Abstract
Recently, medical plants have been widely used as replacements for antibiotics in disease treatment. Because of its multiple medicinal uses, peppermint (Mentha piperita, MP) is a common herbal remedy. In the present study, MP powder was used as a feed additive to assess growth performance; hematological; biochemical and immune parameters; intestinal histology; and interleukin 1β (IL-1β) gene expression, as well as protection against Vibrio alginolyticus infection in Oreochromis niloticus. O. niloticus (n = 120, 25.66 ± 0.16 g) were fed diets containing 0 (CTR), 2, 3, or 4% MP for 60 days. The results revealed that the inclusion of 2% MP significantly improved the growth indices, intestinal morphological parameters, and reduced the feed conversion ratio. The 2% MP treatment significantly (P < 0.05) increased hematological parameters (red blood cell (RBC) count, white blood cell (WBC) count, packed cell volume% (PCV%), hemoglobin) compared with those of the CTR (P < 0.05). Additionally, feeding fish 2% MP diets decreased the levels of cholesterol and LDL (low-density lipoprotein). There were significant increases in immune responses (serum protein and phagocytic activity and index) and non-significant increases in the expression of IL-1β in the 2% MP group comparing with the other groups and the CTR group (P < 0.05). At the end of the feeding trial (60 days), fish were challenged with a virulent strain of Vibrio alginolyticus and the results showed that the mortality rate decreased in the 2% MP treatment group, followed by the 3% and 4% MP groups. Overall, the results revealed that the dietary inclusion of 2% MP can exhibit growth-promoting and immunostimulant effects for sustainable aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture has grown worldwide to capture a larger portion of the market for edible fisheries products. One of the most highly cultivated fish species in Egypt and around the world is O. niloticus (Khalil et al. 2021; Kord et al. 2021). Aqua-culturists are very interested in this species because of its high marketability, capacity to adapt to different environmental conditions, and disease resistance (Kord et al. 2021). However, a high fish population density and a lack of adequate proper sanitation not only pose hazards to fish but also increase the possibility of infections spreading to the surrounding aquatic environment (Li et al. 2021). The most popular method for controlling bacterial diseases is the use of antibiotics, but this approach is frequently very expensive, suppresses the immune system (Abu-Zahra 2023; 2024), pollutes the environment, leaves chemical residues in fish tissues that may be hazardous to human health, and promotes the evolution of antibiotic-resistant bacteria (Polianciuc et al. 2020). Immunostimulants might be good alternatives to chemotherapeutics and antibiotics, but only at a certain level because they leave behind residues in the environment. The current focus of disease management in aquaculture should be on finding ecologically friendly strategies that can enhance aquatic welfare, one of which is feed additives.
The study of dietary plant extracts has recently become a very busy topic of research. Plant extracts have great promise for application in aquaculture because they are known to have beneficial impacts on the health and immunity of fish. The potential of these extracts as antimicrobial agents and substitutes for chemical agents in aquaculture has recently undergone revision (Kokoska et al. 2019; Álvarez-Martínez et al. 2021). Due to their antioxidant, antibacterial, and immunostimulatory properties (Parham et al. 2020), herbal plants can play a substantial role in the treatment and prevention of fish diseases. Currently, aquaculture uses more than 60 plant species to support the enhancement of fish health and disease prevention (Van Hai 2015). However, additional studies are essential for evaluating the application of these products due to the diversity of metabolites and chemical characteristics that plants can produce, as well as their ecological impacts (Neelavathi et al. 2013).
The Mentha piperita (peppermint or mint), produced by hybridization of spearmint and water mint, belongs to family Lamiaceae. MP, a plant evaluated for use in aquaculture, is known for its many medicinal uses as a popular herbal remedy. Its antimicrobial (Mahboubi and Haghi 2008), antioxidative, and antiparasitic effect (Anjos and Isaac 2020) and cholesterol-lowering properties (Hardari et al. 2010) have long been confirmed.
MP is frequently used to treat digestive issues, and some of its active ingredients, such as menthol, stimulate the gastrointestinal mucosa to relax (Magouz et al. 2021). This relaxation outcome can lead to increased release of digestive enzymes and positive alterations in intestinal motility (Magouz et al. 2021). Many studies have confirmed the capability of peppermint (powder or aqueous extracts) to enhance the digestibility and stimulate fish growth and health of Lates calcarifer (Talpur 2014), O. niloticus (Dawood et al. 2020; Magouz et al. 2021), Rutilus frisii kutum (Adel et al. 2015), and Rutilus caspicus (Paknejad et al. 2020).
Vibriosis is a fatal bacterial disease that affects fish and causes high mortality in addition to significant economic losses (Mohd Nor et al. 2019). Vibriosis is a common disease of marine environment and frequently dispersed in brackish or seawaters. The disease epidemics only emerge when fish are exposed to infectious pathogen in the presence of extremely stressful conditions (Abdelsalam et al. 2021). Because of their highly flexible genome which can horizontally transmit virulence genes, vibriosis is one of the most common pathogenic diseases affecting fish (Xu et al. 2017). Many fish species, such as O. niloticus and Clarias gariepinus, are susceptible to epizootic infections with high mortalities brought on by Vibrio spp. (Abdelsalam et al. 2021). Vibrio alginolyticus, V. mimicus, V. anguillarum, and V. vulnificus are frequently implicated in infection and mortalities among wild and farmed fish (Abdelsalam et al. 2021).
Therefore, this study was designed to evaluate the impacts of MP on growth parameters, biological indices (hematological, biochemical, and immune parameters), and disease resistance and survival of O. niloticus infected with Vibrio alginolyticus. Few studies have investigated the optimal levels of MP dietary supplement for fish. We explored whether this plant could be used as a natural fish immunostimulant and growth enhancer.
Materials and methods
Bacteriological examination
A total of 100 randomly apparently healthy and diseased O. niloticus were obtained from different farms in Kafrelsheikh Province, Egypt, from March to August 2022. Fish were transferred alive to the lab in a well-aerated tank; they were anesthetized, subjected to spinal cord transaction, and immediately subjected to bacteriological examination.
All the fish samples were internally examined, and Vibrio spp. were isolated from the liver, spleen, heart, kidney, skin, and gills (Alapide-Tendencia and Dureza (1997)) using TSB (trypticase soya broth) supplemented with 3% NaCl. The samples were then incubated at 30 °C for 24 h before being streaked on TCBS (thiosulfate citrate bile salts) media. After 24 h of incubation at 30 °C, the plates were checked for the presence of characteristic Vibrio spp. colonies.
Identification of bacterial isolates
All the purified isolates were identified by their morphology, such as shape, motility, and Gram stain. Biochemical identification was carried out using the following subsequent tests: indole production, citrate utilization, string test, arginine hydrolysis, methyl red, oxidase, triple sugar iron, urease, hydrogen sulfide production, Voges Proskauer, gelatin liquefaction, nitrate reduction, ornithine decarboxylase, B-galactosidase (ONPG), arginine decarboxylase, L-lysine decarboxylase, sensitivity to the Vibrio static agent O/129, and salt tolerance (Parveen and Tamplin 2013).
Identification of the Vibrio 16SrRNA gene and V. alginolyticus species–specific genes and some virulence genes via polymerase chain reaction (PCR)
Using primers targeting 663 bp of the 16S rRNA gene specific for the genus Vibrio, five isolates suspected to be Vibrio spp. were molecularly confirmed. Three isolates suspected to be V. alginolyticus (n = 3) were molecularly confirmed using primers targeting 737 bp of the collagenase genes and primers targeting 250 bp for the trh genes as shown in Table 1.
DNA extraction
DNA extraction from the samples was carried out using the QIAamp DNA Mini Kit (Qiagen, Germany, GmbH), with some adjustments made to the manufacturer’s instructions. The oligonucleotide primers used (Metabion Germany) are detailed in Table 1.
PCR amplification
The primers used in the 25-µl reaction mixture consisted of 12.5 µl of Emerald Amp Max PCR Master Mix (Takara, Japan), 6 µl of DNA template, 1 µl of each primer at a concentration of 20 pmol, and 4.5 µl of water. The reaction was conducted utilizing a Biosystem 2720 heat cycler.
Analysis of the PCR products
1 × TBE buffer was used at room temperature to electrophorese the PCR products on a 1.5% agarose gel (Applichem, Germany, GmbH) with gradients of 5 V/cm. Using a gel documentation system, 15 µl of the PCR products was added to each gel slot for gel analysis (Alpha Innotech, Biometra). Computer software was used to analyze the data once the gel was photographed.
Fish rearing conditions
O. niloticus (n = 120; 25.66 ± 0.16 g) that were apparently healthy was obtained from a private fish farm (Kafrelsheikh Province, Egypt). Fish were transferred to the local laboratory of the Animal Health Research Institute in Kafrelsheikh Province in a well-aerated tank, where they were housed in glass aquaria (50 × 40 × 40 cm). The tanks were provided with dechlorinated tap water. The water temperature was held at 25 ± 2 °C and electric pumps were used to maintain constant aeration. Debris was siphoned and half of the water was renewed daily and fully twice weekly. The fish were acclimatized for 14 days. During the adaptation period, the fish were fed a basal diet ad libitum. Water parameters were frequently monitored and adjusted using water-analyzing device, where dissolved oxygen, pH, and temperature recorded 6.4 ± 1.2 mg/l, 6.8 ± 0.2, and 25 ± 1 °C, respectively.
Experimental design
Four experimental diets (Table 2) were prepared from commercial items according to Jobling (2011) to meet the nutritional needs of O. niloticus. After being removed from their natural habitat, the M. piperita leaves were correctly recognized. The leaves were first dried in the shade for 3 to 4 days and then at 55 °C. After that, the plants were ground into a uniform powder and completely combined with the basal diet (Bhatnagar and Saluja 2019). The basal diet served as the CTR. The other 3 experimental diets were prepared to contain MP powder in the following proportions: 2%, 3%, and 4% of feed instead of corn grains. A cereal grinding machine (FFC-45, JIMO, China) was utilized to grind and sieve the diet materials. After the components had been ground and MP powder was added, an appropriate amount of water was added to create a dough. The pellets (1.5 mm) were prepared using an experimental extruder (Model SYSLG30-IV), which was dried for one day at ambient temperature, wrapped in plastic bags, and kept at − 2 °C till usage.
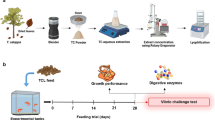
Fish were distributed into 4 groups each in triplicate (n = 30 fish/group; 10 fish/replicate). The CTR group was fed a basal diet without any additives; the treated groups were fed an MP-supplemented diet (MP diet) at 2, 3, or 4% of feed for groups 2–4, respectively. During the feeding trial (60 days), feed was introduced to the fish twice a day (at 9:00 a.m. and 2:00 p.m.) at a rate of 3% of the fish live body weight. Excessive care was taken to ensure that all of the feed was eaten. The fish were weighed at the beginning (W1) and then every 14 days for the next 60 days. During each period, the feed intake was modified based on the average body weight. Figure 1 illustrates the experimental design.
Growth performance
According to Azab et al. (2018), the growth parameters were calculated by the subsequent equations:
Sampling
At the end of the feeding period (60 days), all fish were fasted for 24 h before sampling and anesthetized with 150 mg/l MS222 (Du et al. 2022). Nine fish were haphazardly taken from each group for collection of two sets of blood samples from the caudal blood vessels. The first was collected with syringes containing a drop of 10% EDTA in EDTA tubes (Abu-Zahra et al. 2023) to estimate hematological parameters, phagocytic activity, and the phagocytic index. The other set of blood was drawn without anticoagulant, and the serum was collected by centrifugation at 3000 rpm for 10 min, collected in Eppendorf tubes, and kept at − 20 °C until examination.
The fish were anesthetized by submerging in a saturated benzocaine solution and cold water and then killed via spinal cord transection. Tissue samples from the intestine (n = 9/group) were stored in 10% formalin until histopathological examination. The fish were dissected on ice to obtain liver tissues (n = 9/group) which were quickly placed in liquid nitrogen and kept at − 80 °C till the extraction of RNA.
Hematological parameters
Hematological parameters were estimated according to Faggio et al. (2014). RBCs (red blood cells; × 106/mm3) and WBCs (white blood cells; × 103/mm3) were counted using a hemocytometer, hemoglobin content (Hb; g/100 ml) was estimated by the cyanmethemoglobin method, and PCV (packed cell volume; %) was assessed by the microhematocrit technique. On sterile slides, thin blood films were taken to assess the differential leucocyte count. After the slides were allowed to dry, modified Wright's stain was applied.
Serum biochemical and immune parameters
Using commercial kits, total serum protein, triglyceride, cholesterol (CHO), and HDL (high-density lipoprotein) and LDL (low-density lipoprotein) were estimated according to Coles (1974), Gottfried and Rosenberg (1973), Zak et al. (1954), and Bergmenyer (1985) respectively. Glucose levels were estimated via the colorimetric method using commercial kits. The commercial kits used and their catalogue number are listed in Table 3.
The phagocytic assay was carried out following the methodology of Siwicki et al. (1994) and Park and Jeong (1996) with minor adjustments. For each treatment, blood samples were taken to determine the number of phagocytic cells and phagocytosed bacteria. The PA (phagocytic activity; % cell with engulfed bacteria) and PI (phagocytic index; number of engulfed bacteria/cell) were estimated by counting 100 phagocytes per slide under a microscope and were calculated as follows:
Serum lysozyme activities were evaluated using the turbidimetric assay designated by Abu-Elala et al. (2013). Then, 25 µl of serum was added to 0.75 mg/ml lyophilized Micrococcus lysodeikticus as a substrate in phosphate buffer adjusted to pH 5.75 in 96-well plates. After incubating for 0 to 15 min at 25 °C, the absorbance was detected via a spectrophotometer at 450 nm. A unit of lysozyme activity was defined as a 0.001/min decrease in absorbency.
Serum bactericidal activity (SBA) was determined following the methods of Biller-Takahashi et al. (2013). After 24-h incubation period, the colonies from the resulting incubated mixture were counted on TSA plates in duplicate (two plates per sample) to determine the number of live bacteria. Better serum bactericidal activity will be shown with a lower bacterial count.
Gene expression
Using the RNAxPlus reagent, total RNA was extracted from each sample’s liver tissues in accordance with the manufacturer’s instructions. A spectrophotometer (NanoDrop ND-1000, Thermo Scientific, USA) was used to measure the amount of extracted RNA at 260/280 nm (Panigrahi et al. 2011). Electrophoresis on a 1.5% agarose gel stained with ethidium bromide was used to measure the quality of the RNA. cDNA (200 ng) was generated using the Genet Bio cDNA® synthesis kit instructions (Korea). Real-time PCRs were carried out in triplicate using a standard technique that included 40 cycles of denaturation, annealing, and extension at 95 °C for 15 s each. The initial denaturation step took place at 95 °C for 10 min. β-actin was utilized as a non-regulatory reference gene.
Quantitative real-time PCR
Using the standard methodology outlined by Miandare et al. (2013), RT‒qPCR (quantitative reverse transcription PCR) was used to estimate the levels of interleukin 1-β (IL-1β) gene expression in liver tissues. Standard curves were created using seven serial dilutions of pooled cDNA, ranging from 1:10 to 1:2000. Using the 2−ΔΔCt technique, the fold change in the relative mRNA expression of the genes was computed (Livak and Schmittgen 2001). The primers used for the analysis of IL-1β gene expression in O. niloticus are tabulated in Table 4.
Histopathological examination
The samples were obtained from the anterior, middle, and posterior parts of the intestine. The formalin-fixed intestinal tissues were processed and embedded in paraffin wax. Then, the sections were cut into 5-mm-thick sections and stained with H&E (Hematoxylin and Eosin) (Gargiulo et al. 1998). A Leica EC3 digital camera linked to a Leica DM 5000 light microscope was used to microphotograph the stained tissues.
Challenge assay
After a feeding period of 60 days, 20 fish from each treatment group were intraperitoneally injected with 0.2 ml/ fish of 1 × 106 CFU of V. alginolyticus (Younes et al. 2016). The strain was earlier isolated from diseased O. niloticus and characterized via phenotypic and molecular methods. The isolates were kept in glycerol at − 20 °C until usage. The bacterial suspensions were made using McFarland standard turbidity tubes (El Latif et al. 2019). During the challenge, the fish were fed a CTR diet alone or plus MP. Dead fish were collected and aseptically examined to determine the cause of death. Mortalities were only taken into account when V. alginolyticus was recovered from infected fish. Mortality rate and RPS (relative percent survival) were recorded for 10 days and were assessed according to the following formulas (Amend 1981):
Re-isolation and identification of V. alginolyticus were performed on moribund fish that survived at the end of the challenge in each group (kidney, heart, liver, gills, spleen, and skin) by bacteriological methods as described previously.
Biosafety measures
Once the experiment was complete, all the dead fish and leftover fish were burned in the laboratory stationary incinerator. The biosafety procedures followed the pathogen regulation directive for infectious materials (Vibrio spp.).
Statistical analysis
The results are displayed as the mean ± SE (standard error). The significance of the data was evaluated by using one-way ANOVA followed by Duncan’s multi-range test to compare the means at P < 0.05 using the software program SPSS version 22. Prior to the data analysis, the normality and homogeneity of variance were assessed. The figures were drawn using the software program SPSS version 22 and Excel 2016.
Results
Colonial and microscopical characters
Typical colonial morphology for V. alginolyticus on TCBS agar was smooth, yellow (sucrose positive), and 2–3 mm in diameter. On the microscopic examination, V. alginolyticus was Gram negative comma-shaped (curved) bacilli, non-sporulating, non-capsulated, arranged singly or in chains, motile by single polar flagella.
Molecular identification of Vibrio isolates
Five isolates supposed to be Vibrio spp. were all positive (Fig. 2A) for the 16SrRNA gene, which is specific for the genus Vibrio. As shown in Table 5 and Fig. 2B, three isolates were amplified for the species-specific primers for V. alginolyticus (collagenase) and the virulence gene (trh). All V. alginolyticus isolates were positive for the species primer (collagenase) and virulence gene (trh). Results of agar disc diffusion tests of Vibrio spp. isolated fromO . niloticus are presented in Table 6.
Agarose gel electrophoresis of A 16SrRNA gene amplification for the molecular identification of Vibrio isolates with an amplicon size of 663 bp (n = 5 samples); ladder: 100 bp. B PCR amplification of the collagenase (737 bp) and trh (250 bp) genes of v. alginolyticus; lane L:100–600 bp DNA ladder; N: negative control; P: positive control; lanes 1–3 (on the right): positive collagenase gene (737 bp). Lanes 1–3 (on the left): Positive trh gene (250 bp). C PCR amplification of sodB (248 bp) and ctxAB (536 bp) of V. cholerae; lane L:100–600 bp DNA ladder; N: negative control; P: positive control; lanes 1–3 (on the right): positive sodB gene (248 bp); lanes 2 and 3 (on the left): positive ctxAB gene (536 bp)
Growth performance
Table 7 shows that compared with the CTR diet, the 2% MP supplement in the O. niloticus diet considerably (P < 0.05) amended the final body weight, gain%, SGR, FCR, PER, and EEU (approximately 7.6%, 20.4%, 13.8%, 14.2%, 16.9%, and 14.7% respectively) compared with those in the CTR group. However, compared with those in the CTR treatment, the growth and feed efficiency parameters were insignificantly lower in the 3 or 4% MP treatment groups (P > 0.05). No mortalities were detected in the treated or CTR groups during the feeding trial.
Hematological parameters
Table 8 shows the variations in hematological parameters in O. niloticus fed diets containing different concentrations of MPs. Two percent MP inclusion significantly (P < 0.05) augmented Hb%, RBCs, WBCs, and PCV% by approximately 27.2%, 27.3%, 16.8%, and 27.2%, respectively, while the other MP inclusion levels (3% or 4%) had no noticeable impact on hematological parameters compared to those of the CTR group. Moreover, all dietary inclusion levels of MPs had no substantial impact on the differential leukocyte count compared with that in the CTR group.
Biochemical parameters
Table 9 shows that the addition of 2% MP to the O. niloticus diet considerably (P < 0.05) decreased the serum total CHO and LDL levels by approximately 18.4% and 30.8%, respectively, while it noticeably (P < 0.05) elevated the total serum protein level by approximately 10.4%; however, the inclusion of other MP levels (3% or 4%) had no substantial effect on the abovementioned serum parameters compared with those in the CTR group. Moreover, all the MP inclusion levels had no effect on the serum triglyceride, HDL, or VLDL levels but did significantly (P < 0.05) reduce the CHO/HDL ratio compared to that in the CTR group. There were no significant (P > 0.05) differences in the serum glucose concentration between the CTR group and the MP-containing diet group except for the fish fed the 4% MP-containing diet.
Immune parameters
Figure 3 shows the variations in immune responses in the O. niloticus experimental groups. The addition of 2% MP significantly (P < 0.05) augmented the phagoctic index and activity (Fig. 3a, b), while the addition of 3 or 4% MP to the O. niloticus diet non-significantly (P > 0.05) increased the phagocytic index and activity compared to those in the CTR group. Moreover, compared with those in the CTR group, the serum lysosomal activity and bactericidal activity (Fig. 3c, d) in the MP groups did not significantly differ (P > 0.05). The lower viable bacterial colony counts in the MP-fed fish relative to those in the CTR group indicated bactericidal improvement.
Immune response of O. niloticus fed diets containing different concentrations of M. piperita (MP). a Phagocytic activity %. b Phagocytic index No. c Lyzosome activity (U/ml). d Serum bactericidal activity (SBA) %. The values are the means; the error bars represent the SE (n = 9/group). The different subscripts above the bars (P < 0.05) indicate significant differences between treatments
Gene expression
Figure 4 shows the fold change in IL-1β gene expression in the O. niloticus groups. The inclusion of 2% MP in the O. niloticus diet did not significantly (P > 0.05) upregulate IL-1β gene expression, while the inclusion of more MP (3% or 4%) non-significantly (P > 0.05) downregulated IL-1β gene expression compared to that in the CTR group.
Gene expression of interleukin 1-β (IL-1β) in the O. niloticus fed diets containing different concentrations of M. piperita (MP). The values are the means; the error bars represent the SE (n = 9/group). The different subscripts above the bars (P < 0.05) indicate significant differences between treatments
Intestinal morphological measurements
Intestinal morphometric measurements of the intestine are presented in Table 10 and Fig. 5. The results of the statistical analysis revealed that dietary inclusion of 2% MP significantly (P < 0.05) increased the number of goblet cells and the length of the villi in the anterior, middle, and posterior parts of the intestine (approximately 37.2% and 37.0%; 30.0% and 25.9%; 44.4% and 57.3%, respectively) compared to those in the CTR group. In contrast, 3% or 4% MP addition reduced villi length and goblet cell number in all intestinal parts in a dose-dependent manner, while all MP inclusion levels non-significantly (P > 0.05) improved villi width and inter villi space in all intestinal parts in a dose-dependent manner except for 2% MP addition which non-significantly (P > 0.05) reduced the inter villi space in all intestinal parts compared to those in the CTR group.
Photomicrograph of H&E-stained intestinal portions, × 100, scale bar = 100 μm. (A1) Anterior portion of the CTR group showing normal villi. (A2) Middle portion of the CTR group showing thin villi lined with pseudostratified epithelium. (A3) Posterior portion of the CTR group showing normal villi. (B1) Anterior portion of the 2% MP group showing a marked increase in villi length. (B2) Middle portion of the 2% MP group showing a marked increase in villi length. (B3) Posterior portion of the 2% MP group showing a marked increase of villi length. (C1) Anterior portion of the 3% MP group showing normal villi. (C2) Middle portion of the 3% MP group showing long and thin villi. (C3) Posterior portion of the 3% MP group showing normal villi. (D1) Anterior portion of the 4% MP group showing normal villi with blunted ends. (D2) Middle portion of the 4% MP group showing normal villi. (D3) Posterior portion of the 4% MP showing normal villi
Challenge assay
Clinical examination of the challenged fish (Fig. 6) revealed loss of appetite, swimming toward the surface, imbalance, fin hemorrhage, tail erosion, ulceration at the base of the pelvic and pectoral fins, and eye protrusion. The severity and frequency of lesions were more severe in the CTR (Fig. 6A) and the other two infection groups (Fig. 6C and D) than in the 2% MP group (Fig. 6B).
Clinical examination of O. niloticus challenged groups. A CTR group showing fin hemorrhage and rot, ulcers at the base of the pelvic and pectoral fins, and eye protrusion. B 2% MP group showing tail erosion. C 3% MP group showing tail erosion and detachment and hemorrhagic fins. D 4% MP group showing hemorrhagic and eroded tail and hemorrhage at the operculum
Internal examination of the infected O. niloticus (Fig. 7) revealed pale and hemorrhagic livers, enlarged spleens, distended gallbladders, severe inflammation and hemorrhages in the intestine, and pale gills. Almost all the treated groups exhibited the same pattern and the CTR group exhibited the most severe changes.
Postmortem examination of O. niloticus challenged groups. E CTR showing pale liver, severe inflammation and hemorrhage in the intestine, and pale gills. F 2% MP group showing splenomegaly, hemorrhagic liver, and distended gall bladder. G 3% MP group showing splenomegaly, hemorrhagic liver, and distended gall bladder. H 4% MP showing splenomegaly, hemorrhagic liver, and distended gall bladder
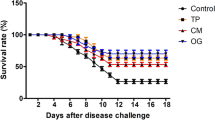
The mortality rate (Fig. 8) was significantly lower in the 2% MP group (0%) than in the 3% MP and 4% MP groups (10% and 5%, respectively). The CTR group had the highest mortality rate (25%). The 3% MP and 4% MP groups achieved RLP of 60% and 80% which was raised to 100% in 2% MP group. Additionally, V. alginolyticus was reisolated in pure cultures from moribund fish.
Discussion
Vibriosis is among the most common zoonotic diseases that human contract from fish and other aquaculture animals. The 16 SrRNA gene was used to identify the genus Vibrio which includes many bacterial species and species-specific genes used for the confirmatory detection of Vibrio spp. and for their differentiation from closely related Vibrio spp. In our investigation, all 5 random suspected isolates examined for the 16SrRNA gene produced a light band at 663 bp. The collagenase gene has been frequently used as a biomarker in the molecular identification of V. alginolyticus and is capable of degrading the basal epithelial membrane and conjunctiva tissue (Mustapha et al. 2012). Additionally, among the essential virulence components of V. alginolyticus is the trh gene. In our study, all V. alginolyticus isolates tested possessed the collagenase and trh genes (100%). Currently, microbial diseases are one of the main obstacles to aquaculture. Due to these limitations, massive amounts of chemicals (such as antibiotics and disinfectants) are used to limit fish mortality and prevent significant financial losses. These therapies, which have been widely used over the past few decades, are currently under heavy criticism due to their proven negative effects on fish (Abu-Zahra 2023; 2024), aquatic environments, and even human health.
The use of medicinal plants in aquafeeds is becoming more popular for a number of reasons, such as their immunostimulatory properties, antioxidant and antimicrobial benefits, eco-friendliness, and ability to increase feed palatability (Adel et al. 2015). The 2% MP treatment (treated with MP at a rate of 2%) significantly improved the body weight, WG, FCR, and PER compared to that of the CTR treatment (with no MP). The explanation could be that MP improved the digestibility and appropriate usage of nutrients, which in turn influenced protein synthesis and, ultimately, growth performance, as well as intestinal morphology, which may have enhanced the ability of the fish to absorb and digest feed. Our findings agreed with those of Adel et al. (2015) and Paknejad et al. (2020), who showed significantly higher growth rates in Caspian roach fingerlings fed on diet containing 2–4 g MP/kg compared to CTR. The same outcomes were also observed in numerous investigations in which extracts from medicinal herbs were used to stimulate feeding and promote growth in aquatic animals (Aguiar et al. 2023; Talpur 2014; Adel et al. 2015). Similar findings were obtained by Mahmoud et al. (2017) who found that addition of curcumin to O. niloticus diets exhibited a significant upsurge in growth performance compared to control group. Also, our data are similar with the studies of Nya and Austin (2009), Talpur and Ikhwanuddin (2013), and El-Kassas et al. (2022), who fed fish on diets supplemented with garlic and ginger diet and moringa diets to rainbow trout, L. calcarifer and O. niloticus, respectively. The authors observed significantly higher growth rates in treated fish compared to the CTR. A comparison of the results of the groups containing 2% MP with those of the groups containing 3% MP, 4% MP, and CTR, 2% MP revealed significant differences with respect to the various growth parameters. These findings demonstrated that 2% MP is an ideal diet for O. niloticus. The obtained data agreed with those obtained by Abdel-Tawwab (2012), who concluded that 2 g of American ginseng/kg diet led to best growth rates of O. niloticus compared to the higher inclusion levels and the CTR.
Hematological parameters are important tools for diagnosing disorders, determining nutrient levels, and assessing hygienic conditions and fish health. Our results revealed that the use of 2% MP in the diet increased Ht, Hb, RBC, and WBC levels. One of the key markers of fish health is the WBC count, which can indicate the existence or absence of infection as well as the kind of response to infection as well as other physiological and pathological elements. Infectious diseases, inflammation, nutrition (Munteanu and Schwartz 2022), stress, age, temperature (Mugwanya et al. 2022), sex, and hormonal changes are among the variables that impact WBC counts. One of the most vital defenses against infection is the nonspecific immune system. One of the primary components of peppermint, menthol, has antifungal and antibacterial effects. Therefore, this chemical may be related to the increase in nonspecific immunity. The data obtained revealed that including peppermint in diet enhanced RBC counts, which may be related to the effects of polyphenolic substances. These compounds can form complexes with iron, facilitating its access to RBCs (Paknejad et al. 2020). Additionally, polyphenols have an antioxidant impact on RBC membranes by providing a physical barrier against free radicals (Paknejad et al. 2020). Our results are in line with those of Talpur and Ikhwanuddin (2012) and of Talpur and Ikhwanuddin (2013), who also observed an increase in WBC count when L. calcifer were fed a diet supplemented with garlic and ginger. Moreover, MP inclusion insignificantly reduced neutrophil % and increased lymphocyte % compared to CTR. This effect may be related to the properties of MP active ingredient (menthol) to reduce inflammation (Chumpitazi et al. 2018). The present data is supported by El-Kassas et al. (2022), who found that moringa leaves inclusion in O. niloticus diet increase lymphocyte and decrease neutrophil number compared to CTR.
The observed growth promotion and other immune-related activities that are discussed below may also be connected to those results on blood cells. Since relatively little information is currently available about the immunomodulatory properties of peppermint, our findings support excessive studies evaluating the potential of peppermint oils and their constituents as immune response modulators.
The present study revealed that the serum glucose concentration significantly (P < 0.05) reduced in the fish fed a diet containing 4% MP compared to that in the other treatment groups. A possible explanation for the decrease in the serum glucose concentration may be related to the stimulatory effect of MP on insulin secretion (Talpur 2014). Additionally, our results revealed a significantly higher serum protein concentration in the 2% MP group than in the CTR group. Moreover, the increases in total serum protein concentration are consistent with the findings of earlier study on Lates calcarifer fed MP-incorporated feed (Talpur 2014). Serum protein levels indicate blood osmolarity, renal function, and the capacity of the liver to synthetize proteins. Therefore, increased serum protein at 2% MP indicates a clearer capacity for protein synthesis, which corresponds with increased growth. Our results revealed that the inclusion of MPs up to 3% led to a significant decrease in blood CHO levels, LDL levels, and the CHO/HDL ratio. Reduced hepatic cholesterol synthesis and elevated hepatic 7α hydroxylase activity could explain this difference. According to the present study, cardiovascular activity may be enhanced due to the decreases in CHO and LDL. These results were in agreement with those of Talpur (2014).
One of the most significant defense mechanisms against pathogenic bacteria is phagocytosis. Our results revealed enhanced phagocytic activity and index in the 2% MP treatment group, which is consistent with the findings of Talpur (2014), who reported considerably greater phagocytic activity after feeding an MP diet to L. calcarifer. These findings suggested that the fish’s nonspecific immunity has improved. As an antibacterial enzyme, lysozyme degrades the peptidoglycan links in the bacterial cell wall and is thus a key defensive molecule in the fish innate immune system. Serum bactericidal activity is an essential component of the immune response and is included in the destruction of fish pathogenic microorganisms. In the current study, there were no significant increases in the serum lysosomal activity in any of the treated groups compared with that in the CTR group. These results are inconsistent with those of Paknejad et al. (2020), Adel et al. (2015), and Talpur (2014). The bactericidal activity of the MPs in the present study was related to the existence of flavonoids, tannins, and other bioactive compounds. Our findings are consistent with those of Talpur and Ikhwanuddin (2012) and (2013), who observed the elevated serum bactericidal activity in L. calcifer following feeding on garlic, neem leaf, and ginger, respectively. Additionally, the inclusion of 2% MP in O. niloticus feed caused a non-significant increase in expression of IL-1β, demonstrating its immunostimulatory effect. To the best of our knowledge, MP leaves have anti-inflammatory proprieties, which is not well studied at the transcriptomic level in O. niloticus. So, our study represents the first report on the effects of MP leaves dietary inclusion on the transcriptomic profile of inflammatory mediator genes, such as IL-1β. Our results revealed non-significant upregulations of the IL-1β mRNA transcripts by dietary inclusion of 2% MP. This effect might be linked to increasing the % of lymphocytes. Moreover, this pro-inflammatory response may be attributed to menthol content of MP (Chumpitazi et al. 2018).
The use of 2% MP in O. niloticus feed led to a greater height and width of intestinal villi, an increase in the number of goblet cells, and a decrease in the inter villi space in all parts of the intestine suggesting improved development of the absorptive area of the intestine. A greater length of intestinal villi in fish leads to improved intestinal health, increased nutrient absorption efficiency, and consequently improved growth performance (Ringø et al. 2022; Huerta-Aguirre et al. 2019). Goblet cells have the potential to protect the mucosal layer of fish from infections, injury, and dehydration, through the expulsion of mucus (Yang and Yu 2021). Moreover, high MP concentrations (3% and 4%) in O. niloticus feed reduced the number of goblet cells, the length and width of intestinal villi, and reduced the intervilli space in all parts of the intestine, consequently decreasing nutrient absorption by decreasing the contact between absorptive cells and nutrients (Adeoye et al. 2016).
Feeding fish experimental diets for 60 days triggered a decrease in morbidity after challenge with V. alginolyticus. Fish fed the MP diet at various concentrations exhibited significantly lower morbidity rates than did those fed the CTR diet. The highest number of fish showing clinical signs was observed in the CTR (15 fish) and 4% MP treatment (12 fish). The 2% MP treatment had the lowest morbidity rate (30%). The mortality rate of O. niloticus was quite lower (25%) in the CTR group, and the inclusion of MP significantly decreased the mortality rate to 0% in the 2% MP groups. These findings demonstrated the influence of water salinity on disease occurrence, which may be explained by the fact that Vibrio spp. are typically found in seawater. These results matched those of Al-Sunaiher et al. (2010), who reported that O. niloticus experimentally infected with V. alginolyticus had a 50% mortality rate within 10 days of injection. The clinical lesions post-infection were nearly similar to those found in naturally infected ones and were less severe in the groups fed MP than in the CTR group. These results may be attributed to enhanced innate immunity, such as increased WBC counts, enhanced of phagocytic activity and index, and increased expression of IL-1β. Similar results were obtained by Talpur (2014). The primary active ingredient of MP is essential oil. It contains a variety of bioactive chemicals with demonstrated biological activity, particularly antimicrobial properties ((Mancianti and Ebani 2020). Because these chemicals can scavenge free radicals, they may be used as therapeutic agents for a variety of pathogens. An earlier study demonstrated the detrimental impacts of A. hydrophila on survival following a challenge, and the inclusion of MP and Bacillus coagulans in the diet has been associated with improved immunological response and growth (Bhatnagar and Saluja 2019). The most obvious clinical and internal lesions in the challenged fish were loss of appetite, swimming toward the surface, imbalance, fin hemorrhage and rot, exophthalmia, pale and hemorrhagic liver, splenomegaly, distended gall bladder, and severe intestinal inflammation and hemorrhage. These results were almost similar to those reported by Sumithra et al. (2019) and Yanuhar et al. (2022) who infected O. niloticus and humpback grouper (cromileptes altivelis) with V. vulnificus and V. harveyi and V. alginolyticus respectively.
Many herbiotics have been described to have various potentials as growth and immune stimulators in aquaculture. In the present study, we focused on M. piperita (MP) as a feed additive for O. niloticus. MP may be superior to other plants because of its high availability, better economic value, and appetite accelerator. Also, it has many properties, such as increasing production, being non-hazardous, not causing fish skin pigmentation (as curcumin at 2% in our unpublished study), and overfeeding of fish does not cause any digestive issues (as garlic) (Banerjee et al. 2003).
Conclusion
In our study, we developed a novel understanding of the use of herbiotics in aquaculture. Our study revealed that MP, a natural growth promoter, is a viable substitute for traditional synthetic growth promoters in aquafeed. MP significantly enhanced O. niloticus growth performance, intestinal morphology, health status, and disease resistance. These results demonstrated the possibility of adding 20 g MP /kg of diet, which could lead to a more sustainable aquaculture sector. Completive study with biochemical analyses of nutrient components of this plant is required to formulate medicinal plants supplemented diets for fish and may be a beneficial way to produce organic fish. It would be interesting to investigate the amelioratory role of this medication in mitigating the degenerative effects followed by infection. Additionally, further investigations are needed to examine the effects of dietary MP on large sizes of fish under conditions of the field.
Data availability
The authors confirm that the data supporting the findings of this study are available within the manuscript, figures, and tables.
References
Abdelsalam M, Ewiss MAZ, Khalefa HS et al (2021) Coinfections of Aeromonas spp., Enterococcus faecalis, and Vibrio alginolyticus isolated from farmed Nile tilapia and African catfish in Egypt, with an emphasis on poor water quality. Microb Pathog 160:105213. https://doi.org/10.1016/j.micpath.2021.105213
Abdel-Tawwab M (2012) The use of American ginseng (Panax quinquefolium) in practical diets for Nile tilapia (Oreochromis niloticus): growth performance and challenge with Aeromonas hydrophila. J Appl Aquac 24:366–376. https://doi.org/10.1080/10454438.2012.733593
Abu-Elala N, Marzouk M, Moustafa M (2013) Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int J Vet Sci Medi 1:21–29. https://doi.org/10.1016/j.ijvsm.2013.05.001
Abu-Elala NM, Abd-Elsalam RM, Marouf S et al (2016) Eutrophication, ammonia intoxication, and infectious diseases: interdisciplinary factors of mass mortalities in cultured Nile tilapia. J Aquat Anim Health 28:187–198. https://doi.org/10.1080/08997659.2016.1185050
Abu-Zahra NIS, Atia AA, Elseify MM, Soliman S (2023) Biological and histological changes and DNA damage in Oreochromis niloticus exposed to oxytetracycline: a potential amelioratory role of ascorbic acid. Aquacult Int. https://doi.org/10.1007/s10499-023-01356-5
Abu-Zahra NIS, Elseify MM, Atia AA, Al-sokary ET (2024) Impacts of florfenicol on immunity, antioxidant activity, and histopathology of Oreochromis niloticus: a potential protective effect of dietary spirulina platensis. Vet Res Commun 48:125–138. https://doi.org/10.1007/s11259-023-10189-9
Adel M, Abedian Amiri A, Zorriehzahra J et al (2015) Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilus frisii kutum). Fish Shellfish Immunol 45:841–847. https://doi.org/10.1016/j.fsi.2015.06.010
Adeoye AA, Yomla R, Jaramillo-Torres A et al (2016) Combined effects of exogenous enzymes and probiotic on Nile tilapia (Oreochromis niloticus) growth, intestinal morphology and microbiome. Aquaculture 463:61–70. https://doi.org/10.1016/j.aquaculture.2016.05.028
Aguiar G, Carneiro C, Campelo D et al (2023) Effects of dietary peppermint (Mentha piperita) essential oil on growth performance, plasma biochemistry, digestive enzyme activity, and oxidative stress responses in juvenile Nile tilapia (Oreochromis niloticus). Fishes 8:374. https://doi.org/10.3390/fishes8070374
Alapide-Tendencia EV, Dureza LA (1997) Isolation of Vibrio spp. from Penaeus monodon (Fabricius) with red disease syndrome. Aquaculture 154:107–114. https://doi.org/10.1016/s0044-8486(97)00045-8
Al-Sunaiher AE, Ibrahim ASS, Alsalamah AA (2010) Association of Vibrio species with disease incidence in some cultured fishes in the Kingdom of Saudi Arabia. In: Wasj; 8(5):653–660. https://www.researchgate.net/publication/268375138_Association_of_Vibrio_Species_with_Disease_Incidence_in_Some_Cultured_Fishes_in_the_Kingdom_of_Saudi_Arabia. Accessed 24 Jan 2024
Álvarez-Martínez FJ, Barrajón-Catalán E, Herranz-López M, Micol V (2021) Antibacterial plant compounds, extracts and essential oils: an updated review on their effects and putative mechanisms of action. Phytomedicine 90:153626. https://doi.org/10.1016/j.phymed.2021.153626
Amend D (1981) Potency testing of fish vaccines. International symposium in fish biologics: Serodiagnostics and vaccines. Environ Sci 49:447–454
Anjos ACP, Isaac A (2020) The efficacy and dosage of Mentha piperita essential oil in the control of Monogenean parasites in Oreochromis niloticus. J Parasit Dis 44:597–606. https://doi.org/10.1007/s12639-020-01233-5
Azab A, Khalaf-Allah H, Khattaby AER et al (2018) Effect of stocking density and feeding rate on growth performance and total production of Nile Tilapia, Oreochromis niloticus reared in earthen ponds. Egypt J Aquacult 8:33–54
Banerjee SK, Mukherjee PK, Maulik SK (2003) Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res 17:97–106. https://doi.org/10.1002/ptr.1281
Bergmenyer H (1985) Methods of enzymatic analysis Bergmenyer HU. Methods of Enzymatic analysis, 3 rd ed. Int J Biochem 17:154–160. https://doi.org/10.1016/0020-711x(85)90098-9
Bhatnagar A, Saluja S (2019) Synergistic effects of autochthonous probiotic bacterium and Mentha piperita diets in Catla catla (Hamilton, 1822) for enhanced growth and immune response. Fish Aquat Sci 22. https://doi.org/10.1186/s41240-019-0130-7
Biller-Takahashi JD, Takahashi LS, Pilarski F et al (2013) Serum bactericidal activity as indicator of innate immunity in pacu Piaractus mesopotamicus (Holmberg, 1887). Arquivo Brasileiro De Medicina Veterinária e Zootecnia 65:1745–1751. https://doi.org/10.1590/s0102-09352013000600023
Chumpitazi BP, Kearns GL, Shulman RJ (2018) Review article: the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther 47:738–752. https://doi.org/10.1111/apt.14519
Coles EH (1974) Veterinary clinical pathology. PP2 W. B. Sounders Company, Philadelphia, London, Toronto. 11 13. In: Internet Archive. https://archive.org/details/veterinaryclinic00cole
Dawood MAO, El-Salam Metwally A, Elkomy AH et al (2020) The impact of menthol essential oil against inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Fish Shellfish Immunol 102:316–325. https://doi.org/10.1016/j.fsi.2020.04.059
Du X, Zhang W, He J et al (2022) The impact of rearing salinity on flesh texture, taste, and fatty acid composition in largemouth bass Micropterus salmoides. Foods 11:3261. https://doi.org/10.3390/foods11203261
El Latif AMA, Elabd H, Amin A et al (2019) High mortalities caused by Aeromonas veronii: identification, pathogenicity, and histopathological studies in Oreochromis niloticus. Aquacult Int 27:1725–1737. https://doi.org/10.1007/s10499-019-00429-8
El-Kassas S, Aljahdali N, Abdo SE, et al. (2022) Moringa oleifera leaf powder dietary inclusion differentially modulates the antioxidant, inflammatory, and histopathological responses of normal and Aeromonas hydrophila-infected mono-sex Nile tilapia (Oreochromis niloticus). Front Vet Sci 9. https://doi.org/10.3389/fvets.2022.918933
Faggio C, Piccione G, Marafioti S et al (2014) Monthly variations of hematological parameters of Sparus aurata and Dicentrarchus labrax reared in Mediterranean land off-shore tanks. Cah Biol Mar 55:437–443
Gargiulo AM, Ceccarelli P, Dall’aglio C, Pedin V (1998) Histology and ultrastructure of the gut of the tilapia (Tilapia spp.), a hybrid teleost. Anatomia Histologia Embryologia 27:89–94. https://doi.org/10.1111/ahe.1998.27.issue-5
Gottfried SP, Rosenberg B (1973) Improved manual spectrophotometric procedure for determination of serum triglycerides. Clin Chem 19:1077–1078. https://doi.org/10.1093/clinchem/19.9.1077
Hardari A, Nobakht A, Safamehr A (2010) Investigation the effects using Nettle (Urtica dioica), Menta pulagum (Oreganum valgare) and Zizaphora (Thymyus vulgaris) medicinal plants and there mixtures on biochemical and immunity parameters of broilers. Proc. 4th Iran. Cong. Anim. Sci, 214–217. https://www.researchgate.net/publication/288266855_Investigation_the_effects_using_Nettle_Urtica_dioica_Menta_pulagum_Oreganum_valgare_and_Zizaphora_Thymyus_vulgaris_medicinal_plants_and_there_mixtures_on_biochemical_and_immunity_parameters_of_broiler
Huerta-Aguirre G, Paredes-Ramos KM, Becerra-Amezcua MP et al (2019) Histopathological analysis of the intestine from Mugil cephalus on environment reference sites. Pollution of water bodies in Latin America. Springer International Publishing, Cham, pp 319–328
Jobling M (2011) National Research Council (NRC): nutrient requirements of fish and shrimp. Aquacult Int 20:601–602. https://doi.org/10.1007/s10499-011-9480-6
Khalil HS, Momoh T, Al-Kenawy D et al (2021) Nitrogen retention, nutrient digestibility and growth efficiency of Nile tilapia ( Oreochromis niloticus ) fed dietary lysine and reared in fertilized ponds. Aquac Nutr 27:2320–2332. https://doi.org/10.1111/anu.13365
Kokoska L, Kloucek P, Leuner O, Novy P (2019) Plant-derived products as antibacterial and antifungal agents in human health care. Curr Med Chem 26:5501–5541. https://doi.org/10.2174/0929867325666180831144344
Kord MI, Srour TM, Omar EA, et al. (2021) The immunostimulatory effects of commercial feed additives on growth performance, non-specific immune response, antioxidants assay, and intestinal morphometry of Nile tilapia, Oreochromis niloticus. Front Physiol 12. https://doi.org/10.3389/fphys.2021.627499
Li L, Shen Y, Yang W et al (2021) Effect of different stocking densities on fish growth performance: a meta-analysis. Aquaculture 544:737152. https://doi.org/10.1016/j.aquaculture.2021.737152
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Magouz FI, Mahmoud SA, El-Morsy RAA et al (2021) Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 530:735944. https://doi.org/10.1016/j.aquaculture.2020.735944
Mahboubi M, Haghi G (2008) Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J Ethnopharmacol 119:325–327. https://doi.org/10.1016/j.jep.2008.07.023
Mahmoud HK, Al-Sagheer AA, Reda FM et al (2017) Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 475:16–23. https://doi.org/10.1016/j.aquaculture.2017.03.043
Mancianti F, Ebani VV (2020) Biological activity of essential oils. Molecules 25:678. https://doi.org/10.3390/molecules25030678
Miandare HK, Farahmand H, Akbarzadeh A et al (2013) Developmental transcription of genes putatively associated with growth in two sturgeon species of different growth rate. Gen Comp Endocrinol 182:41–47. https://doi.org/10.1016/j.ygcen.2012.11.013
Mohd Nor N, Mohd Yazid SH, Mohd Daud H et al (2019) Costs of management practices of Asian seabass (Lates calcarifer Bloch, 1790) cage culture in Malaysia using stochastic model that includes uncertainty in mortality. Aquaculture 510:347–352. https://doi.org/10.1016/j.aquaculture.2019.04.042
Mugwanya M, Dawood MAO, Kimera F, Sewilam H (2022) Anthropogenic temperature fluctuations and their effect on aquaculture: a comprehensive review. Aquacul Fish 7:223–243. https://doi.org/10.1016/j.aaf.2021.12.005
Munteanu C, Schwartz B (2022) The relationship between nutrition and the immune system. Front Nutr 9. https://doi.org/10.3389/fnut.2022.1082500
Mustapha S, Moulay Mustapha E, Brahim B, Nozha C (2012) Characterization of Vibrio alginolyticus Trh positive from Mediterranean environment of Tamouda Bay (Morocco). World Environment 2:76–80. https://doi.org/10.5923/j.env.20120204.04
Neelavathi P, Venkatalakshmi P, Brindha P (2013) Antibacterial activities of aqueous and ethanolic extracts of Terminalia Catappa leaves and bark against some pathogenic bacteria. Int J Pharm Pharm Sci 5:114–120
Nya EJ, Austin B (2009) Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32:971–977. https://doi.org/10.1111/j.1365-2761.2009.01101.x
Paknejad H, Hosseini Shekarabi SP, Shamsaie Mehrgan M et al (2020) Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol Biochem 46:1883–1895. https://doi.org/10.1007/s10695-020-00839-z
Panigrahi A, Viswanath K, Satoh S (2011) Real-time quantification of the immune gene expression in rainbow trout fed different forms of probiotic bacteria Lactobacillus rhamnosus. Aquac Res 42:906–917. https://doi.org/10.1111/j.1365-2109.2010.02633.x
Parham S, Kharazi AZ, Bakhsheshi-Rad HR et al (2020) Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 9:1309. https://doi.org/10.3390/antiox9121309
Park KH, Jeong HD (1996) Enhanced resistance against Edwardsiella tarda infection in tilapia (Oreochromis niloticus) by administration of protein-bound polysaccharide. Aquaculture 143:135–143. https://doi.org/10.1016/0044-8486(95)01224-9
Parveen S, Tamplin ML (2013) Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio cholerae. Guide to Foodborne Pathogens 148–176. https://doi.org/10.1002/9781118684856.ch9
Polianciuc SI, Gurzău AE, Kiss B, et al. (2020) Antibiotics in the environment: causes and consequences. Med Pharm Rep. https://doi.org/10.15386/mpr-1742
Ringø E, Harikrishnan R, Soltani M, Ghosh K (2022) The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 12:3016. https://doi.org/10.3390/ani12213016
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41:125–139. https://doi.org/10.1016/0165-2427(94)90062-0
Sumithra TG, Reshma KJ, Anusree VN et al (2019) Pathological investigations of Vibrio vulnificus infection in genetically improved farmed tilapia (Oreochromis niloticus L.) cultured at a floating cage farm of India. Aquaculture 511:734217. https://doi.org/10.1016/j.aquaculture.2019.734217
Talpur AD (2014) Mentha piperita (Peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquaculture 420–421:71–78. https://doi.org/10.1016/j.aquaculture.2013.10.039
Talpur AD, Ikhwanuddin M (2012) Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 364–365:6–12. https://doi.org/10.1016/j.aquaculture.2012.07.035
Talpur AD, Ikhwanuddin M (2013) Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol 34:254–264. https://doi.org/10.1016/j.fsi.2012.11.003
Tarr CL, Patel JS, Puhr ND et al (2007) Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol 45:134–140. https://doi.org/10.1128/jcm.01544-06
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
Xu Y, Wang C, Zhang G et al (2017) ISCR2 is associated with the dissemination of multiple resistance genes among Vibrio spp. and Pseudoalteromonas spp. isolated from farmed fish. Arch Microbiol 199:891–896. https://doi.org/10.1007/s00203-017-1365-2
Yang S, Yu M (2021) Role of goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res 14:3171–3183. https://doi.org/10.2147/jir.s318327
Yanuhar U, Nurcahyo H, Widiyanti L, et al. (2022) In vivo test of Vibrio alginolyticus and Vibrio harveyi infection in the humpback grouper (Cromileptes altivelis) from East Java Indonesia. Vet World 1269–1282. https://doi.org/10.14202/vetworld.2022.1269-1282
Younes AM, Fares M, Gaafar AY, Mohamed LA (2016) Isolation of Vibrio alginolyticus and Vibrio vulnificus Strains from Cultured Oreochromis niloticus Around Qarun Lake, Egypt. Global Veterinaria 16: 1–5. In: International Digital Organization for Scientific Information. https://www.researchgate.net/publication/297694707_Isolation_of_Vibrio_alginolyticus_and_Vibrio_vulnificus_Strains_from_Cultured_Oreochromis_niloticus_Around_Qarun_Lake_Egypt
Zak B, Dickenman RC, White EG et al (1954) Rapid estimation of free and total cholesterol. Am J Clin Pathol 24:1307–1315. https://doi.org/10.1093/ajcp/24.11_ts.1307
Acknowledgements
The authors express their appreciation and hearty thanks to all Animal Health Research Institute Dokki-Giza, Kafrelsheikh Provincial Lab staff for the facilities they provided and their kind assistance.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Nagwa I.S. Abu-Zahra: methodology, formal analysis, writing—original draft, writing—review and editing, resources, supervision, investigation, visualization, Abeer M. ElShenawy: ideas, writing—original draft, formulation of overarching research goals and aims, writing—review. Gehan I.E Ali: resources, investigation, visualization, validation, writing—review, Eman T. Al-sokary: ideas, formulation of overarching research goals and aims, project administration, writing—review, Mohamed A. Mousa: resources, investigation, visualization, validation, writing—Review, Hala A. M. Abd El-Hady: ideas, writing—original draft, formulation of overarching research goals and aims, writing—Review.
Corresponding author
Ethics declarations
Animal welfare and ethics statement
The experimental methodology, protocols, and animal care used in the present study all followed the relevant guidelines and regulations of the Animal Health Research Institute, Agriculture Research Center, Giza, Egypt.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abu-Zahra, N.I.S., ElShenawy, A.M., Ali, G.I.E. et al. Mentha piperita powder enhances the biological response, growth performance, disease resistance, and survival of Oreochromis niloticus infected with Vibrio alginolyticus. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01469-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01469-5