Abstract
Antibiotics are crucial in the control of bacterial infections on fish farms. However, the influence of licensed antibiotics on DNA damage, histological changes, and protein differentiation in some farmed fish species is unidentified. The current study aimed to investigate the effect of therapeutic (80 mg/kg body weight/day) and high doses (160 mg/kg body weight/day) of oxytetracycline (OTC) on some biological indices, protein differentiation, histological changes, and DNA damage in Oreochromis niloticus (n = 360; 40 ± 3.5 g) and the potential positive effects of dietary ascorbic acid (AA) and whether it is capable of reducing the deleterious impacts of OTC and working synergistically to increase fish disease resistance and decrease morbidity and mortality rates. In the current study, fish were fed diets with or without OTC (80 or 160 mg/kg body weight/day) and AA (100 mg/kg diet/day) for 28 days, followed by a bacterial stress test with two different virulent strains for 14 days. Treatments include CTR (control; without additives), AA, OTC80, OTC160, OTC80 + AA, and OTC160 + AA. The results revealed that the AA group had the best antioxidative (significant increase in SOD, CAT, and GPx) and immunomodulatory effects (an increase in IgM, nitroblue tetrazolium, and total leucocytic count). Furthermore, the hematological parameters, immune indices (NBT), and antioxidant enzymes were significantly reduced by OTC160 (p ≤ 0.05). The results also showed that OTC160 notably augmented the activity of liver (ALT, AST, and LDH) and kidney (creatinine and urea) damage markers (p ≤ 0.05). OTC160 revealed considerable hepatic and renal tissue damage and significantly higher hepatocyte DNA damage. Pathological changes and DNA damage were directly correlated to the dosage of OTC exposure, where they were more severe in OTC160. OTC80 showed insignificant changes in most parameters. Compared to CTR, the inclusion of AA in the O. niloticus diet significantly restored the biological indices, histological changes, and DNA damage in OTC-treated groups. The morbidity and mortality percentages after bacterial challenge were significantly lower in the OTC80 + AA group, followed by the OTC160 + AA group than in the CTR. These results corroborate the use of AA as a potent antioxidant and immune stimulant and a potential co-adjuvant to OTC in the diet of O. niloticus to reach optimal resistance to disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensive aquaculture techniques make fish much more susceptible to infectious diseases, which causes financial losses due to the cost of chemotherapy and fish mortality (Pepi and Focardi 2021). Bacterial diseases may result in significant losses in tilapia production, particularly during the winter season. Farmed fish may have a lower disease risk by improving their resistance to infections through better nutritional formulations, vaccinations, and immunostimulants (Al-Khalaifah et al. 2020). Antibiotic therapy is crucial in aquaculture for maintaining fish health and controlling bacterial infections. To date, the US Food and Drug Administration has only permitted three antibiotics for use in aquaculture: florfenicol, oxytetracycline (OTC), and ormetoprim and sulfadimethoxine (USFWS 2015).
OTC is one of the antibiotics authorized for usage in aquaculture and is frequently used as an effective therapy for fish bacterial diseases, for instance, Vibrio anguillarum, Aeromonas hydrophila, Aeromonas salmonicida, and Pseudomonas species (Nakano et al. 2018; Leal et al. 2019). The oral route is the most popular method of administering antibiotics in aquaculture since there is little chance of ecological contamination (Leal et al. 2019). The OTC therapeutic dose for specific fish species or diseases was between 5 and 82.5 mg/kg body weight/day for 10 successive days (USFWS 2015). The excessive and improper use of antibiotics in aquaculture contributes to the spread of antibiotic resistance and residues, which can remain long in water, tissues, or sediments after drug use is stopped (Bojarski et al. 2020).
The use of immunomodulators in fish feeds is an approach for not only improving defense responses to threats to which fish are exposed but also for finding alternatives to antibiotics and chemotherapeutics used for treating fish diseases (Oliva-Teles 2012). Vitamins are organic compounds required for life since normal growth, reproduction, and health all depend on trace amounts of them. Ascorbic acid (Vitamin C) modulates immune functions such as cell proliferation, macrophage infiltration, phagocytic activity, lysozyme and cytokines levels, and antibody production (Ibrahim et al. 2020). It is also an effective natural antioxidant that is essential in scavenging reactive oxygen species (ROS) and protecting the fish from oxidative damage (Hajirezaee et al. 2020). Additionally, AA plays a vital role in recovery from toxicant and environmental stressor exposure (Hajirezaee et al. 2020). Fish are deficient in the L-gluconolactone oxidase enzyme, which is responsible for the de novo synthesis of AA, so they must get their AA requirements from external sources (Fracalossi et al. 2001).
Several markers are used for assessing the health status of fish and the therapeutic efficacy of the drug used. Hematological parameters are good tools for assessing physiological and pathological alterations in fish. The changes in hematology could be caused by feeding habits, pollutants, and environmental factors. There is a delicate balance called redox balance between the antioxidant defenses and pro-oxidant forces. Oxidative stress arises when ROS increases in the absence of oxidant defense. To combat ROS damage, fish employ a variety of defense mechanisms, including antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GRx) (Ahmed et al. 2017). Comet assays are used to review and assess the health and genetic condition of many fish species because of their high sensitivity in determining DNA damage in a single cell (Kousar et al. 2022). The most genotoxic compounds have been found to produce ROS, which interacts with DNA and disrupts it. Protein electrophoresis is a blood test that measures specific proteins. The test uses electrical charges to separate proteins in the blood.
NBT is a fast and low-cost test that focuses on phagocytes’ capacity to reduce the dye by producing ROS which are responsible for pathogen elimination in vivo. One of the most effective methods of protection against fish diseases is the capability of macrophages to destroy pathogens. As a result, the bacterial stress test is the most reliable test of immune function with the maximum level of biological significance because it evaluates an integrated immune reaction at the organismal level (Köllner et al. 2002). Aeromonas hydrophila and Vibrio parahaemolyticus are significant public health concerns and the primary reason for bacterial disease in O. niloticus. A. hydrophila is the main cause of MAS (motile Aeromonas septicemia) in a broad variety of fish species. Vibriosis is a potentially fatal bacterial disease that affects fish globally, leading to high mortalities and severe financial losses.
To our knowledge, no data on the role of dietary AA in OTC-induced stress in O. niloticus is currently available. Thus, this study was designated to evaluate the potential immunostimulatory and antibacterial effects of AA when given alone or combined with OTC to assess its role in modifying the deleterious impacts of OTC through investigating the changes in biological indices, protein differentiation, DNA damage, histological changes, and disease resistance against different bacterial infections. To the best of the authors’ knowledge, it is the first study to investigate the effect of OTC, AA, and their combination on serum protein differentiation. So far, only a few studies have been conducted on the genotoxicity of antibiotics on aquatic animals.
Material and methods
Feed formulation
A commercial fish feed (30% protein, 5.4% fiber, 7.93% ether extract, 8.17% Ash, 48.5% non-protein nitrogen, and digestible energy equals 3373 (kcal/kg)3, 2mm in diameter) was used for the preparation of experimental diets. Six experimental diets were formulated; one diet served as a control without any additives, and the other five diets were prepared to contain 100 mg AA/kg diet, 80 mg OTC/kg biomass/day, 160 mg OTC/kg biomass/day, 80 mg OTC/kg biomass/day + 100 mg AA/kg diet, and 160 mg OTC/kg biomass/day + 100 mg AA/kg diet. OTC (oxytetracycline hydrochloride 20%, ADWIA Co., Egypt) and ascorbic acid (99.8%, CAT: sA178, Piochem, Egypt). OTC was given following the manufacturer’s instructions. OTC contains 200 mg/g of powdered oxytetracycline hydrochloride, so 400 or 800 mg of powdered oxytetracycline hydrochloride per 1 kg of fish mass was used to achieve the recommended dose of 80 or 160 mg/kg Bwt/day. Briefly, a pre-weighed quantity of pellets (2% Bwt) was soaked in approximately 1 mL of vegetable oil containing 400 or 800 mg of powdered oxytetracycline hydrochloride, gently mixed, and air-dried following the method of Trushenski et al. (2018) and Julinta et al. (2019) for OTC oral administration. The medicated feed was freshly prepared daily. The control diet without additives and AA diet were also prepared with the same method.
Experimental fish and design
Only one set of healthy juvenile O. niloticus (n = 360, 40 ± 3.5 g) was purchased from a private farm in Kafrelsheikh province, Egypt. All fish, regardless of sex, were distributed after 2 weeks of adaptation to lab conditions in six groups in 18 glass aquaria (50 × 40 × 40cm), each in triplicate (n = 60/group, 20/tank), as described in Table 1. The debris and wastes were siphoned daily, and about 50% of the aquarium water was changed with dechlorinated 24-h stored water every 2 days. During the adaption period, fish were fed until satiation on a commercial diet of 30% protein. Throughout the experiment, fish were fed 2% of their body weight for 28 days. The feed intake was visually evaluated after each feeding. Fish were weighed biweekly, the variations in weight gain were calculated, and the increase in feed intake was determined by the biomass in each tank. Uneaten food was gathered on each feeding day, collected tank-wise, dried, and weighed. Water parameters during acclimation and the experimental period were regularly checked. Dissolved oxygen was measured using an oxygen meter (HI76407-Hanna Instruments Inc., RI, USA) and recorded at 7.1 ± 0.4 mg/L. Temperature and pH were measured using the digital waterproof pHep® pH/temperature tester (HI98128-Hanna Instruments Inc., RI, USA) and recorded at 25 ± 2 °C and 7.3 ± 0.3, respectively. Observations of external signs, feed intake, and mortality were recorded daily.
Sampling
At the end of the feeding trial (28 days), all fish were starved for 24 h before sampling. Blood was collected from caudal veins with anticoagulant (EDTA) for hematological parameters and NBT (nitro blue tetrazolium reduction test), which was done within 24 h. Another set of blood was collected without anticoagulant for serum purposes. The serum was obtained by centrifugation at 4000 rpm/5 min, collected in Eppendorf tubes, and stored at − 20 °C for further analysis (done within 14 days).
The fish were sacrificed via spinal cord transection after being anesthetized by immersion in freezing water and a saturated benzocaine solution. Tissue samples (liver and kidney) were kept either in 10% formalin till their histological examination or frozen at − 20 °C until comet assay.
Bacterial inoculum preparation and experimental challenge
The therapeutic efficacy of OTC and AA was assessed at the end of the feeding period (28 days), by challenging O. niloticus with a pathogenic strain of A. hydrophila and V. parahaemolyticus which were kindly provided by Microbiological Unite, Kafrelsheikh Provincial Lab, Animal Health Research Institute, Egypt. The strains were previously isolated from diseased O. niloticus and were identified using phenotypic and molecular techniques. The isolates were stored in glycerol at − 20 °C until use. The bacterial suspensions were prepared using McFarland standard turbidity tubes, following El Latif et al. (2019). Loopfuls from A. hydrophila and V. parahaemolyticus aliquots were inoculated into Tryptic soya broth (incubated at 25 °C for 24 h) and alkaline saline peptone water (incubated at 41.5 °C for 18 h), respectively. A loopful from each broth was inoculated into Aeromonas medium base for A. hydrophila (incubated at 25 °C for 24 h) and TCBS (thiosulfate citrate bile salts sucrose agar) for V. parahaemolyticus (incubated at 37 °C for 24 h). The required turbidity degree was achieved by matching them against McFarland standard turbidity tubes (0.5 McFarland, equals 1.5 × 108). The required bacterial dose was then prepared by dilution with normal saline.
After the feeding trial, each group was divided into two subgroups of 20 fish each. Subgroup A challenged intraperitoneally with 0.2 ml of 1 × 107 CFU A. hydrophila and subgroup B was intraperitoneally challenged with 0.2 ml of 1 × 106 CFU V. parahaemolyticus. During the challenging time, fish were fed control diets alone, including AA and/or OTC. Fish were observed for 14 days to track clinical signs, morbidity, and mortality rates (MR%) daily. MR% and RPS (relative percent of survival) are evaluated according to the following equations:
Hematological indices
The hematological indices were all measured according to Faggio et al. (2014). RBCs (red blood cell count; × 106/mm3), TLC (total leukocyte count; × 103/mm3), and PLT (platelets count; × 103/mm3) were counted using a modified Neubauer’s hemocytometer. Hb concentration (hemoglobin; g/100ml) was determined by the cyanmethemoglobin method and PCV (packed cell volume; %) was assessed using a micro-hematocrit centrifuge. Thin blood films were prepared on clean slides for the assessment of differential leucocyte count. The slides were allowed to dry before being stained with a modified Wright’s stain.
Immune-related indices
Following the manufacturer’s procedures, the serum IgM and IgE levels were determined using the sandwich ELIZA (Table 2). OD (optical density) is measured spectrophotometrically at wavelength 450 nm. The OD value is relative to the quantity of specific Ig.
NBT was estimated as described by Esteban et al. (2001). Briefly, 1 × 106 peripheral blood leucocytes/well were treated with an equivalent NBT volume of 0.2% for 2 h at 28 °C. The supernatants were disposed of, and the cells were fixed for 5 min in 100% (v/v) methanol. Each well received two washes with 125 mL of 70% (v/v) methanol. The fixed cells were left to dry. Per well, 120 ml of 2 N potassium hydroxide (KOH) and 140 ml of dimethyl sulfoxide (Sigma-Aldrich, USA) were used to dissolve the reduced NBT (in the form of the blue precipitate formazan). Using an ELISA assay or reader, the turquoise-blue solution was evaluated at 630 nm.
Oxidative stress biomarkers
Serum antioxidant capacity was evaluated by detecting the levels of SOD (superoxide dismutase), GPx (glutathione peroxidase), and CAT (catalase) activities. Using diagnostic kits from Bio-diagnostic CO., Egypt. SOD, GPx, and CAT were colorimetrically determined at 560, 340, and 510 nm, respectively, according to Nishikimi et al. (1972), Paglia and Valentine (1967), and Aebi (1984).
Serum biochemical parameters
Serum biochemical markers for liver function (ALT, AST, and LDH) and kidney function (creatinine and urea) were determined by a colorimetric method (Tietz 1995). Total protein and albumin were estimated according to Doumas et al. (1981). Globulin was calculated by subtracting albumen from total protein. Kits used for the estimation of serum biochemical parameters are shown in Table 3.
Protein electrophoresis was done using the CAPI 3 PROTEIN (E) 6 kit by the SEBIA CAPILLARYS 3 TERA automated instrument in an alkaline buffer (pH 9.9), following the manufacturer's instructions.
Histological examination and single-cell gel electrophoresis
Paraffin blocks including fixed tissues of the hepatopancreas and posterior kidney were cut into sections (3–7 mm thick), continuously sliced, and stained with H&E (hematoxylin and eosin). The stained tissues were microphotographed using a digital camera (Leica EC3) linked to a light microscope (Leica DM 5000).
DNA damage was detected in the hepatocyte via the comet assay (single-cell gel electrophoresis), as described by Singh et al. (1988). In brief, tissues were placed on microscope slides in 0.6% normal melting point and low melting point agarose gel, and then immersed in lysing solution (2.5mol NaCl, 100 Mm Na2EDTA, newly added 1%Triton- × 100, and 10% DMSO) at 4 °C for 1 h for denaturation and unwinding of DNA. Slides were immersed in electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, PH. 13.0) for 30 min at 4 °C, and then neutralized with Tris–HCl buffer (400 Mm Tris–HCl, PH 7.4) before final staining with a fluorescent dye (20ug/ml ethidium bromide). The DNA damage was observed using a fluorescent microscope (Nikon Microscope-Eclipse, E600 with Y-FL EPI-Fluorescence attachment, Japan) outfitted with a 515–560-nm excitation filter, a 590-nm barrier filter, and an automatic digital imaging system running Comet assay TM software (perceptive Instrument, UK).
Statistical analysis
The data is expressed as mean ± standard deviation (SD). For testing the data, a one-way ANOVA (SPSS® version 22, SPSS Inc., IL, USA) was used, followed by Duncan’s post hoc test to compare the means, considering the significance of the treatment effects at a probability level of p ≤ 0.05.
Method validation
To assess biological variation and repeatability, each treatment was conducted in triplicates, consisting of three samples obtained from different fish for each triplicate (n = 9 samples/treatment).
Results
Hematological parameters
Table 4 shows the variations in hematological parameters (Hb, RBCs, PCV, TLC, and PLT) and differential leukocyte counts after feeding fish diets treated with AA and/or OTC. The best enhancement was observed in the AA group, followed by the OTC + AA groups. The OTC160 group exhibited a significant decrease in Hb, RBCs, and PCV% compared to CTR, except for platelet counts, which were significantly higher than CTR. Likewise, TLC, lymphocytes, and MID counts were noticeably higher in either AA or OTC + AA groups than in OTC (OTC80/OTC160) and CTR groups. The latter groups exhibited insignificant differences between their TLC and DLC, except MID counts were significantly higher in the OTC160 group than in the other two groups (OTC80 and CTR).
Immune response and antioxidant activity
Effects of dietary AA and/or OTC on immune response are presented in Fig. 1a. The obtained data showed a significantly higher IgM and NBT in fish fed AA alone or combined with OTC, with the highest levels in AA, followed by AA + OTC as compared to CTR. OTC80 showed a significant increase in IgM levels without significant NBT activity changes. The OTC160 group substantially decreased NBT level compared to the CTR group. OTC160 showed a considerable increase in IgE levels compared to other groups.
The results of antioxidant activity are presented in Fig. 1b. AA and OTC80 + AA groups had significantly higher antioxidant enzyme (GPx, CAT, and SOD) activities than CTR. The OTC80 + AA group improved slightly compared to the AA group, while insignificant differences were found between OTC80 and CTR. OTC160 displayed significant decreases in all the measured antioxidant enzymes. OTC160 + AA showed improvement toward control values.
Biochemical parameters
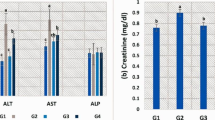
Compared to CTR, OTC160 had significantly higher liver enzymes (ALT, AST, and LDH), followed by OTC160 + AA (Table 5). In contrast, all the other groups showed insignificant differences. Again, high doses of OTC with or without AA (OTC160 / OTC160 + AA) exhibited a significant increase in urea and creatinine concerning the levels of CTR. Therapeutic doses of OTC (OTC80 / OTC80 + AA) also significantly increased the urea levels, while AA showed a significant decrease in urea levels (Table 5). Fish were actively feeding, except OTC160 which showed decreased feed intake % (84.67 ± 3.06%). No mortalities were recorded during the experiment in the treatment or control groups except for 3 mortalities in OTC160.
Protein profile and protein electrophoresis
Figure 2 shows the impact of dietary AA and/or OTC on protein profiles. The obtained data showed significantly higher serum levels of total protein and globulin in fish fed AA alone or combined with OTC, with the highest levels in AA and OTC80 + AA, followed by the OTC160 + AA group compared to CTR. Albumen level and percent increased in all OTC-treated groups except OTC80 + AA, which showed only an increase in albumen level with an insignificant increase in albumen percent.
The protein electrophoresis results (Table 6) revealed that AA caused a significant increase in beta1% and gamma %, while OTC80 revealed a significant increase in beta1%. OTC + AA groups showed a substantial decrease in alpha 2%. Alpha 1% and beta 2% showed insignificant changes compared to CTR in all fish groups (Fig. 3).
Protein electrophoresis of O. niloticus experimental groups, a CTR; b AA showed a significant increase in beta 1 % and gamma %; c OTC80 showing a significant increase albumin % and beta 1 %; d OTC160 showing a significant increase albumin %; e OTC80+AA showing a substantial decrease in alpha 2 %; f OTC160+AA showing a significant increase in albumin % and a substantial decrease in alpha 2 %
Internal and histological examination
Internal examination of OTC-treated groups (Fig. 4) revealed hemorrhagic spots on the liver, hemorrhagic abdomen, and enlarged spleen. The severity of signs was less in the OTC80 group than in the OTC160 group, which was more severe. Including AA in the diets of OTC-treated groups significantly improves the picture of internal organs.
Internal examination of O. niloticus experimental groups. a OTC80 showing hemorrhagic spots on the liver, b OTC160 showing hemorrhagic abdomen, adhesion of internal organs, and hemorrhagic liver, c OTC80 + AA showing normal internal organs except the enlarged spleen, d OTC160 + AA showing pale hemorrhagic liver
Histological changes in the hepatopancreas and posterior kidney were briefly tabulated (Table 7). They revealed normal hepatopancreatic histoarchitecture where central veins surrounded hepatocytes and pancreatic acini in the control group (Fig. 5a). Normal histoarchitecture was similarly detected in the hepatopancreas of the AA, OTC80, and OTC80 + AA groups (Fig. 5b, c, and e). However, in the OTC160 group, considerable tissue damage was seen in the form of marked necrosis of hepatic cells and pancreatic acini accompanied by mononuclear inflammatory cell infiltration and severe vacuolar degeneration of hepatic cells (Fig. 5d). This disordered tissue damage was somewhat restored in the OTC160 + AA group, which presented only vacuolation of hepatocytes and normal hepatopancreas (Fig. 5f).
Representative photomicrograph of H&E-stained hepatopancreas (H indicates hepatocytes and HP indicates hepatopancreas). Scale bar = 50 μm. a Hepatopancreas of CTR group showing normal hepatopancreatic histoarchitecture where hepatocytes cords (H) and pancreatic acini surround central vein, b hepatopancreas of AA group showing normal hepatopancreatic histoarchitecture, c hepatopancreas of OTC80 showing normal hepatopancreatic histoarchitecture, d hepatopancreas of OTC160 group showing marked necrosis of pancreatic acini accompanied with mononuclear inflammatory cells infiltration (arrowhead) and severe vacuolar degeneration of hepatic cells (arrow), e hepatopancreas of OTC80 + AA group showing normal hepatopancreatic histoarchitecture, f hepatopancreas of OTC160 + AA group showing vacuolation of hepatocytes (arrow) and normal hepatopancreas
Like hepatopancreas, the posterior kidney of CTR, AA, and OTC80 + AA groups had a normal renal histoarchitecture composed of renal tubules (Fig. 6a, b, and c). The posterior kidney of the OTC80 group displayed mild vacuolation of the renal tubular epithelium (Fig. 6d), and that of the OTC160 group showed a marked degree of renal tubular degeneration (Fig. 6e). Once more, the kidney of the OTC160 + AA group presented signs of improvement, with foci of degenerative changes within some renal tubules (Fig. 6f).
Representative photomicrograph of H&E-stained posterior kidney (RT shows renal tubules). Scale bar = 50 μm. a The posterior kidney of the CTR group showing normal renal histoarchitecture where composed of renal tubules, b posterior kidney of the AA group showing normal renal histoarchitecture, c posterior kidney of OTC80 group showing mild vacuolation of the renal tubular epithelium (arrows), d posterior kidney of OTC160 group showing a marked degree of renal tubular degeneration (arrowheads), e posterior kidney of OTC80 + AA group showing normal renal histoarchitecture, f posterior kidney of OTC160 + AA group showing foci of degenerative changes within some renal tubules (arrows)
Comet assay screening DNA damage in hepatocytes of O. niloticus experimental groups. a Comet length (um); b head diameter (um); c % DNA in head; d tail moment; e tail length (um); f % DNA in tail; values are means; the error bars represent SD (n = 9/group). Significant differences between treatments are indicated by different subscripts above the bars (p ≤ 0.05)
Comet assay and DNA damage
The comet assay results on DNA damage (Fig. 7) revealed that OTC160 displayed a significantly (p ≤ 0.05) higher percent of DNA damage in hepatocytes, represented by decreased % DNA in the head, increased tail length, % DNA in the tail, and increased tail moment. This damage was partially restored by including AA in O. niloticus diets (OTC160 + AA). The OTC80 and OTC80 + AA groups showed damage in the form of an increase in tail length and an increase in the tail moment only in the OTC80 group. There were no significant differences between the AA and CTR groups (Fig. 7).
Experimental infection
The clinical examination of the infected fish (Fig. 8) with both strains (A. hydrophila and V. parahaemolyticus) revealed hemorrhagic spots and skin ulcers, tail erosion, exophthalmia, and ocular opacity. Post-mortem examination of challenged fish revealed congestion of internal organs, hemorrhagic liver, and enlarged spleen (Fig. 8). The frequency and severity of signs were less severe in treated groups than CTR and in V. parahaemolyticus infected groups than in A. hydrophila infected groups.
Clinical and internal examination of experimentally challenged O. niloticus groups. a CTR infected with A. hydrophila showing congested internal organs, hemorrhagic liver, distended gall bladder, and enlarged spleen; b OTC80 + AA infected with A. hydrophila showing hemorrhage and erosion of the oral region and tail; c OTC160 infected with A. hydrophila showing ocular opacity and hemorrhage; d CTR infected with V. parahaemolyticus showing hemorrhagic liver, distended gall bladder, enlarged spleen, and tail erosion
The morbidity and mortality percentages (Fig. 9) were significantly lower in the OTC80 + AA group, followed by the OTC160 + AA group and the OTC80 group than in the CTR group challenged with both examined strains. The dietary AA achieved RPS of 50% in the case of A. hydrophila infection and 33.33% in the case of V. parahaemolyticus, which was increased to 100% when combined with OTC80 treatment (OTC80 + AA), while OTC80 treatment alone had RPS of 70% and 83.33% in the case of A. hydrophila infection and V. parahaemolyticus, respectively, and OTC160 treatment achieved a low RPS of 30% in the case of A. hydrophila infection and 0% in the case of V. parahaemolyticus, which was raised to 80% and 83.33% when combined with AA (OTC160 + AA).
Morbidity, mortality rates, and RPS of experimentally challenged O. niloticus groups (n = 20/subgroup). a O. niloticus subgroups challenged with A. hydrophila, b O. niloticus subgroups challenged with V. parahaemolyticus. Significant differences between treatments are indicated by different subscripts (p ≤ 0.05)
Discussion
Because of its broad-spectrum antibacterial properties, OTC is usually added to fish feeds in countries where it is not prohibited as a growth promoter. This prolonged therapy, however, causes OTC deposits in fish tissue, which has serious consequences for both fish and consumers (Reda et al. 2013). OTC has been shown to cause oxidative stress and liver dysfunction. OTC has the potential to promote the production of ROS, which promotes lipid peroxidation in the hepatocyte membranes, leading to further outflow of intracellular enzymes into the bloodstream (Nakano et al. 2018). Furthermore, OTC induced adverse effects on immune response, nephrotoxicity, genotoxicity, histological alterations, and impaired antioxidant capacity and hematological parameters in fish species (Reda et al. 2013). This drives us to seek appropriate neoadjuvants to act synergistically with OTC and diminish negative effects. AA is a micronutrient that is essential for fish growth and physiological processes. Dietary acidifiers are well-recognized to improve OTC absorption (Ng and Koh 2016). We investigated whether AA might improve OTC’s antibacterial potential and, as a result, the fish’s immunity and assessed the potential role of dietary AA in reducing OTC-induced stress in O. niloticus.
Our findings demonstrated that the AA and OTC + AA groups significantly increased RBCs, Hb, PCV%, and PLT counts more than the OTC and control groups. The enhancement might be attributed to acidic pH levels produced by acidifiers, which effectively liberate essential minerals like potassium, calcium, ferrous, and copper from feed (Khajepour and Hosseini 2012). Also, this could be owing to AA’s antioxidative capabilities, which extend the life span of RBCs through its effective ROS scavenging ability (Nayak et al. 2007). Our results corroborate the findings of Affonso et al. (2007) that feeding Brycon amazonicus diets enriched with AA substantially increased RBCs and PCV values. An upsurge in RBC, PCV, and Hb is related to decreased hemolysis or increased hematopoiesis. Our findings showed that high doses of OTC (OTC160) caused the anemic condition in O. niloticus, possibly due to the inhibition of erythropoiesis and increased lysis of RBCs (Mişe Yonar et al. 2020). Similarly, Omoregie and Oyebanji (2002) detected a substantial reduction in RBCs, PCV, and Hb in O. niloticus that fed OTC added to diets.
The numbers of TLC and DLC are useful tools for assessing fish immunity. Here, we conclude that AA upsurges the immune responses and disease resistance. This conclusion depends on our results that dietary AA can increase TLC, particularly lymphocytes, which are involved in antibody production, hence increasing the immunological response of fish against infection. A comparable upsurge in lymphocyte count was detected in poultry fed an acidified diet (Wang et al. 2010). In the current trial, OTC treatment insignificantly affected the TLC and DLC values, with a significant increase in MID in the OTC160 group. In contrast to our results, Dobšíková et al. (2013) demonstrated that OTC reduces the TLC in Cyprinus carpio. These variations may be related to differences in exposure periods and fish species.
In the current study, the AA when added alone or combined with OTC improved O. niloticus immunity, as evidenced by high levels of total protein, globulin, beta 1%, gamma %, IgM, and NBT. In agreement with our findings, many studies revealed that dietary AA increased the fish’s immunity (Hardie et al. 1991; Moradi et al. 2022). In this study, OTC160 significantly decreased NBT with no effect on IgM and this result confirms that high doses of OTC reduced phagocytosis. Furthermore, OTC80 was reported to increase IgM levels with an insignificant effect on NBT. Other studies have found that OTC downregulates antibody production, TLC, and phagocytic activity in Cyprinus carpio and O. niloticus (Kasagala and Pathiratne 2008; Reda et al. 2013, 2016), respectively. Likewise, Siwicki et al. (1989) reported suppression of the NBT activity and phagocytosis of O. mykiss spleen phagocytes following I/P injection of OTC (10 mg/kg). In contrast to our results, oral administration of OTC to Scophthalmus maximus at 200 mg OTC kg/BW/day for 12 days did not influence the NBT or phagocytosis activity (Tafalla et al. 2002), while Lundén et al. (2002) discovered in vitro that commonly used antibiotics (oxolinic acid, OTC, florfenicol, and sulphadiazine combined with trimethoprim) markedly decreased NBT activity in O. mykiss at the highest drug dose tested (100 g/ml). These confused results could be attributed to the multiple conditions used in different trials, such as species of fish, dosage, route of antibiotic administration, and water temperature, all of which affect drug absorption.
Lundén et al. (2002) discovered that OTC is reserved in the pronephros, a major fish lymphoid organ; thus, OTC immune suppression may be attributed to OTC penetration and impairment of immune cell function. However, when OTC was combined with AA, the immunological response and resistance to infection were maximized. This was evident in decreased rates of morbidity and mortality in the AA + OTC group compared to fish fed each alone. These findings indicate that innate immunity in fish is among the targets of OTC. To our knowledge, it is the first study to evaluate the effect of OTC on fish IgE. Our results demonstrated a significant elevation in IgE levels in fish serum treated with OTC160, which indicated a type of sensitivity toward high doses of OTC.
Increased ROS levels are well known to cause oxidative stress, which can impact fish health and cause tissue damage. Some key antioxidant enzymes, like SOD, GPx, and CAT help prevent ROS production. Our results revealed a significant decrease in the activity of all measured antioxidant enzymes (SOD, GPx, and CAT) in the OTC160 group compared to the OTC80 group, which showed insignificant differences from the CTR. These observed changes in the activities of antioxidant enzymes could be due to an increase in ROS production, resulting in excessive usage of these enzymes during ROS removal. While Nazeri et al. (2017) similarly pointed to the antioxidant activity of tetracycline, Abraham et al. (2021) reported a non-significant decrease in SOD activity in the hepatic and muscular tissues affected by the therapeutic dose of OTC for 10 days. The reduced antioxidant activities as observed by the parameters measured may have hindered detoxification and increased OTC toxicity at high doses in O. niloticus at the cellular level. In contrast, feeding fish with AA significantly restored serum antioxidant enzyme activities, demonstrating the protective role of dietary AA against oxidative damage induced by OTC. It could be owing to AA’s high antioxidative activity, allowing it to neutralize ROS and reduce oxidative stress. Through H + donation, AA binds to ROS and recovers free radicals. AA was also demonstrated to operate as a reducing agent, principally by inhibiting the transfer of metal ions like Fe and Cu ions, which interact with H2O2 to form hydroxyl radicals (Babior 2000). Many earlier studies have found that AA as a feed additive increases CAT, SOD, and GPX activity in Acipenser baerii (Xie et al. 2006) and Pelteobagrus fulvidraco (Liang et al. 2017). In contrast, Chen et al. (2015) reported that dietary AA would not affect the GPx activity of juvenile largemouth bass. Based on earlier comparable situations, species differences could explain this phenomenon.
Serum biochemical enzyme activity (AST, ALT, and LDH) can provide useful information about liver tissue health status, as changes in the activities of these enzymes reveal liver tissue damage. The biochemical outcomes demonstrated the histological findings of the hepatopancreas and posterior kidney. In the current study, high doses of OTC (OTC160/OTC160 + AA) significantly increased liver enzyme activities compared to the CTR and these results suggest OTC is a probable bother in amino acid and carbohydrate metabolism in the liver, which is the main organ of detoxification. This increase could be attributed to oxidative damage induced by OTC, which affects hepatocyte permeability, causing the outflow of these enzymes into fish blood (Yonar 2012). In conformity with our findings, increased liver enzymes in OTC-treated fish have previously been detected in Oncorhynchus kisutch and O. mykiss (Nakano et al. 2018; Moradi et al. 2022). The influence of OTC on these biomarkers was more distinct in the OTC160 compared to the OTC80 in a dose-dependent manner. OTC exposure resulted in changes in the activities of enzymes involved in gluconeogenesis in other fish species. OTC altered the activity of LDH, one of the enzymes related to gluconeogenesis, in zebrafish (Oliveira et al. 2013) and rainbow trout (Rodrigues et al. 2018). Thus, OTC may increase gluconeogenesis, which causes excessive consumption of energy and may result in a decline in organ weight.
High serum creatinine and urea levels demonstrate renal dysfunction, a low glomerular filtration rate, and muscular atrophy. The oral dosing with high doses of OTC (OTC160) notably increased the serum creatinine and urea levels in O. niloticus, while OTC80 only significantly elevated the serum urea levels demonstrating the nephrotoxic potential of OTC. Dietary inclusion of AA (100 mg/kg diet) significantly reduced these parameters toward control value and the histological findings of the hepatopancreas and posterior kidney proved this protective role. In this report, liver and kidney biomarkers significantly decreased following the inclusion of AA in O. niloticus diets, suggesting the efficacy of this nutrient in protecting hepatocytes and renal cells from damage. This was consistent with findings from previous studies on thornfish, Terapon jarbua (Chien & Hwang 2001), juvenile cobia (Zhou et al. 2012), and O. niloticus (Ibrahim et al. 2020).
Serum protein electrophoresis is a laboratory method for identifying the levels of specific proteins in serum. This enables them to be monitored and analyzed separately. It entails exposing serum in a special gel to an electric charge. This causes the various protein types to relocate and band together. The pattern of serum protein electrophoresis outcomes is determined by the fractions of two main protein types (albumin and globulins). The largest peak, albumin, is located near the + ve electrode. The following 5 constituents (globulins) are identified as alpha 1, alpha 2, beta 1, beta 2, and gamma. The peaks observed for these constituents are oriented near the − ve electrode, with the gamma peak being the nearest to it. The increased levels of beta 1% and gamma % were reported in the AA group. These types of proteins support immunity. Antibodies or immunoglobulins are usually the only proteins found in the normal gamma region. OTC + AA groups showed a substantial decrease in alpha 2% which may be related to liver disease. Albumen level and percent increased in all OTC-treated groups except OTC80 + AA which showed only an increase in albumen level with an insignificant increase in albumen percent.
Similar to our results, poor feed intake and growth were also observed in Clarias gariepinus juveniles (Lawal et al. 2012) and O. niloticus (Roy 2017) at higher doses of OTC. The current study’s findings supported several previous studies that found that the general acceptance of medicated feeds reduced as drug doses increased (Robinson et al. 1990; Toften and Jobling 1997; Julinta et al. 2019). The reduced survival of O. niloticus with increasing OTC dosing which was recorded in the current study supported by Stojanova and Luque (2017), who stated that any drug or antibiotic at higher doses than its allowable level outcomes in a toxicity reaction and becomes toxic to the host organism.
Internally, fish fed high doses of OTC showed hemorrhagic abdomen, adhesion of internal organs, and hemorrhagic liver. These results were consistent with Julinta et al. (2019) who reported pale liver and kidney and liquefaction and discoloration of internal organs in O. niloticus fed the higher doses of OTC.
Histopathology is a well-established method for investigating the toxicological effects of drug treatment in fish because it can distinguish, identify, and evaluate histological changes in specific key organs (Rodrigues et al. 2017). Because the liver and kidney are the organs that respond most readily to toxicants in fish, they are regarded as the best targets for histopathological studies (Rodrigues et al. 2017). In this study, the histological changes were directly correlated to the OTC dose. The most severe pathological changes of the hepatopancreas observed in OTC160 were in the form of marked necrosis of hepatic cells and pancreatic acini, accompanied by mononuclear inflammatory cell infiltration and severe vacuolar degeneration of hepatic cells. The inclusion of AA in the fish diet partially restored the disordered tissue damage in the OTC160 + AA group, which presented only vacuolation of hepatocytes and normal hepatopancreas. Hepatic vacuolization was a pronounced feature that was generally caused by extra fat deposition in the cytoplasm as a result of metabolic dysfunction caused by various toxicants (Velma and Tchounwou 2010). Various authors have reported hepatic vacuolation after exposure to tetracycline in various species, which is consistent with our findings (Nunes et al. 2015; Rodrigues et al. 2017). These hepatotoxic impacts must be regarded, particularly when the fish are exposed to dosages over the prescribed dose or for a longer period than recommended.
During histopathological examination, some necrotic areas were detected in hepatic tissue. The observed hepatocyte necrosis in organisms exposed to high doses of OTC might be a result of the inhibition of DNA synthesis, which is necessary for liver growth and development. Hepatic damage in O. niloticus exposed to 1- and 10-days OTC at 80 and 800 mg/kg/day was also reported. In line with our findings, OTC treatment caused hepatic damage and atrophy in O. niloticus (Julinta et al. 2019; Limbu et al. 2021). The most common inflammatory characteristic was mononuclear inflammatory cell infiltration, which was more common in OTC160, indicating the existence of an inflammatory reaction to the high dose of OTC.
The most obvious changes observed in the posterior kidney varied from mild vacuolation of the renal tubular epithelium (OTC80) to a marked degree of renal tubular degeneration (OTC160). Once more, the kidney of the OTC160 + AA group presented signs of improvement, with foci of degenerative changes within some renal tubules. Previous trials on the OTC impact on renal histology in various fish species have yielded conflicting results, with observed lesions varying from no alterations to severe necrosis (Reda et al. 2013; Soler et al. 1996). In our earlier study, another antibiotic (florfenicol; 10 mg/kg BW/day/10 days) was demonstrated to cause mild hepatocyte vacuolization consistent with glycogen storage with no changes in the renal tissues of O. niloticus (Abu-Zahra et al. 2023). In the current study, histopathological changes were not detected in tissues of the hepatopancreas and posterior kidney affected by AA.
The comet assay, which can detect damaged DNA strands at the individual cell level in an alkaline medium, is one of the most widely used methods for assessing genotoxicity in fish (Singh et al. 1988). It permits the accessing of DNA damage at the level of single cell by determining movement of fragmented DNA in gel-implanted cells (Kousar et al. 2022) and allows investigation of the entire genome throughout the cell cycle, not just during mitosis (Bae et al. 2020). The majority of genotoxic substances have been found to produce ROS that interacts with the DNA and causes its disturbance. In our study, high dose of OTC was reported to cause oxidative stress which plays an important role in inducing genotoxicity and cytotoxicity in hepatocytes. Furthermore, DNA damage in liver cells may be linked to OTC160-induced genetic abnormalities, which may result in the modulation of deviant and physiologically nonfunctional proteins that cause mitochondrion malfunction and nuclear protein damage. The observed OTC genotoxicity also might be clarified by OTC-DNA binding. According to Khan et al. (2003), OTC and tetracyclines overall have a strong DNA affinity and subsequently resulted in the creation of a TC-DNA binary compound that causes changes in the secondary structure of the native DNA duplex. Secondly, ROS generation and toxic metabolites formed during OTC metabolism, which causes oxidative stress and disrupts enzymatic activity, maybe another toxicological mechanism for antibiotic toxicity (Liu et al. 2014). Also, OTC forms bonds with divalent ions for instance Ca2+ and Mg2+ leading to further disruption of several biological processes (Madhu Sharma et al. 2019). More damaged DNA portions move to the comet’s tail section as DNA damage increases in the cell nucleus, causing the tail region to glow more intensely and the tail length to lengthen (Kousar et al. 2022) as detected in the current study. After 28 days of OTC exposure, the antioxidant activity was impaired, resulting in oxidative stress and genotoxicity, such as abnormalities in the RBCs nucleus (Rodrigues et al. 2018). So far, only a few researches on the mutagenic and genotoxic effects of antibiotics on fish have been performed. In another research, Itoh et al. (2006) revealed DNA changes in the comet assay caused by several quinolone antibiotics. Di Cerbo et al. (2016) and Gallo et al. (2017) demonstrated that OTC induces in vitro inflammatory response, genotoxic damage, and apoptosis. In the current study, there was a dose-dependent increase in DNA damage which is consistent with Ahmed et al. (2011) who found that DNA damage in the erythrocytes of O. mossambicus increased with increasing arsenic concentration. The inclusion of AA in O. niloticus significantly reduced hepatocyte DNA damage in OTC-treated groups. Consequently, the results of this study augment the fact that fish like O. niloticus are good in vivo models for tracking the mutagenic effect of drugs on aquatic systems.
Once again, fish-fed AA + OTC had the lowest morbidity and mortality rates after bacterial challenge, implying that AA and OTC have another type of synergism against infections. This can be clarified by the assembly of AA’s antimicrobial activity with that of OTC. Most pathogenic bacteria in the intestine prefer a pH of 7 or slightly higher; nevertheless, beneficial microbes thrive in an acidic pH range (5.8–6.2). As a result, acidifying the gastrointestinal content of tilapia more frequently decreases pathogens (particularly Gram-negative) while increasing beneficial microbes (Ng et al. 2009). Notably, AA increased the RPS of all treatments after infection, corroborating the findings of a previous study on Pelteobagrus fulvidraco Richardson challenged with A. hydrophila (Liang et al. 2017). Furthermore, AA increased resistance to other bacteria, including Escherichia coli (Leal et al. 2017) and Vibrio harveyi (Zhou et al. 2012).
In our study, fish fed on AA were healthier than those fed a control diet, which was demonstrated by the high levels of antioxidants, immune parameters, safety, and survival rates in the challenge test.
Conclusion
To the best of our knowledge, it is the first study to investigate the effect of dietary AA on OTC-induced stress in O. niloticus. The present study revealed that the dietary administration of high doses of OTC in O. niloticus notably impacted the hematological parameters, immunity, and antioxidant activity of O. niloticus. Also, OTC160 induced DNA damage and histological changes in O. niloticus. AA supplementation was reported to reestablish the suppressed immunity, as indicated by increased activities of serum total protein, globulin, IgM, NBT, gamma, and beta protein % and augmented TLC and lymphocyte values. Furthermore, the serum activity of antioxidant enzymes (SOD, CAT, and GPx) increased and that of biochemical enzymes (ALT, AST, and LDH) decreased. Also, AA is capable of reducing DNA damage and histological changes induced by OTC. Thus, dietary supplementation of AA might be an effective approach to reduce the adverse impacts of OTC on O. niloticus and work synergistically to increase fish disease resistance and decrease morbidity and mortality rates. The results of this trial on OTC biosafety would enhance tropical fish safety and might aid in the development of effective fish health approaches. We anticipate that our study’s findings will help better understand the potential environmental risks of antibiotics, and support the progress of management approaches to minimize the impacts of these substances in the ecosystem and the consequences for farmed fish.
Data availability
The authors confirm that the data supporting the findings of this study are available within the manuscript, figures, and tables.
References
Abraham TJ, Julinta RB, Roy A et al (2021) Dietary therapeutic dose of oxytetracycline negatively influences the antioxidant capacity and immune-related gene expression in Nile tilapia Oreochromis niloticus (L.). Environ Toxicol Pharmacol 87:103685. https://doi.org/10.1016/j.etap.2021.103685
Abu-Zahra NIS, Elseify MM, Atia AA, Al-sokary ET (2023) Impacts of florfenicol on immunity, antioxidant activity, and histopathology of Oreochromis niloticus: a potential protective effect of dietary spirulina platensis. Vet Res Commun. https://doi.org/10.1007/s11259-023-10189-9
Aebi H (1984) [13] Catalase in vitro. In: Methods in Enzymology. Elsevier, pp 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Affonso EG, Silva E da C, Tavares-Dias M, et al (2007) Effect of high levels of dietary vitamin C on the blood responses of matrinxã (Brycon amazonicus). Comparative Biochem Physiol Part A: Mol & Integrative Physiol 147:383–388https://doi.org/10.1016/j.cbpa.2007.01.004
Ahmed M, Abdullah N, Yusof HM et al (2017) Improvement of growth and antioxidant status in Nile tilapia, Oreochromis niloticus, fed diets supplemented with mushroom stalk waste hot water extract. Aquac Res 48:1146–1157. https://doi.org/10.1111/are.12956
Ahmed MdK, Habibullah-Al-Mamun Md, Hossain MA et al (2011) Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 84:143–149. https://doi.org/10.1016/j.chemosphere.2011.02.025
Al-Khalaifah HS, Khalil AA, Amer SA et al (2020) Effects of dietary doum palm fruit powder on growth, antioxidant capacity, immune response, and disease resistance of African catfish, Clarias gariepinus (B.). Animals 10:1407. https://doi.org/10.3390/ani10081407
Amend D (1981) Potency testing of fish vaccines. International Symposium in Fish Biologics: Serodiagnostics and Vaccines. Environ Sci 49:447–454
Babior BM (2000) Phagocytes and oxidative stress. Am J Med 109:33–44. https://doi.org/10.1016/s0002-9343(00)00481-2
Bae D-Y, Atique U, Yoon JH, et al (2020) Ecological risk assessment of urban streams using fish biomarkers of DNA damage and physiological responses. Polish J Environ Stud 29:1077–1086. https://doi.org/10.15244/pjoes/104660
Bojarski B, Kot B, Witeska M (2020) Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 13:189. https://doi.org/10.3390/ph13080189
Chen Y-J, Yuan R-M, Liu Y-J et al (2015) Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture 435:431–436. https://doi.org/10.1016/j.aquaculture.2014.10.013
Chien LT, Hwang DF (2001) Effects of thermal stress and vitamin C on lipid peroxidation and fatty acid composition in the liver of thornfish Terapon jarbua. Comp Biochem Physiol B: Biochem Mol Biol 128(1):91–97. https://doi.org/10.1016/s1096-4959(00)00299-2
Di Cerbo A, Palatucci AT, Rubino V et al (2016) Toxicological implications and inflammatory response in human lymphocytes challenged with oxytetracycline. J Biochem Mol Toxicol 30:170–177. https://doi.org/10.1002/jbt.21775
Dobšíková R, Blahová J, Mikulíková I et al (2013) The effect of oyster mushroom β-1.3/1.6-D-glucan and oxytetracycline antibiotic on biometrical, hematological, biochemical, and immunological indices, and histopathological changes in common carp (Cyprinus carpio L.). Fish Shellfish Immunol 35:1813–1823. https://doi.org/10.1016/j.fsi.2013.09.006
Doumas BT, Bayse DD, Carter RJ et al (1981) A candidate reference method for determination of total protein in serum. I. Dev Valid Clin Chem 27:1642–1650. https://doi.org/10.1093/clinchem/27.10.1642
El Latif AMA, Elabd H, Amin A et al (2019) High mortalities caused by Aeromonas veronii: identification, pathogenicity, and histopathological studies in Oreochromis niloticus. Aquacult Int 27:1725–1737. https://doi.org/10.1007/s10499-019-00429-8
Esteban MA, Cuesta A, Ortuño J, Meseguer J (2001) Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol 11:303–315. https://doi.org/10.1006/fsim.2000.0315
Faggio C, Piccione G, Marafioti S et al (2014) Monthly variations of hematological parameters of Sparus aurata and Dicentrarchus labrax reared in Mediterranean land off-shore tanks. Cah Biol Mar 55:437–443
Fracalossi DM, Allen ME, LúciaK Y, Oftedal OT (2001) Ascorbic acid biosynthesis in Amazonian fishes. Aquaculture 192:321–332. https://doi.org/10.1016/s0044-8486(00)00455-5
Gallo A, Landi R, Rubino V et al (2017) Oxytetracycline induces DNA damage and epigenetic changes: a possible risk for human and animal health? PeerJ 5:e3236. https://doi.org/10.7717/peerj.3236
Hajirezaee S, Mohammadi G, Naserabad SS (2020) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Hardie LJ, Fletcher TC, Secombes CJ (1991) The effect of dietary vitamin C on the immune response of the Atlantic salmon (Salmo salar L.). Aquaculture 95:201–214. https://doi.org/10.1016/0044-8486(91)90087-n
Ibrahim RE, Ahmed SAA, Amer SA et al (2020) Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia. Oreochromis Niloticus Aquaculture Reports 18:100545. https://doi.org/10.1016/j.aqrep.2020.100545
Itoh T, Mitsumori K, Kawaguchi S, Sasaki YF (2006) Genotoxic potential of quinolone antimicrobials in the in vitro comet assay and micronucleus test. Mutation Res/Genetic Toxicol Environ Mutagen 603:135–144. https://doi.org/10.1016/j.mrgentox.2005.11.003
Julinta RB, Abraham TJ, Roy A, et al. (2019) Effect of oxytetracycline-dosing on the growth, safety, and intestinal histology of Nile tilapia, Oreochromis niloticus (L.) juveniles. Intl J Curr Microbiol Appl Sci 8:2708–2724. https://doi.org/10.20546/ijcmas.2019.808.313
Kasagala D, Pathiratne A (2008) Effects of waterborne chloramphenicol and oxytetracycline exposure on hematological parameters and phagocytic activity in the blood of Koi carp, Cyprinus Carpio. Diseases in Asian Aquaculture VI. Asian Fisheries Society, Manila, Philippines, Fish Health Section, pp. 283–296. https://www.researchgate.net/publication/288633664_Effects_of_waterborne_chloramphenicol_and_oxytetracyclene_exposure_on_hematological_parameters_and_phagocytic_activity_in_the_blood_of_Koi_carpCyprinus_carpio
Khajepour F, Hosseini SA (2012) Citric acid improves growth performance and phosphorus digestibility in Beluga (Huso huso) fed diets where soybean meal partly replaced fish meal. Anim Feed Sci Technol 171:68–73. https://doi.org/10.1016/j.anifeedsci.2011.10.001
Khan MA, Mustafa J, Musarrat J (2003) Mechanism of DNA strand breakage induced by photosensitized tetracycline-Cu (II) complex. Mutat Res 525:109–119
Köllner B, Wasserrab B, Kotterba G, Fischer U (2002) Evaluation of immune functions of rainbow trout (Oncorhynchus mykiss)—how can environmental influences be detected? Toxicol Lett 131:83–95. https://doi.org/10.1016/s0378-4274(02)00044-9
Kousar S, Javed M, Ambreen F, Abbas S (2022) Determination of genotoxic effects of aluminum on Cirrhinus mrigala and Ctenopharyngodon idella using comet assay and micronucleus test. Iran J Fisher Sci 21:545–567. https://doi.org/10.22092/ijfs.2022.350567.0
Lawal MO, Zaid AA, Ezenwanne DO (2012) The growth rate and histology of catfish (Clarias gariepinus) juveniles fed antibiotics (oxytetracycline) Report and Opinion 4:6. https://www.researchgate.net/publication/315421827_The_growth_rate_and_histology_of_Catfish_Clarias_gariepinus_Juveniles_fed_Antibiotics_Oxytetracycline_and_Furasol_treated_Feed
Leal E, Zarza C, Tafalla C (2017) Effect of vitamin C on innate immune responses of rainbow trout (Oncorhynchus mykiss) leukocytes. Fish Shellfish Immunol 67:179–188. https://doi.org/10.1016/j.fsi.2017.06.021
Leal JF, Santos EBH, Esteves VI (2019) Oxytetracycline in intensive aquaculture: water quality during and after its administration, environmental fate, toxicity, and bacterial resistance. Rev Aquac 11:1176–1194. https://doi.org/10.1111/raq.12286
Liang X-P, Li Y, Hou Y-M et al (2017) Effect of dietary vitamin C on the growth performance, antioxidant ability, and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac Res 48:149–160. https://doi.org/10.1111/are.12869
Limbu SM, Chen L, Zhang M, Du Z (2021) A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: a review. Rev Aquac 13:1015–1059. https://doi.org/10.1111/raq.12511
Liu J, Lu G, Ding J et al (2014) Tissue distribution, bioconcentration, metabolism, and effects of erythromycin in crucian carp (Carassius auratus). Sci Total Environ 490:914–920. https://doi.org/10.1016/j.scitotenv.2014.05.055
Lundén T, Lilius E-M, Bylund G (2002) Respiratory burst activity of rainbow trout (Oncorhynchus mykiss) phagocytes is modulated by antimicrobial drugs. Aquaculture 207(3–4):203–212. https://doi.org/10.1016/s0044-8486(01)00760-8
Madhu Sharma, Jyoti Thakur, Subhash Verma, Pardeep Sharma (2019) Cytotoxic and genotoxic effect of oxytetracycline on fish Cyprinus carpio after acute exposure. Asian Journal of Pharmaceutical and Clinical Research 134–137. https://doi.org/10.22159/ajpcr.2019.v12i12.35740
Mişe Yonar S, Yonar ME, Pala A et al (2020) Effect of trichlorfon on some hematological and biochemical changes in Cyprinus carpio: the ameliorative effect of lycopene. Aquaculture Reports 16:100246. https://doi.org/10.1016/j.aqrep.2019.100246
Moradi S, Javanmardi S, Gholamzadeh P, Tavabe KR (2022) The ameliorative role of ascorbic acid against blood disorder, immunosuppression, and oxidative damage of oxytetracycline in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 48:201–213. https://doi.org/10.1007/s10695-022-01045-9
Nakano T, Hayashi S, Nagamine N (2018) Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environ Sci Pollut Res 25:7121–7128. https://doi.org/10.1007/s11356-015-4898-4
Nayak SK, Swain P, Mukherjee SC (2007) Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunol 23:892–896. https://doi.org/10.1016/j.fsi.2007.02.008
Nazeri S, Farhangi M, Modarres S (2017) The effect of different dietary inclusion levels of rutin (a flavonoid) on some liver enzyme activities and oxidative stress indices in rainbow trout, Oncorhynchus mykiss (Walbaum) exposed to Oxytetracycline. Aquac Res 48:4356–4362. https://doi.org/10.1111/are.13257
Ng W-K, Koh C-B (2016) The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev Aquac 9:342–368. https://doi.org/10.1111/raq.12141
Ng W-K, Koh C-B, Sudesh K, Siti-Zahrah A (2009) Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquac Res 40:1490–1500. https://doi.org/10.1111/j.1365-2109.2009.02249.x
Nishikimi M, Appaji Rao N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Nunes B, Antunes SC, Gomes R et al (2015) Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: antioxidant effects, neurotoxicity and histological alterations. Arch Environ Contam Toxicol 68:371–381. https://doi.org/10.1007/s00244-014-0101-z
Oliva-Teles A (2012) Nutrition and health of aquaculture fish. J Fish Dis 35:83–108. https://doi.org/10.1111/j.1365-2761.2011.01333.x
Oliveira R, McDonough S, Ladewig JCL et al (2013) Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ Toxicol Pharmacol 36:903–912. https://doi.org/10.1016/j.etap.2013.07.019
Omoregie E, Oyebanji SM (2002) Oxytetracycline-induced blood disorder in juvenile Nile tilapia Oreochromis niloticus (Trewavas). J World Aquaculture Soc 33:377–382. https://doi.org/10.1111/j.1749-7345.2002.tb00514.x
Paglia DE, Valentine WN (1967) Studies on quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pepi M, Focardi S (2021) Antibiotic-Resistant bacteria in aquaculture and climate change: a challenge for health in the Mediterranean area. Int J Environ Res Public Health 18:5723. https://doi.org/10.3390/ijerph18115723
Reda RM, Ibrahim RE, Ahmed E-NG, El-Bouhy ZM (2013) Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt J Aquatic Res 39:241–248. https://doi.org/10.1016/j.ejar.2013.12.001
Reda RM, Mahmoud R, Selim KM, El-Araby IE (2016) Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 50:255–262. https://doi.org/10.1016/j.fsi.2016.01.040
Robinson EH, Brent JR, Crabtree JT, Tucker CS (1990) Improved palatability of channel catfish feeds containing Romet-30®. J Aquat Anim Health 2:43–48. https://doi.org/10.1577/1548-8667(1990)002%3c0043:ipoccf%3e2.3.co;2
Rodrigues S, Antunes SC, Correia AT, Nunes B (2018) Oxytetracycline effects in specific biochemical pathways of detoxification, neurotransmission, and energy production in Oncorhynchus mykiss. Ecotoxicol Environ Saf 164:100–108. https://doi.org/10.1016/j.ecoenv.2018.07.124
Rodrigues S, Antunes SC, Nunes B, Correia AT (2017) Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ Toxicol Pharmacol 53:164–176. https://doi.org/10.1016/j.etap.2017.05.012
Roy A (2017) Biosafety and efficacy of oxytetracycline against Aeromonas caviae infection in Nile tilapia, Oreochromis niloticus. Master’s thesis. West Bengal University of Animal and Fishery Sciences, Kolkata
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. https://doi.org/10.1016/0014-4827(88)90265-0
Siwicki AK, Anderson DP, Dixon OW (1989) Comparisons of nonspecific and specific immunomodulation by oxolinic acid, oxytetracycline and levamisole in salmonids. Vet Immunol Immunopathol 23:195–200. https://doi.org/10.1016/0165-2427(89)90122-0
Soler F, Reja A, García-Rubio L et al (1996) Anatomo-pathological effect of oxytetracycline in tench (Tinca tinca). Toxicol Lett 88:104. https://doi.org/10.1016/s0378-4274(96)80375-4
Stojanova J, Luque S (2017) Therapeutic drug monitoring: more than avoiding toxicity. In: Antibiotic Pharmacokinetic/Pharmacodynamic Considerations in the Critically Ill. Springer Singapore, Singapore, pp 173–199.
Tafalla C, Novoa B, Alvarez JM, Figueras A (2002) In vivo and in vitro effect of oxytetracycline treatment on the immune response of turbot, Scophthalmus maximus (L.). J Fish Dis 22:271–276. https://doi.org/10.1046/j.1365-2761.1999.00179.x
Tietz NW (1995) Clinical guide to laboratory tests. 3rd ed. Philadelphia. WB. Saunders, 268–273. Retrieved March 27, 2023, from http://www.sciepub.com/reference/141231
Toften H, Jobling M (1997) Feed intake and growth of Atlantic salmon, Salmo salar L., fed diets supplemented with oxytetracycline and squid extract. Aquac Nutr 3:145–151. https://doi.org/10.1046/j.1365-2095.1997.00081.x
Trushenski JT, Aardsma MP, Barry KJ et al (2018) Oxytetracycline does not cause growth promotion in finfish1. J Anim Sci 96:1667–1677. https://doi.org/10.1093/jas/sky120
USFWS U.S. Fish and Wildlife Service (2015) Approved drugs for use in aquaculture, second ed. U.S. Fish and Wildlife Service’s Aquatic Animal Drug Approval Partnership Program, American Fisheries Society’s Fish Culture and Fish Health Sections, Association of Fish and Wildlife Agencies, and Fisheries and Water Resources Policy Committee’s Drug Approval Working Group. In: U.S. Fish and Wildlife Service. https://fws.gov/. Accessed 10 Jun 2023
Velma V, Tchounwou PB (2010) Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, Carassius auratus. Mutation Res/Genetic Toxicol Environ Mutagen 698:43–51. https://doi.org/10.1016/j.mrgentox.2010.03.014
Wang JP, Lee JH, Yoo JS et al (2010) Effects of phenylacetic acid on growth performance, intestinal microbiota, relative organ weight, blood characteristics, and meat quality of broiler chicks. Poult Sci 89:1549–1555. https://doi.org/10.3382/ps.2009-00235
Xie Z, Niu C, Zhang Z, Bao L (2006) Dietary ascorbic acid may be necessary for enhancing the immune response in Siberian sturgeon (Acipenser baerii), a species capable of ascorbic acid biosynthesis. Comp Biochem Physiol a: Mol Integr Physiol 145:152–157. https://doi.org/10.1016/j.cbpa.2006.05.015
Yonar ME (2012) The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol 32:994–1001. https://doi.org/10.1016/j.fsi.2012.02.012
Zhou Q, Wang L, Wang H et al (2012) Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol 32:969–975. https://doi.org/10.1016/j.fsi.2012.01.024
Acknowledgements
The authors express grateful appreciation and hearty thanks to all Animal Health Research Institute Dokki- Giza, Kafrelsheikh Provincial Lab staff for the facilities they afford and their kind assistance.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
NISAZ: methodology, formal analysis, writing—original draft, writing—review and editing, resources, supervision, investigation, visualization. SS: ideas, writing—original draft, formulation of overarching research goals and aims, writing—review. AAA: resources, investigation, visualization, validation, writing—review. MME: ideas, formulation of overarching research goals and aims, project administration, writing—review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Animal welfare and ethics
The Institutional Aquatic Animal Care and Use Committee, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University approved the experimental protocols for the care and maintenance of live fish (approval number: IAACUC-KSU-2022–0023).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abu-Zahra, N.I.S., Atia, A.A., Elseify, M.M. et al. Biological and histological changes and DNA damage in Oreochromis niloticus exposed to oxytetracycline: a potential amelioratory role of ascorbic acid. Aquacult Int 32, 3889–3916 (2024). https://doi.org/10.1007/s10499-023-01356-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01356-5