Abstract
The current investigation was carried out to determine the impacts of different levels of vitamin K (VK) on the growth performance, hematological parameters, and immunological response of all-male juveniles of Nile tilapia (O. niloticus). VK3 (menadione) was added in five concentrations (0.0, 2.0, 4.0, 8.0, and 12.0 mg kg–1 diet) in five isonitrogenous (30% cp), isocaloric (18.61 MJ kg–1) diets. The fish were fed the diets at a daily rate of 3% of their live weight, divided into 3 meals for 60 days. The results implied that supplemental VK did not provide any growth rate improvements and the efficiency of feed utilization over the control diet. Increasing dietary VK above 2 mg kg–1 feed resulted in significant retardation in fish performance and survival rates. Furthermore, supplemental VK up to 2–4 mg kg–1 increased hematological parameters, physiological functions, immune response, antioxidant capacity, and bone mineralization. Further increase in dietary VK resulted in a significant decline or level off in these parameters. Meanwhile, liver function enzymes increased progressively with dietary VK increasing. In conclusion, these findings suggest that supplemental VK maybe not be necessary for Nile tilapia growth performance, whereas about 2–4 mg kg–1 diet is required for other physiological functions. Increasing VK beyond these levels may pose adverse effects on Nile tilapia. However, further long-term studies are required to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin K (VK) is a group of fat-soluble, quinone-derived compounds, sharing a common 2-methyl-1,4-naphthoquinone ring but differing in the side chain at the C3-position (Lambert and De Leenher 1992; Krossøy et al. 2011). This group includes at least two naturally occurring forms of VK: VK1 (phylloquinone; 2-methyl-3-phytyl-1,4-naphthoquinone) and VK2 (menaquinones; MK; 2-methyl-3-(prenyl)n-1,4-naphthoquinone) (Krossøy et al. 2011). A third, chemically synthesized form of VK is VK3 (menadione; 2-methyl-1,4-naphthoquinone). Vitamin K has several functions in mammals and fish, including regulating the process of blood coagulation, through the production of prothrombin, which is a protein responsible for regulating clotting time for blood (Booth 1997; Jiang and Doolittle 2003). Vitamin K is also engaged in bone metabolism, quality, and health (Zhou et al. 2009) and Ca2+ homeostasis regulation (Oldenburg et al. 2008). Also, it plays a physiological role as an enzymatic co-factor, in addition to its role in inflammation, renal disease, sepsis, neoplasia, and energy metabolism (Arai et al. 2008; Booth 2009).
The VK requirement for cultivated fish is controversial and not fully comprehended. Varying, and sometimes contradictory, outcomes have been published on the impact of VK on farmed fish and its deficiency symptoms. For example, dietary VK deficiency induced a reduction in mineralization of bone and bone mass, leading to bone abnormalities in haddock (Melanogrammus aeglefinus) (Roy and Lall 2007) and mummichog (F. heteroclitus) (Udagawa 2001; 2004). A vitamin K-deficient diet also reduced growth performance and increased mortality in amago salmon (Oncorhynchus rhodurus) (Taveekijakarn et al. 1996). Increased blood coagulation time, hemorrhages, anemia, loss of fin tissue, weak bones, and occurrence of spinal curvature and short tails have also been documented as symptoms of VK deficiency (Taveekijakarn et al. 1996; Udagawa 2004; Lall and Lewis-McCrea 2007). Supplemental VK improved the growth, food digestion, and absorption in common carp (C. carpio), and anti-oxidant capacity in rainbow trout and common carp (Yuan et al. 2016). According to these studies, these fishes lack the ability to de novo synthesize VK, and thus, it needs to be provided with their diets.
On the contrary, several other studies revealed that supplemental VK is possibly not required for farmed fish. For instance, vitamin K-deficient feed did not cause detectable deficiency signs in rainbow trout (Kitamura et al. 1967), channel catfish (Murai and Andrews 1977), and Atlantic salmon (Graff et al. 2002; Krossøy et al. 2009). These researches concluded that the VK requirement of fish could be met by the amount found in basal diets (Graff et al. 2002; Krossøy et al. 2009), suggesting that supplemental VK may be not necessary for farmed fish.
Tilapia is currently the essential cultivated fish in the world, second only to carps (FAO 2020). They are currently farmed in over 120 countries worldwide, with Nile tilapia (Oreochromis niloticus) being the most great farmed tilapia species. This species represents 70– > 80% of total production of tilapia during 2000–2019 (El‐Sayed 2020; FAO 2020). Nile tilapia is also ranked second (after silver carp) in terms of global farmed fish production in 2019 (FAO 2020).
However, the Nile tilapia’s requirement from VK has not been considered. It is unknown whether this species requires VK for healthy growth and physiological functions. As far as the authors know, only a single study (Lee 2003) evaluated the VK requirement of hybrid tilapia (O. aureus × O. niloticus). Therefore, the current study was conducted to assess the impacts of supplemental VK on growth performance, feed efficiency, hematological parameters, immune response, and antioxidant capacity in Nile tilapia juveniles.
Materials and methods
Fish and culture facility
All-mall Nile tilapia (O. niloticus) fingerlings used in the current study were obtained from the nursing unit of Fish Farming and Technology Institute (FFTI), Suez Canal University. Healthy tilapia fingerlings with an initial weight of 30.28 ± 3.75 g were randomly distributed, in triplicates, into 15 indoor fiberglass, circular tanks with a maximum capacity of 3 m3 (1.7 m diameter and 1.4 m high) filled with 1000 L dechlorinated tap water, at a density of 30 fish /m3, under controlled photoperiods (12 L:12 D cycle) and temperature (25 ± 1 °C). The criteria for healthy fingerlings selection are eating when presented with food, not seeming disinterested, having bright coloration without abnormally dark spots, swimming actively with a symmetrical gait, and missing scales or not having tattered fins. They were adapted to the culture conditions for 2 weeks, during which they were fed with a commercial, extruded feed (30% CP; Aller aqua, 6th of October City, Egypt). The tank water used was filtered with sand filters and sterilized using ultraviolet units (Fujan Newland Entech Co., Ltd, China). Aeration was provided by an air blower (Rotary Blower, SWR, China) through diffuser stones. After the acclimation period, each tank’s fish were netted, counted, weighed, and the average initial weights were recorded.
Water quality parameters, including temperature, dissolved oxygen (DO), ammonia (NH4-N), nitrates (NO3-N), nitrites (NO2-N), and pH, were monitored weekly (ExStik II D-0600, FLIR Systems, Inc., USA) (Milwaukee MW-100). Twenty percent of the water capacity was changed per day. The scales of these parameters’ values throughout the study were as follows: DO = 6.0–7 mg L–1, NH4–N = 0.050–0.060 mg L–1, NO3–N = 7.0–8.3 mg L–1, NO2–N = 0.02–0.05 mg L–1, and pH = 8.0–8.4.
Experimental diets and feeding regime
VK3 (menadione) was added in five concentrations (0.0, 2.0, 4.0, 8.0, and 12.0 mg kg–1 diet) in five experimental isonitrogenous (30% cp), isocaloric (18.61 MJ kg–1), designated as D0, D2, D4, D8, and D12, respectively (Table 1). The diets were prepared as described by El-Sayed et al. (2000) and El-Sayed et al. (2013). The fish were fed the test diets at a daily rate of 3% of the fish’s live weight divided into 3 meals (9:00, 12:00, and 15:00 h) for 60 days.
Each tank’s fish were sampled and weighed at 10-day intervals, their average weights were recorded and the daily amount of feed for each tank was readjusted accordingly. After the feeding trial ended, each tank’s all fish were collected, counted, and weighed, and the average final weights were recorded.
Calculation of fish growth parameters
Growth performance and feed utilization efficiency were calculated as follows:
-
Percent weight gain = 100 (final weight (g) – initial weight (g) /initial weight (g).
-
Specific growth rate (SGR, % /day) = 100 (Ln Wf − Ln Wi) ∕ t), where Wi and Wf are the initial and final weights (g), and t is the time of the experiment (days).
-
Feed conversion ratio (FCR) = dry feed intake (g)/ fish live weight gain (g).
-
Protein efficiency ratio (PER) = fish live weight gain (g)/dry protein fed (g).
-
Protein productive value (PPV) = 100}protein gain (g) /protein fed (g){.
-
Survival rate (SV) = (final stocking density / initial stocking density) × 100
Proximate composition analyses
After the trial ended, from each tank, five fish were randomly collected and frozen at − 20 °C for final body composition analyses. Initial body analysis was completed on a pooled sample of 10 fish per treatment which was weighed and frozen before the study. Proximate analyses of whole-body protein, lipid, moisture, and ash were performed according to standard AOAC (2005) methods. Briefly, for protein was measured as nitrogen by a semi-automatic Kjeldahl (N × 6.25; VELP Scientific, UDK 126, Italy) following acid digestion. Lipid content was measured gravimetrically after Soxhlet extraction with petroleum ether (40–60 °C) as a solvent. For moisture content determination, fish samples were dried at 105 °C to constant weight. Ash content was determined after ignition in a muffle furnace at 550 °C for 6 h. All analyses were performed in triplicate samples for each parameter.
Hematological and immunological analyses
After the feeding trial ended, blood samples were collected from the caudal vein of five fish from each tank, using heparinized syringe needles (15 units/mL; 5000 IU, Amoun Pharmaceutical Co., Cairo, Egypt). From each tank, blood samples were split into two parts; one part was stored in heparinized Eppendorf tubes and used for hematology (red blood cell counting, total leucocytic count, hemoglobin, and hematocrit). The second part was kept in unheparinized tubes, left to clot at 4 °C and centrifuged at 5000 rpm (5 min) at room temperature, for separating plasma which was stored at − 18 °C and used for plasma analysis.
Red blood cell count (RBCs) was counted under light microscope using a Neubauer hemocytometer after blood dilution with phosphate-buffered saline (pH, 7.2) (Shalaby et al. 2019; Abdel-Tawwab et al. 2020). Total leucocytic count (WBCs) (Rey V´azquez and Guerrero 2007) and hematocrit (PCV) were immediately determined according to Rehulka (2000) after sampling by placing fresh blood in glass capillary tubes, centrifuging it for 5 min in a microhematocrit centrifuge, and measuring the packed cell volume. Concentration of hemoglobin (Hb) was determined colorimetrically according to Jain (1993). Mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) were calculated according to Haney et al. (1992) as follows:
Biochemical and hepatic functions analyses
Plasma Ca2+ (mmol/l) was measured using a Stat Profile pHOx plus analyser (Nova Bio. Co., Waltham, USA), whereas total calcium in plasma (mmol/l) was measured using ICP-AES and ALP was measured using DGKC (Deutsche Gesellschaft für Klinische Chemie) method. Plasma PTH (Para thyroid hormone) level (nmol/l) was measured with a homologous radioimmunoassay (RIA) according to Rotllant et al. (2003). The TAC (total antioxidant capacity) was determined spectrophotometrically (Roberta et al. 1999). TP (total protein) was measured by Vitros TP device using dry and wet biochemical slides with Clinical Chemistry Analyzer (Microlab 300, ELI Tech Group) (Mariana et al. 2011). Globulin and creatinine were determined using Clinical Chemistry Analyzer (Microlab 300, ELI Tech Group). Activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined colorimetrically according to Reitman and Frankel (1957).
Immunological assays
Serum lysozyme activity (LYZ) was measured by turbidometric assay according to Ellis (1990) using M. luteus suspension (Sigma, USA). Lysozyme activity for all samples was calculated from a standard curve prepared from the white of a chicken egg lysozyme (Sigma, USA).
Statistical analysis
The current results were stated as (mean ± standard error of mean (SE)). One-way analysis of variance (ANOVA) was carried out to test the significant difference (P < 0.05) between treatments. Statistical analyses were conducted using (SPSS Inc.) program v22.0 (Chicago, IL). Duncan’s Multiple Range Test was applied to compare means when F-values from the ANOVA were significant (P < 0.05).
Results
Fish performance
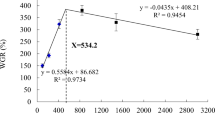
Nile tilapia growth performance was significantly affected (P < 0.05) by dietary vitamin K (VK) (Table 2). The control diet (D0) and D2 produced significantly better performance than other diets (P < 0.05). However, D0 and D2 did not change significantly (P < 0.05). Further increase in supplemental VK resulted in significant retardation in growth rates, feed efficiency, and survival rates (P < 0.05). The best vitality and fastest readily consumption of the diets were observed in D2 followed by D0 while the worst vigor and longest readily consumption of the diets were observed in D12.
Body composition
The Nile tilapia whole-body chemical composition in the current study was impacted significantly by VK doses (Table 3). Body moisture, protein, and lipids tended to decrease, whereas body ash increased (P < 0.05), with increasing VK levels. However, these parameter values did not change significantly at 0 and 2 mg VK/kg.
Hematological, immunological, and hepatic and kidney functions analyses
Dietary VK supplementation had a significant impact on hematological parameters (P < 0.05) (Table 4). The concentrations of red blood cells (RBCs) count, hemoglobin concentration (Hb), hematocrit (PCV), mean cell volume (MCV), and white blood cells (WBC), mean cell hemoglobin (MCH) and total antioxidant capacity (TAC) significantly increased (P < 0.05) with increasing VK levels up to 2 mg/kg, and decreased or leveled off with a further increase in VK levels.
Immunological parameters and hepatic and kidney function analyses were also significantly affected (P < 0.05) by dietary VK levels (Table 5). Total protein and albumin resort to raise with increasing VK levels up to 4 mg/kg (D4) and decreased afterward. Globulin concentration was also significantly decreased at D4 (P < 0.05), whereas at other VK levels, it did not significantly differ (P < 0.05). Supplemental VK significantly enhanced plasma total calcium (P < 0.05), but increasing VK from 2 to 12 mg kg–1 did not alter Ca concentrations (P < 0.05). The ionized calcium was significantly increased at 2 mg/kg (D2) (P < 0.05) and decreased afterward. Lysozyme activity also increased up to 12 mg VK (P < 0.05). PTH showed a significant rise with increasing VK levels up to 4 mg/kg (D4). Otherwise, AST, ALT, and creatinine levels significantly enhanced with increasing VK levels (P < 0.05).
Discussion
Controversial, varying, and sometimes conflicting outcomes have been reported on VK requirements of farmed fish. The current investigation revealed that supplemental VK did not result in any increase in fish performance, and feed utilization, even at the lowest concentration (2 mg kg–1). Moreover, fish performance was significantly retarded with a further increase in dietary VK levels. These findings suggest that the Nile tilapia requirement of VK is low and could be met by the amount of VK found in the basic diet. Comparable results were reported in channel catfish (Ictalurus punctatus) (Murai and Andrews 1977), rainbow trout (Kitamura et al. 1967), and Atlantic salmon (Graff et al. 2002; Krossøy et al. 2009), where VK-deficient diets did not cause deficiency signs, suggesting that supplemental VK might not be necessary for these fishes.
Fish may require extremely low VK concentrations for optimum performance. For example, Krossøy et al. (2009) demonstrated that only 0.1 mg phylloquinone (VK1) kg–1 feed was sufficient to meet the requirement for regular growth and bone veracity in juvenile Atlantic salmon. Less than 0.2 mg VK kg–1 has also been suggested as a minimum requirement for Atlantic cod (G. morhua) (Grahl-Madsen and Lie 1997). Along the same line, no considerable impact of dietary (VK3) on the growth of yellow croaker juvenile (Pseudosciaena crocea) was observed (Cheng et al. 2015). Also, no external signs of insufficiency of VK or toxicity were detected (Cheng et al. 2015). However, VK was desired for the period of blood coagulation (< 3.45 mg kg–1), and for the maximum accumulation of menaquinone-4 in fish muscle (10.42 mg kg–1) and liver (10.55 mg kg–1). Similar results were also found in white shrimp (L. vannamei), where growth performance was not considerably affected by supplemental VK3 from 9.97 to 156.02 mg kg–1 diet (Dai et al. 2022a, b).
On the other hand, excessive dietary VK can be toxic, causing various toxicity symptoms in fish, including a reduction in growth rates (Grisdale-Helland et al. 1991; Grahl-Madsen and Lie 1997), oxidation-mediated liver toxicity, and bone abnormality (Udagawa 2001). These findings may explain the reduced growth in Nile tilapia feed excessive VK in the current study. The reduction (or leveling off) of the biochemical parameters (protein, albumin, globulin, lysozyme), together with the significant increase in liver function enzyme activities, at high VK doses (beyond 4 mg kg–1 feed), may also support this assumption, thereby suggesting that excessive dietary VK may be toxic to Nile tilapia. However, further, long-term investigations are in demand to verify these results.
It should be mentioned, however, that toxicity symptoms of VK may depend on the source of supplemental VK used. For example, Udagawa (2001) found that high doses of dietary VK3 (menadione sodium bisulfite—MSB) caused a high incidence of bone abnormality in mummichog (F. heteroclitus), compared with phylloquinone (VK1) which showed no significant variation in bone deformities. Similar toxicity symptoms of Menadiones (VK3) and its peers have also been reported in mammals (Rebhun et al. 1984; Suttie, 1991), whereas no symptoms of toxicity were detected for VK1 or VK2. These findings imply that phylloquinone is more suitable as a vitamin K source than MSB in fish feed.
In contrast with the outcomes above, several other studies revealed that supplemental VK is necessary for farmed fish. Dietary VK enhanced the growth rates and feed consumption in common carp (Yuan et al. 2016) and anti-oxidant capacity in rainbow trout (Stephensen et al. 2002) and common carp (Yuan et al. 2016). VK deficiency resulted in reduced growth performance and increased mortality in amago salmon (O. rhodurus) (Taveekijakarn et al. 1996), and also caused bone abnormality in haddock (M. aeglefinus) (Roy and Lall 2007) and mummichog (F. heteroclitus) (Udagawa 2001, 2004). Hemorrhages, anemia, increased blood coagulation time, loss of fin tissue, and occurrence of spinal curvature have also been documented as symptoms of VK deficiency (Taveekijakarn et al. 1996; Udagawa 2004; Lall and Lewis-McCrea 2007). These studies suggest that these fishes may lack the ability to de novo synthesize VK, and thus, supplemental VK becomes necessary.
Hematology can provide important and reliable information on metabolic disorders, stress, nutrient deficiencies, and adaptation mechanisms to environmental variations; thereby, they are a practical tool for monitoring fish health (Makled et al. 2017; El-Sayed et al. 2021). The present hematological parameters values, including Hb%, RBC, WBC, PCV, MCV, MCH, blood platelets, and TAC, were significantly increased in Nile tilapia fed VK-supplemented diets up to 2 mg kg–1 feed. Beyond this level, these limitations were significantly reduced or leveled off. Similar findings were discovered in lake trout, where 2 mg VK kg–1 feed was sufficient for normal coagulation and satisfactory PCV in lake trout (Poston 1976). It has also been suggested that VK deficiency causes time-prolonged coagulation and anemia (Taveekijakarn et al. 1996; Steinberg 2022) .
Although supplemental VK was not necessary for regular growth performance in the current study, about 2–4 mg kg–1 were required for optimum immunological responses and for maintaining the antioxidant defence mechanism. This could be due to that VK suppresses the formation of lipid peroxide (Tampo and Yonaha 1996). These peroxides are free radicals, strongly involved in lipid peroxidation and protein oxidation (Kohen and Nyska 2002). Supplemental VK is effective in preventing reactive oxygen species (ROS) through the production of enzymes of antioxidant (such as CAT, SOD, GPx, and GST), thereby blocking the accumulation of free radical and exerting a protective defense against oxidative stress (Fang et al. 2002; Yuan et al. 2016). Similar to this, dietary VK3 had no effect on the growth performance of L. vannamei, but significantly improved their immunological response and the capacity of antioxidant (Dai et al. 2022a, b). Thus, it is evident that VK plays a significant role in mediating the activities of antioxidant enzymes and improves immune responses of farmed fish and shrimp.
In addition to the previous functions, VK plays an essential function in other biological processes in fish, such as bone mineralization (Udagawa 2000; Roy and Lall 2007). However, VK requirement for regular growth and survival in fish is generally lower than the requirement for normal bone development (Udagawa 2000). The same author (Udagawa 2001) found that when a VK-free diet was fed to mummichog (F. heteroclitus) larvae, it caused severe incidences of deformities in the backbone and caudal skeleton. Moreover, the larvae from broodfish fed a VK-deficient diet had vertebral deformities compared to larvae from broodfish were fed a VK-rich diet (Udagawa 2004). Vitamin K-deficient diets also caused the formation of weak and thin bones, and induced abnormalities in bone structure, including vertebral fusion and row irregularity (Udagawa 2001, 2004). Also, in haddock (M. aeglefinus) feeds, VK insufficiency reduced the mineralization of bone and caused bone deformities (Roy and Lall 2007). In current consideration, the increase in body ash, total plasma Ca, and ionized Ca with supplemental VK agrees with these findings, thereby supporting this vitamin role in bone structure and mineralization.
Conclusion
In conclusion, the present findings detected that supplemental VK is possibly not necessary for growth and feed efficiency of Nile tilapia, assuming that the VK contents in the basal feed may fulfill the requirement of these fish. On the other hand, about 2–4 mg VK kg–1 diet is required for optimum physiological functions, immune response, bone mineralization, and the capacity of antioxidant.
Data availability
Data are available on request due to privacy.
References
Abdel-Tawwab M, Khalifa E, Diab AM, Khallaf MA, Abdel-Razek N, Khalil RH (2020) Dietary garlic and chitosan alleviated zearalenone toxic effects on performance, immunity, and challenge of European seabass, D. labrax, to Vibrio alginolyticus infection. Aquac Int 28:493–510. https://doi.org/10.1007/s10499-019-00477-0
AOAC (2005) Association of official analytical chemists. Official Methods of Analysis (16th edn). Author Arlington, VA, USA
Arai H, Nagai K, Doi T (2008) Role of growth arrest-specific gene 6 in diabetic nephropathy. Vitam Horm 78:375–392
Booth S (1997) Skeletal functions of vitamin K-dependent proteins: not just for clotting anymore. Nutr Rev 55:282–284
Booth SL (2009) Roles for vitamin K beyond coagulation. Annu Rev Nutr 29:89–110
Cheng L, Zhang W, Lin S, Xu W, Mai K (2015) Effects of dietary vitamin K on growth performances, blood coagulation time and menaquinone-4 (MK-4) concentration in tissues of juvenile large yellow croaker P crocea. Aquaculture Res 46(5):1269–1275
Dai T, Jiao L, Tao X, Lu J, Jin M, Sun P, Zhou Q (2022) Effects of dietary vitamin D3 supplementation on the growth performance, tissue Ca and P concentrations, antioxidant capacity, immune response and lipid metabolism in L vannamei larvae. British J Nutri 128(5):793–801
Dai T, Zhang X, Li M, Tao X, Jin M, Sun P, ... & Jiao L (2022b) Dietary vitamin K 3 activates mitophagy, improves antioxidant capacity, immunity and affects glucose metabolism in L. vannamei. Food & Function, 13(11), 6362–6372
Ellis AE (1990) Lysozyme Assays. Techniques in Fish Immunol 1:101–103
El-Sayed AFM (2020) Tilapia Culture, 2nd edn. Elsevier/Academic Press, London, UK and San Diego, CA, USA, p 348
El-Sayed AF, Nmartinez I, Moyano FJ (2000) Assessment of the effect of plant inhibitors on digestive proteases of Nile tilapia using in vitro assays. Aquacult Int 8:403–415
El-Sayed AF, El-Ghobashy AE, El-Mezayen MM (2013) Effect of feed colour on growth and feed utilization of N ile tilapia (O. niloticus L.) larvae and fingerlings. Aquaculture Nutri 19(6):870–876
El-Sayed AFM, Tammam MS, Makled SO (2021) Lecithin-containing bioemulsifier boosts growth performance, feed digestion and absorption and immune response of adult Nile tilapia (O niloticus). Aquaculture Nutri 27(3):757–770
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879
FAO (2020) The State of World Fisheries and Aquaculture 2020. FAO. https://doi.org/10.4060/ca9229en
Graff IE, Waagbo R, Fivelstad S, Vermeer C, Lie O, Lundebye AK (2002) A multivariate study on the effects of dietary vitamin K, vitamin D3 and calcium, and dissolved carbon dioxide on growth, bone minerals, vitamin status and health performance in smolting Atlantic salmon Salmo salar L. J Fish Dis 25:599–614
Grahl-Madsen E, Lie Ø (1997) Effects of different levels of vitamin K in diets for cod (G. morhua). Aquaculture 151(1–4):269–274
Grisdale-Helland B, Helland S, A sgard, T. (1991) Problems associated with the present use of menadione sodium bisulfited and vitamin A in diets for Atlantic salmon. Aquaculture 92:351–358
Haney DC, Hursh DA, Mix MC, Winton JR (1992) Physiological and hematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J Aquat Anim Health 4:48–57. https://doi.org/10.1577/1548-8667
Jain NC (1993) Essentials of Veterinary Hematology. Lea and Febiger, Philadelphia, pp 76–250
Jiang Y, Doolittle RF (2003) The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci USA 100:7527–7532
Kitamura S, Suwa T, Ohara S, Nakagawa K (1967) Studies on vitamin requirements of rainbow trout II. The deficiency symptoms of fourteen kinds of vitamins. Bull Jpn Soc Sci Fish 33:1120–1125
Kohen R, Nyska A (2002) Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30(6):620–650
Krossøy C, Waagbo R, Fjelldal PG (2009) Dietary menadione nicotinamide bisulphite (vitamin K3) does not affect growth or bone health in first feeding fry of Atlantic salmon (S. salar L). Aquac Nutr 15:638–649
Krossøy C, Waagbø R, Ørnsrud R (2011) Vitamin K in fish nutrition. Aquacult Nutr 17:585–594
Lall SP, Lewis-McCrea LM (2007) Role of nutrients in skeletal metabolism and pathology in fish – an overview. Aquaculture 267:3–19
Lambert WE, De Leenher AP (1992) Vitamin K. In: De Leenher AP, Lamberts WE, Nelis HJ (eds) Modern Chromatographic Analysis of Vitamins, 2nd edn. Marcel Dekker Inc, New York, USA, pp 214–251
Lee JY (2003) Vitamin K requirements of juvenile hybrid tilapia (O. niloticus × O. aureus) and grouper (E. malabaricus). Master Thesis, National Taiwan Ocean University, Keelung, Taiwan, 89 pp
Makled SO, Hamdan AM, El-Sayed AM, Hafez EE (2017) Evaluation of marine psychrophile, Psychrobacter namhaensis SO89, as a probiotic in Nile tilapia (Oreochromis niloticus) diets. Fish and Shellfish Immunol 61:194–200. https://doi.org/10.1016/j.fsi.2017.01.001
Mariana L, Cristea V, Coprean D, Mirela M, Tanţi P and Elena B (2011) Biochemical determinations and oxidative stress evaluation on Oncorhynchus mykiss grown in recirculating system. Lucrări Ştiinţifice - vol. 55, Seria Zootehnie
Murai T, Andrews J (1977) Vitamin K and anticoagulant relationship in catfish diets. Bull Jpn Soc Sci Fish 43:785–794
Oldenburg J, Marinova M, Müller-Reible C, Watzka M (2008) The vitamin K cycle. Vitam Horm 78:35–62
Poston HA (1976) Optimum level of dietary biotin for growth, feed utilization, and swimming stamina of fingerling lake trout (Salvelinus namaycush). J Fisheries Board of Canada 33(8):1803–1806
Rebhun W, Tennant B, Dill S, King J (1984) Vitamin K3 induced renal toxicosis in the horse. J Am Vet Med Assoc 184:1237–1239
Rehulka J (2000) Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout. O Mykiss Aquaculture 190:27–47. https://doi.org/10.1016/S0044-8486(00)00383-5
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Rey V´azquez G, Guerrero GA (2007) Characterization of blood cells and hematological parameters in C dimerus (Teleostei, Perciformes). Tissue Cell 39:151–160
Roberta R, Pellegrini N, Proteggente A, Pannala A, Yang M and Rice-Evans C (1999) Antioxidant activity applying an improved abts radical cation decolorization assay Free Radical Biology & Medicine, Vol. 26, Nos. 9/10,1999, 1231–1237
Rotllant J, Worthington GP, Fuentes J, Guerreiro PM, Teitsma CA, Ingleton PM, Balment RJ, Canario AVM, Power DM (2003) Determination of tissue and plasma concentrations of PTHrP in fish: development and validation of a radioimmunoassay using a teleost 1–34 N-terminal peptide. Gen Comp Endocrinol 133:146–153
Roy P, Lall S (2007) Vitamin K deficiency inhibits mineralization and enhances deformity in vertebrae of haddock (M. aeglefinus). Comp Biochem Physiol 148:174–183
Shalaby A, Ghareeb A, Abd El-Rahman M, Abd El-Hamid E (2019) Effect of different levels of folic acid on the growth and some physiological aspects of Nile tilapia “O. niloticus.” Egypt J Aquac 9:33–45. https://doi.org/10.21608/eja.2019.19184.1008
Steinberg CE (2022) Vitamin K ‘keep the hematologist away!’ Organic Macro-and Micro-Nutrients, Aquatic Animal Nutrition, pp 951–959
Stephensen E, Sturve J, Förlin L (2002) Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comp Biochem Physiol c: Toxicol Pharmacol 133(3):435–442
Suttie, L. W., Machlin, L. J. (1991). Vitamin K In: Handbook of vitamins. Chapter 3, 111
Tampo Y, Yonaha M (1996) Enzymatic and molecular aspects of the antioxidant effect of menadione in hepatic microsomes. Arch Biochem Biophys 334(1):163–174
Taveekijakarn P, Miyazaki T, Matsumoto M, Arai S (1996) Studies on vitamin K deficiency in amago salmon (O. rhodurus) (Jordan & McGregor). J Fish Dis 19:209–214
Udagawa M (2000) Review: Physiological role of vitamin K in fish. JARQ 34(4):279–284
Udagawa M (2001) The effect of dietary vitamin K (phylloquinone and menadione) levels on the vertebral formation in mummichog Fundulus heteroclitus. Fisheries Sci 67:104–109
Udagawa M (2004) The effect of parental vitamin K deficiency on bone structure in Mummichog Fundulus heteroclitus. J World Aquac Soc 35:366–371
Yuan J, Feng L, Jiang WD, Liu Y, Jiang J, Li SH, Zhou XQ (2016) Effects of dietary vitamin K levels on growth performance, enzyme activities and antioxidant status in the hepatopancreas and intestine of juvenile J ian carp (Cyprinus carpio var Jian). Aquaculture Nutrition 22(2):352–366
Zhou HY, Hong JL, Shu P, Ni YJ, Qin MJ (2009) A new dicoumarin and anticoagulant activity from Viola yedoensis Makino. Fitoterapia 80(5):283–285
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed F. Abdelhamid: material preparation, data collection, and analysis; Ahmed G. A. Gewida: material preparation, data collection, and analysis; Abdel-Fattah M. El-Sayed: supervision, reviewing, and editing; Mohamed F. Badran: conceptualization, methodology, and writing.
Corresponding author
Ethics declarations
Ethical approval
The Committee of Scientific Research Ethics, Faculty of Agriculture granted ethical approval in compliance with Suez Canal University norms for the ethics of scientific research (Ref. No.: 65/2022).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhamid, A.F., Gewida, A.G.A., El-Sayed, AF.M. et al. Impacts of different levels of vitamin K on the growth performance, hematological parameters, and immunological response of juvenile Nile tilapia (Oreochromis niloticus). Aquacult Int 32, 477–488 (2024). https://doi.org/10.1007/s10499-023-01169-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01169-6