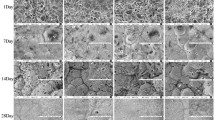
Localization of PCNA, CD44, osteocalcin, Mdm2, p53, and caspase-3 on the surface of implant with calcium phosphate and hydroxyapatite coating was studied by immunocytochemical method in a model of femur fracture in rats. PCNA+, Ost+, CD44+, and Mdm2+ cells were found in the periosteum, in the layer of the outer surrounding plates, and in the connective tissue of the Haversian canals. Cell density increased on day 7 after fracture and then decreased by day 30. The number of p53+ and CASP3+ cells reached a maximum on day 14 (they were predominantly located in the periosteum and bone plates adjacent to it) and decreased by day 30. Calcium phosphate coating stimulated proliferative activity of cells at the early stages of the regeneration phase and apoptotic death at the later stages. Components of coating can be viewed as a positioning clue for differentiation of mesenchymal stromal cells. The effectiveness of reparative osteogenesis is determined by the balance of proliferative and destructive factors at the site of the fracture healing. This process can be optimized with various nanostructured materials with osteoinductive properties, in particular bioresorbable calcium phosphate coatings on titanium implants. However, the influence of these components on the state of cambial cells, their differentiation, and positioning in the repair zone is unknown.
Similar content being viewed by others
References
Kalinichenko SG, Matveeva NY, Kostiv RE, Puz’ AV. Role of Vascular Endothelial Growth Factor and Transforming Growth Factor-β2 in Rat Bone Tissue after Bone Fracture and Placement of Titanium Implants with Bioactive Bioresorbable Coatings. Bull. Exp. Biol. Med. 2017;162(5):671-675. doi: https://doi.org/10.1007/s10517-017-3684-3
Kostiv RE, Kalinichenko SG, Matveeva NYu. Trophic factors of bone growth, their morphogenetic characterization and clinical significance. Tikhookean. Med. Zh. 2017;(1):10-16. doi: https://doi.org/10.17238/PmJ1609-1175.2017.1.10-16. Russian.
Plekhova NG, Lyapun IN, Drobot EI, Shevchuk DV, Sinebryukhov SL, Mashtalyar DV, Gnedenkov SV. Functional State of Mesenchymal Stem Cells upon Exposure to Bioactive Coatings on Titanium Alloys. Bull. Exp. Biol. Med. 2020;169(1):147-156. doi: https://doi.org/10.1007/s10517-020-04841-6
Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD, Yu X, Kumbar SG. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr. Pharm. Des. 2013;19(19):3420-3428. doi: https://doi.org/10.2174/1381612811319190008
Beck GR Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, includ-ing Nrf2. Exp. Cell Res. 2003;288(2):288-300. doi: https://doi.org/10.1016/s0014-4827(03)00213-1
Beutel BG, Danna NR, Granato R, Bonfante EA, Marin C, Tovar N, Suzuki M, Coelho PG. Implant design and its effects on osseointegration over time within cortical and trabecular bone. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(6):1091-1097. doi: https://doi.org/10.1002/jbm.b.33463
Bolander J, Chai YC, Geris L, Schrooten J, Lambrechts D, Roberts SJ, Luyten FP. Early BMP, Wnt and Ca2+/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials. 2016;86:106-118. doi: https://doi.org/10.1016/j.biomaterials.2016.01.059
Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J. Bone Miner. Res. 2009;24(4):597-605. doi: https://doi.org/10.1359/jbmr.081210
Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8(2):272-288. doi: https://doi.org/10.7150/ijbs.2929
Dumic-Cule I, Peric M, Kucko L, Grgurevic L, Pecina M, Vukicevic S. Bone morphogenetic proteins in fracture repair. Int. Orthop. 2018;42(11):2619-2626. doi: https://doi.org/10.1007/s00264-018-4153-y
García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112-121. doi: https://doi.org/10.1016/j.bone.2015.07.007
Garg P, Mazur MM, Buck AC, Wandtke ME, Liu J, Ebraheim NA. Prospective review of mesenchymal stem cells differentiation into osteoblasts. Orthop. Surg. 2017;9(1):13-19. doi: https://doi.org/10.1111/os.12304
Granero-Moltó F, Myers TJ, Weis JA, Longobardi L, Li T, Yan Y, Case N, Rubin J, Spagnoli A. Mesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cells. 2011;29(10):1537-1548. doi: https://doi.org/10.1002/stem.697
Han QQ, Du Y, Yang PS. The role of small molecules in bone regeneration. Future Med. Chem. 2013;5(14):1671-1684. doi: https://doi.org/10.4155/fmc.13.133
Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015;94:3-12. doi: https://doi.org/10.1016/j.addr.2015.09.008
Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008;3(2):131-145. doi: https://doi.org/10.2174/157488808784223032
Hyzy SL, Olivares-Navarrete R, Ortman S, Boyan BD, Schwartz Z. Bone morphogenetic protein 2 alters osteogenesis and anti-inflammatory profiles of mesenchymal stem cells induced by microtextured titanium in vitro. Tissue Eng. Part A. 2017;23(19-20):1132-1141. doi: https://doi.org/10.1089/ten.TEA.2017.0003
Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54(2):264-271. doi: https://doi.org/10.1016/j.bone.2012.11.038
Kalinichenko SG, Matveeva NY. Morphological characteristics of apoptosis and its significance in neurogenesis. Neurosci. Behav. Physiol. 2008;38(4):333-344. doi: https://doi.org/10.1007/s11055-008-0046-7
Kalinichenko SG, Matveeva NY, Kostiv RY, Edranov SS. The topography and proliferative activity of cells immunoreactive to various growth factors in rat femoral bone tissues after experimental fracture and implantation of titanium implants with bioactive biodegradable coatings. Biomed. Mater. Eng. 2019;30(1):85-95. doi: https://doi.org/10.3233/BME-181035
Komori T. Cell death in chondrocytes, osteoblasts and osteocytes. Int. J. Mol. Sci. 2016;17(12):2045. doi: https://doi.org/10.3390/ijms17122045
Li G, White G, Connolly C, Marsh D. Cell proliferation and apoptosis during fracture healing. J. Bone Miner. Res. 2002;17(5):791-799. doi: https://doi.org/10.1359/jbmr.2002.17.5.791
Li W, Liu Y, Wang B, Luo Y, Hu N, Chen D, Zhang X, Xiong Y. Protective effect of berberine against oxidative stress-induced apoptosis in rat bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2016;12(6):4041-4048. doi: https://doi.org/10.3892/etm.2016.3866
Olivares-Navarrete R, Hyzy SL, Haithcock DA, Cundiff CA, Schwartz Z, Boyan BD. Coordinated regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by endogenous bone morphogenetic proteins. Bone. 2015;73:208-216. doi: https://doi.org/10.1016/j.bone.2014.12.057
Palumbo C, Ferretti M, De Pol A. Apoptosis during intramembranous ossification. J. Anat. 2003;203(6):589-598. doi: https://doi.org/10.1046/j.1469-7580.2003.00247.x
Toosi S, Behravan J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors. 2020;46(3):326-340. doi: https://doi.org/10.1002/biof.1598
Wang T, Zhang X, Bikle DD. Osteogenic differentiation of periosteal cells during fracture healing. J. Cell. Physiol. 2017;232(5):913-921. doi: https://doi.org/10.1002/jcp.25641
Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2006;172(1):115-125. doi: https://doi.org/10.1083/jcb.200507106
Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int. Orthop. 2013;37(12):2491-2498. doi: https://doi.org/10.1007/s00264-013-2059-2
Yumoto K, Nifuji A, Rittling SR, Tsuchiya Y, Kon S, Uede T, Denhardt DT, Hemmi H, Notomi T, Hayata T, Ezura Y, Nakamoto T, Noda M. Osteopontin deficiency suppresses tumor necrosis factor-α-induced apoptosis in chondrocytes. Cartilage. 2012;3(1):79-85. doi: https://doi.org/10.1177/1947603511421502
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 2, pp. 136-143, June, 2021
Rights and permissions
About this article
Cite this article
Matveeva, N.Y., Kalinichenko, S.G. & Kostiv, R.E. Dynamics of Renewal of Cell Populations of the Bone Tissue on the Surface of Titanium Implants with Bioactive Coating during Fracture Modeling in Rats. Bull Exp Biol Med 171, 559–565 (2021). https://doi.org/10.1007/s10517-021-05269-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05269-2