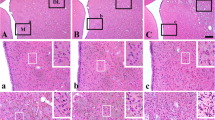
The quantitative content of HIF-1α- and HIF-2α-immunopositive brain neurons in Wistar rats was studied 1, 15, and 30 days after modeling of myocardial infarction. In rats of the control group, the immunohistochemical markers HIF-1α and HIF-2α in the prefrontal cortex of the brain were determined in few pale-colored neurons and capillaries. One day after myocardial infarction simulation, the number of HIF-1α+ neurons increased, and on day 15 it reached the maximum level: the concentration of immunopositive neurons and capillaries increased by 24.7 and 18.4%, respectively, in comparison with the control. After 30 days, the number of HIF-1α+ structures decreased, but remained above the control values. The number of neurons and capillaries positively stained for HIF-2α peaked only on day 30 of the postinfarction period.
Similar content being viewed by others
References
Yakushin SS, Nikulina NN, Seleznev SV. Miocardial Infarction. Moscow, 2019. Russian.
Reed GW, Rossi JE, Cannon C.P. Acute myocardial infarction. Lancet. 2017;389:197-210. doi: https://doi.org/10.1016/S0140-6736(16)30677-8
Vysotsky SV, Pankova EP, Platonova NV, Seledtsov AM, Tikhova MV. Mental disorders that developed against the background of myocardial infarction. Kompleks. Probl. Serd.-Sosud. Zabolev. 2013;(3):90-92. Russian.
Levin OS. Cognitive disorders in the therapeutical practice: cardiovascular diseases. Consilium Medicum. 2009;11(2):55-61. Russian.
Chertok VM, Nevzorova VA, Zakharchuk NV, Chertok AG. The comparative characteristic of the neurons containing the hypoxia-inducible factor lalpha and 2alpha in the brain of rats at hypertension. Tsitologiya. 2018;60(11):883-888. Russian. doi: https://doi.org/10.1134/S0041377118110032
Chertok VM, Nevzorova VA, Zakharchuk NV. Comparative study of HIF-1α- and HIF-2α-immunopositive neurons and capillaries in rat cortex under conditions of tissue hypoxia. Bull. Exp. Biol. Med. 2018;165(4):516-520. doi: https://doi.org/10.1007/s10517-018-4207-6
Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J. Neurosci. 2008;28(8):1988-1993. doi: https://doi.org/10.1523/JNEUROSCI.5323-07.2008
López-Hernández B, Posadas I, Podlesniy P, Abad MA, Trullas R, Ceña V. HIF-1α is neuroprotective during the early phases of mild hypoxia in rat cortical neurons. Exp. Neurol. 2012;233(1):543-554. doi: https://doi.org/10.1016/j.expneurol.2011.11.040
Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors — similar but not identical. Mol. Cells. 2010;29(5):435-442. doi: https://doi.org/10.1007/s10059-010-0067-2
Nevzorova VA, Chertok VM, Brodskaya TA, Roshchenko RV, Plekhova NG. Experimental modeling of myocardial infarction in old rats. Tikhookean. Med. Zh. 2022;(2):72-74. Russian. doi: https://doi.org/10.34215/1609-1175-2022-2-72-74
Mohindra V, Tripathi RK, Singh RK, Lal KK. Molecular characterization and expression analysis of three hypoxia-inducible factor alpha subunits, HIF-1α, -2α and -3α in hypoxia-tolerant Indian catfish, Clarias batrachus [Linnaeus, 1758]. Mol. Biol. Rep. 2013;40(10):5805-5815. doi: https://doi.org/10.1007/s11033-013-2685-1
Chertok VM, Zakharchuk NV, Chertok AG. Cellular-molecular mechanisms of the regulation of angiogenesis in the brain. Neurosci. Behav. Physiol. 2019;49(5):544-554. doi: https://doi.org/10.1007/s11055-019-00768-2
Nevzorova VA, Chertok VM, Brodskaya TA, Selyukova PA, Zakharchuk NV. Mitochondrial dysfunction and vascular aging in comorbid pathology. Tikhookean. Med. Zh. 2022;(1):10-16. Russian. doi: https://doi.org/10.34215/1609-1175-2022-1-10-16
Bon EI, Zimatkin SM, Maksimovich NYv. Effect of hypoxia on morphofunctional characteristics of brain neurons and molecular markers of ischemic hypoxia. Vestn. Smolensk. Gos. Med. Akad. 2021;20(1):51-56. doi: https://doi.org/10.37903/vsgma.2021.1.8
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 175, No. 1, pp. 117-121, January, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chertok, V.M., Nevzorova, V.A. & Kotsyuba, A.E. HIF-1α- and HIF-2α-Immunopositive Neurons and Capillaries in the Prefrontal Cerebral Cortex of Rats with Experimental Myocardial Infarction. Bull Exp Biol Med 175, 101–105 (2023). https://doi.org/10.1007/s10517-023-05819-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-023-05819-w