and genetic diversity of introduced populations of guppies Poecilia reticulata in Australia. Mol. Ecol. 14:3671–3682
Mackay, T. F. C., Hackett, J. B., Lyman, R. F., Wayne, M. L., and Anholt, R. R. H. (1996). Quantitative genetic variation of odor-guided behavior in a natural population of Drosophila melanogaster. Genetics 144:727–735
Moore, A. J., Brodie, E. D., and Wolf, J. B. (1997). Interacting phenotypes and the evolutionary process. 1. Direct and indirect genetic effects of social interactions. Evolution 51:1352–1362
Park, Y. G., Clifford, R., Buetow, K. H., and Hunter, K. W. (2003). Multiple cross and inbred strain haplotype mapping of complex-trait candidate genes. Genome Res. 13:118–121
Price, E. O. (1984). Behavioral-aspects of animal domestication. Quart. Rev. Biol. 59:1–32
Reznick, D., Butler, M. J., and Rodd, H. (2001). Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am. Natural. 157:126–140
Reznick, D. A., Bryga, H., and Endler, J. A. (1990). Experimentally induced life-history evolution in a natural-population. Nature 346:357–359
Robison, B. D., and William, R. (2005). A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Can. J. Fish. Aquat. Sci. 62:2046–2054
Rodd, F. H., and Sokolowski, M. B. (1995). complex origins of variation in the sexual- behavior of male Trinidadian guppies, Poecilia reticulata – interactions between social-environment, heredity, body-size and age. Anim. Behav. 49:1139–1159
Rybicki, S. (2005). Personal communication, Olean, NY
Sinervo, B., Bleay, C., and Adamopoulou, C. (2001). Social causes of correlational selection and the resolution of a heritable throat color polymorphism in a lizard. Evolution 55:2040–2052
Watanabe, T., Yoshida, M., Nakajima, M., and Taniguchi, N. (2005). Linkage mapping of AFLP and microsatellite DNA markers with the body color- and sex-determining loci in the guppy (Poecilia reticulata). Zool. Sci. 22:883–889
West-Eberhard, M. J. (1983). Sexual selection, social competition, and speciation. Quart. Rev. Biol. 58:155–183
Wolf, J. B., Brodie, E. D., and Moore, A. J. (1999). Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. Am. Natural. 153:254–266
Yamamoto, T., and Reinhardt, U. G. (2003). Dominance and predator avoidance in domesticated and wild masu salmon Oncorhynchus masou. Fish. Sci. 69:88–94
ACKNOWLEDGMENTS
We wish to thank Eíllen Rodriguez for assistance with filming and animal care and CISAB for financial and technical support. Dr. Stephanie Welter provided valuable feedback on experimental design. Dr. Mathias Kölliker provided invaluable statistical advice. The members of the Brodie lab and Dr. Teresa Dzieweczynski provided valuable feedback on a previous draft of the manuscript. We also wish to thank two anonymous reviewers for their feedback in strengthening the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bleakley, B.H., Martell, C.M. & Brodie, E.D. Variation in Anti-Predator Behavior Among Five Strains of Inbred Guppies, Poecilia reticulata . Behav Genet 36, 783–791 (2006). https://doi.org/10.1007/s10519-005-9044-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-005-9044-5