Abstract
This study investigated the impacts of water application of a commercial Bacillus species probiotics, SANOLIFE®PRO-W (containing Bacillus subtilis and B. licheniformis), on water quality, digestive enzymes, growth performance, antioxidants, innate immunity, body composition, and resistance of whiteleg shrimp against Fusarium solani infection. A total of 240 animals (2.00 ± 0.07 g) were stocked into twelve 1-m3 concrete ponds and divided into four triplicate treatments. For 56 days, the rearing water was supplemented with Bacillus species probiotic at 0, 0.01, 0.02, and 0.03 g/m3 and defined as control, T1, T2, and T3 groups, respectively. Results revealed that Bacillus species probiotics increased dissolved oxygen and decreased total ammonia, nitrite, and unionized ammonia in the rearing ponds. However, adding probiotic Bacillus to rearing water significantly increased the digestive enzyme (chymotrypsin, trypsin, protease, lipase, and amylase) and growth performance parameters compared to the control. On the other hand, the antioxidant levels of superoxide dismutase, catalase, and glutathione peroxidase were increased significantly, while the malondialdehyde concentrations were decreased significantly compared to the control. Furthermore, Bacillus probiotics boosted the innate immunity of shrimp manifested by increased activities of lysozyme, prophenoloxidase, respiratory burst, and the total hemocyte count compared with the control treatment. Of interest, shrimp mortalities decreased after F. solani infection to 35% in the T3 group compared to 100% in the control group. In conclusion, water application of Bacillus species probiotics, especially at 0.02 and 0.03 g/m3, improved the water quality, welfare, and resistance of whiteleg shrimp to F. solani infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The whiteleg shrimp, Litopenaeus vannamei, is among the commercially valuable penaeid shrimps cultured in several countries worldwide (FAO 2020; Islam et al. 2020). Nevertheless, owing to intensive cultivation and climate shifts (Byers 2021; Kibenge 2019), L. vannamei farming has been affected by several pathogens, which have devastated the business of the shrimp aquaculture industry (Khoa et al. 2004; Kibenge 2019; Zhang et al. 2014; Zou et al. 2020). Black spot disease (BSD) is among the diseases affecting shrimp production as it claimed numerous lives, particularly in the late rearing stages (Pazir et al. 2022). This disease was first reported in the kuruma prawn, Marsupenaeus japonicus, by Ishikawa (1968) and was caused by a fungal infection. Hatai and Egusa (1978) identified the causal agent of BSD as Fusarium solani, which was then regarded as one of the most severe infections of M. japonicus in Japan (Khoa et al. 2005; Yao et al. 2022). Fusarium species are ubiquitous fungal agents affecting plants, soil, freshwater, and brackish water (Lightner 1996; Palmero et al. 2009). These fungi may spread to various plants globally, causing agricultural losses (Figueroa et al. 2018; Moretti et al. 2017; Summerell 2019).
Probiotics are solo or mixed cultures of microbial populations that, when supplied in sufficient quantities, may promote the development and health of the host (El-Saadony et al. 2021; Lara-Flores et al. 2010; Salminen et al. 1999; Yilmaz et al. 2022). Due to their unique advantages and health benefits, probiotics have been marketed and sold as an immediate water supplement and feed additive (Jahangiri and Esteban 2018; LaPatra et al. 2014). Applying probiotics directly to water is an effective method for improving water quality, but applications as feed supplements have also given positive outcomes (Jahangiri and Esteban 2018). Probiotics positively influenced the water quality parameters such as dissolved oxygen, hardness, pH, temperature, and osmotic pressure (Cha et al. 2013; Das et al. 2008).
Bacillus species are commonly used as effective probiotic supplements in aquaculture (Abdel-Tawwab et al. 2022; Liu et al. 2012). Bacillus can produce several extracellular enzymes and withstand extreme temperatures and dehydration (Yu et al. 2009). These probiotics have been extensively explored recently and may contribute greatly to aquatic organisms’ physiological, morphological, hematological, and immunological conditions when administered at optimal levels in the rearing water (Rahman et al. 2021).
The primary target of this research was to examine the influence of applying three water supplementation levels of Bacillus species probiotic (SANOLIFE®PRO-W) on the water quality, growth performance, innate immunity, antioxidant activities, and disease resistance of whiteleg shrimp to F. solani infection.
Materials and methods
Animals and experimental design
Apparent healthy 240 shrimps with an average initial body weight of 2.0 ± 0.07 g/shrimp were acquired from a special farm in Ismailia Governorate, Egypt. Shrimps were acclimatized for 14 days in an outdoor 2000-L fiberglass tank and were fed on a commercial diet of Skretting Company, Belbeis, Sharkia, Egypt. Nutritional ingredients of this diet composed of fishmeal (72% crude protein), soybean meal (48% crude protein), corn gluten (63% crude protein), wheat bran, yellow corn, soybean oil, starch, vitamin, and mineral premix. The proximate chemical composition for the diet was 89.59% dry matter, 40% crude protein, 8.5% ether extract, 3.34% fiber, 6.52% ash, 41.56% NFE, and 18.94 MJ/kg growth energy. Afterward, shrimps were randomly stocked at twelve 1-m3 concrete ponds (20 individuals per each one) in a special farm in Ismailia Governorate, Egypt, to represent four triplicate experimental treatments. Bacillus species probiotics, which are commercially sold as SANOLIFE®PRO-W (a mixture of Bacillus subtilis and B. licheniformis at 5 × 105 CFU/g; INVE Aquaculture, Belgium), were added to the rearing water at levels of 0, 0.01, 0.02, and 0.03 g/m3, representing T0 (control), T1, T2, and T3 respectively. Five liters of clean water was mixed with those different levels of the probiotic, and water (5 L) was sprinkled over the pond’s surface biweekly for 56 days. Water was changed daily at a rate of 5% for each pond. Shrimps were fed on the commercial diet at 7% in the first 28 days and 6% in the next 28 days of the shrimp biomass. The given feed was divided equally into three portions and offered to animals three times a day (8.00, 12.00, and 16.00 h). The total bacterial count in SANOLIFE®PRO-W was determined using the standard pour plate method (APHA 1998).
Water quality measurements
Water quality indices were determined daily at 9:00 h below 30 cm of the ponds’ surface. The water temperature (T; °C) and dissolved oxygen (DO) were determined by an oxygen meter (970 portable DO meter, Jenway, London, UK). A pH meter determined the pH (Digital Mini-pH Meter, model 55, Fisher Scientific, Denver, CO, USA). The salinity was assessed by a refractometer (Erma, Japan). At the same time, the unionized ammonia (NH3) was calculated utilizing the HACH comparison device according to Boyd (1982), and total ammonia nitrogen (TAN) of the pond water was determined by an ammonia test kit (Advance Pharma, Thailand).
Growth efficiency of shrimp
The growth performance was evaluated biweekly via sampling and weighing ten animals from each pond. The body weight gain (WG), daily weight gain (DWG), specific growth rate (SGR), and survival % were calculated using the following equations:
Proximate chemical analysis of body
When the experiment was ended, five shrimps from each pond were weighed and dried in a furnace at 105 °C for 180 min, and then crushed and tested according to AOAC (1995) for moisture, crude protein, crude fat, and ash.
Digestive enzyme activities
Hepatopancreases from shrimps were weighed, and 1.0 g was blended in 1.5 mL of distilled water. Before being examined, these samples were stored in 1-mL Eppendorf tubes at − 20 °C. Supernatants were obtained after centrifugation at 13,000 × g for 5 min at 4 °C. Triplicate tests were performed after diluting the homogenate with the appropriate buffers. Enzyme activity units were measured in milligrams of soluble protein (U mg−1).
Trypsin and chymotrypsin activities were measured kinetically (Geiger 1984; Tseng et al. 1982) using N-α-benzoyl-dl-arginine p-nitroanilide (BAPNA, Sigma, B4875) and N-succinyl-ala-ala-pro-phe (SAPPNA, Sigma, S7388) as substrates. A spectrophotometer recorded both reactions at 407 and 405 nm every 0.9 s for 3 min. Reaction and incubation temperatures were 25 °C. Trypsin and chymotrypsin activities produced 1 μmol of 4-nitroaniline per minute per milligram of protein. Calculations used an ε405 = 10.2 L mmol−1 cm−1 extinction coefficient (Geiger 1984; Geiger and Fritz 1981).
Protease enzyme activities were determined via azocasein (Sigma A2765, Sigma Chemical, St. Louis, USA) as a substrate, as Svåsand et al. (2007) described. Lipase enzyme activity was determined following the method of Versaw et al. (1989) using β-naphthyl caprylate (Sigma, N8875). A 0.001 U/min rise in absorbance at 440 nm and 540 nm was utilized to quantify protease and lipase activity.
Using the method described by Rick and Stegbauer (1974), alpha-amylase activity was measured. Maltose generated a calibration curve (Kanto Chemical, Tokyo, Japan). At 550 nm, a spectrophotometer measured the concentration of the samples and the standards used to create the calibration curve (Jenway, Essex, UK). Amylase value was determined as mmol of maltose emitted per minute per µg of protein.
The innate immunity induces
Hemolymph was collected from 10 animals from each treatment. Hemolymph was extracted from the cardio-coelom near the hindmost side of the carapace by a 1-mL medically disinfected needle and syringe. Anticoagulant (0.34 M NaCl, 0.01 M KCl, 0.01 M EDTA-Na2, and 0.01 M HEPES, pH 7.45 and 780 mOsm. kg−1) was included in the syringe (Vargas-Albores et al. 1993). Hemolymph was collected using a 1:1 anticoagulant ratio. After pipetting 1 mL of hemolymph into an Eppendorf tube and centrifugation at 3000 rpm for 15 min at 4 °C, the blue supernatant was packed into fresh tubes as a plasma sample at a temperature of 80 °C.
A total hemocyte count (THC) was determined by a hemocytometer and examined under a stereomicroscope (Supamattaya et al. 2000). The plasma lysozyme content was evaluated by lysis of the Gram-positive bacteria Micrococcus luteus solution (M3770-25G Lot 117K8707, Sigma-Aldrich), following Zanuzzo et al. (2017).
The respiratory burst (RB) activity was determined in a portion of the whole hemolymph with pre-cooling anticoagulant by the method described by Anderson and Siwicki (1995). Prophenoloxidase (PO) activity was assessed spectrophotometrically by measuring l-DOPA-derived dopachrome production (Liu et al. 2004). Phagocytosis (PP) and the phagocytic index were measured using the procedures outlined by Rengpipat et al. (2000).
Antioxidant activity and lipid peroxidation
Enzymatic antioxidant activities, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzyme activities, were evaluated in the hepatopancreatic samples. CAT activity was measured using the auto-oxidation of pyrogallol described by Marklund and Marklund (1974). The CAT activity was detected after the H2O2 reduction at 240 nm, as Claiborne (1985) described. The GPx activity was measured by tracking the rates of NADPH oxidation by the two-step reaction with glutathione reductase at 340 nm utilizing an extinction coefficient of 6.22 mM−1 cm−1, following Gunzler and Flohe (1985). The units of enzyme activity were reported as specific activities (IU/mg protein). We employed the technique mentioned by Draper and Hadley (1990) for generating TBARS through an acid-heating reaction to measure lipid peroxidation using malondialdehyde. MDA equivalents are shown as nmol/mg protein.
Resistance to F. solani infection.
Preparation of the fungal strain
F. solani was obtained from the Microbiological Unit, Fish Diseases Department, Animal Health Institute, Agricultural Research Center, Dokki, Giza, Egypt. F. solani was cultured on potato dextrose agar (PDA) for 7 days at 25 °C. Then, 20 mL of sterilized distilled water was inserted into each culture plate to collect the conidial material in 30-mL tubes. After that, the samples were filtered to confirm fungal conidia. Finally, enumeration was performed to evaluate the count of the conidia and then optimized to 5 × 104/mL in sterilized distilled water.
Experimental infection procedures
Treated shrimps were obtained and grouped at a rate of 10 individuals per 50-L tank; three replicates represented each treatment. Shrimps were artificially infected in the mid-lateral part of the carapace or the abdominal tergal and pleural plates, carapace, and gills, as described by Hose et al. (1984). Shrimp wounding was performed by removal of the trailing edge of the carapace or the abdominal tergal and pleural plates, carapace, and gills. A cotton swab dipped in a thick solution of F. solani conidiospores (5 × 104 conidia/mL; obtained from a 7-day-old Sabouraud dextrose agar culture) was used to swab wounds quickly. Before returning the shrimp to their aquariums, the leaking hemolymph was permitted to clot and capture the spores. In addition, a cotton swab infected with F. solani was spun (under mild pressure) against the gills. The control treatment was not infected but injured like the other groups. Over 14 days, animals were given the same corresponding diets used from starting the experiment. Abnormal behaviors and shrimp mortalities were detected. Salinity, dissolved oxygen, and water temperature were preserved throughout the challenge tests under the same conditions as the feeding experiment. Every 2 days, leftover feed and waste were removed.
Statistical analysis
Shapiro–Wilk and Bartlett’s tests verified data normality and variance homogeneity before statistical analysis. Each parameter’s mean and SEM were determined. After a one-way analysis of variance (ANOVA) by IPM SPSS (Version 26), Duncan’s test was applied to compare means at P < 0.05 (Dytham 2011).
Results
Water quality parameters
All water quality indices were suitable for shrimp culture (Table 1). Total ammonia nitrogen (TAN) and unionized ammonia were affected by Bacillus species probiotics as their values decreased by increasing the probiotic levels. The control treatment had a high level of TAN and ammonia, while its lowest levels were observed at T2 and T3, which received 0.02 and 0.03 g/m3 of Bacillus species probiotics. On the other hand, the DO levels were significantly increased with probiotic addition, while pH was reduced. In contrast, the probiotic addition to water did not significantly affect the water temperature and salinity levels (P > 0.05).
Digestive enzyme activities
The hepatopancreatic digestive enzymes of shrimp were increased significantly by applying probiotics in the rearing water (P < 0.05; Table 2). The chymotrypsin, trypsin, proteases, lipase, and alpha-amylase enzyme activities were improved significantly with increasing probiotics levels than the control group. There was no substantial variance among T2 and T3 for all digestive enzymes except for chymotrypsin.
Growth performance and feed utilization
When compared with the control group, the result showed that water application of the probiotics significantly enhanced (P < 0.05) the growth performance and the weight of shrimp (Table 3). Final weight, weight gain, weight gain %, ADG, SGR, and feed intake significantly increased with water application, without differences between T2 and T3 (P > 0.05). Conversely, FCR and survival rate were significantly enhanced in probiotic groups (P < 0.05) than the control, without any difference between T2 and T3.
Whole-body chemical composition
Compared with the control group, the total protein and ash contents of the probiotic-treated shrimp were improved. However, the lipid content was significantly reduced (Table 4). There was no substantial variance in moisture content among the different treatments (P > 0.05).
Antioxidant status and lipid peroxidation
The activities of SOD, CAT, and GPx enzymes were significantly improved by adding probiotic Bacillus to water than the control treatment (P < 0.05; Table 5). Conversely, the MDA levels were decreased with increasing probiotic Bacillus levels in pond water than the control treatment (P < 0.05).
Innate immunity status
All immunological parameters significantly improved by increasing the probiotic Bacillus than the control treatment (P < 0.05; Table 6). THC, PO activity, RB activity, lysozyme activity, and phagocytic index were significantly increased in shrimps reared in ponds with probiotic Bacillus than in the control group, particularly at T3 and T4.
Resistance to F. solani infection
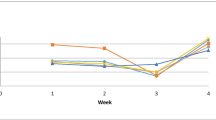
Shrimps reared in water containing Bacillus species probiotics were more resistant to F. solani infection than the control group, which displayed the highest mortality rates. When the probiotic doses increased in the rearing water, fatality rates dropped for each treatment (Fig. 1). The survival rate of shrimp in the T3 group was the highest (75%); however, it was 0% for the control group without probiotic addition (Fig. 2).
Discussion
In the present study, the water quality parameters were within the levels approved for shrimp culture (Boyd and Tucker 1998). The water application of Bacillus species probiotics to pond water lowered TAN and NH3 levels. This is owing to the presence of the Bacillus mixture, which plays essential functions in the nitrogen cycle via ammonification (Hui et al. 2019), nitrification (Rout et al. 2017), and denitrification (Verbaendert et al. 2011) as well as nitrogen fixation (Yousuf et al. 2017). Hence, Bacillus sp. can eliminate the various kinds of nitrogen from aquaculture wastewater. B. amyloliquefaciens DT, for instance, transformed organic nitrogen to ammonium (Hui et al. 2019), while B. cereus PB8 eliminated \({\mathrm{NO}}_{-2}-\mathrm N\) from wastewater (Barman et al. 2018).
The most crucial water quality indicator for aquaculture activities is dissolved oxygen (DO) since the aquatic environment is rich with ammonia, phosphorus, organic waste, copper, and other elements that could reduce the DO levels (dos Santos Simoes et al. 2008). In the current study, DO increased markedly with the addition of Bacillus species probiotics in a dose-dependent manner compared to the control, and this is because Bacillus could optimize the decomposition of organic matter load (Hai 2015), thereby recycling nutrients in the water column and reducing sludge accumulation (Soltani et al. 2019). Furthermore, the decomposition of organic matter increases DO (Boyd and Gross 1998; Cha et al. 2013). Our study agrees with previous results which found that Bacillus sp. improved DO levels in rearing water, such as in the case of B. megaterium (Hura et al. 2018), a combination of Bacillus (Hainfellner et al. 2018), Bacillus species mixture (composed of B. subtilis, B. licheniformis, B. megaterium, and B. laterosporus) (Gomes et al. 2008; Zink et al. 2011).
In the present study, it was noticed that the Bacillus mixture decreased pH values compared with the control group, based on the water application dose. These findings might be explained by the fact that Bacillus efficiently transforms organic matter to CO2, which is then used as a carbon source by β- and γ-proteobacteria (Koops and Pommerening-Röser 2001), which convert most of the organic matter into slime or bacterial biomass (Mohapatra et al. 2013; Zorriehzahra et al. 2016). Moreover, CO2 is known to lower pH levels. The basic character of rearing waters is preferable to acidic waters, as Hura et al. (2018) found positive properties in carp culture due to the conservation of alkalinity by B. megaterium. In Bacillus-treated tilapia ponds, a rise in pH was also detected in the study performed by Elsabagh et al. (2018). In acidic circumstances, Bacillus may raise the pH of the water, making it ideal for fish production. Contrary to what was reported for pH, some reports showed that probiotic Bacillus lowered pH toward neutral (Gomes et al. 2008; Nimrat et al. 2012; Wu et al. 2016).
Herein, the Bacillus mixture did not affect the temperature, confirming prior studies that found no substantial temperature regulation by Bacillus sp. (Banerjee et al. 2010; Ghosh et al. 2008; Nimrat et al. 2012). As Velmurugan and Rajagopal (2009) noted, as a conservative measure, the temperature is unaffected by biological activities.
In the present investigation, the Bacillus sp. combination did not affect the water salinity levels. Aftabuddin et al. (2013) concluded that combining B. megaterium and Streptomyces fradiae did not noticeably impact water salinities. Furthermore, Velmurugan and Rajagopal (2009) found that biological processes do not readily influence salinity since it is a conservative water quality indicator. These authors further demonstrated that Bacillus is incapable of modulating salinity.
There is a strong relationship between the kinds and amounts of nutrients in feed and the number and activity of digestive enzymes in aquatic animals. Herein, it was observed that water application of Bacillus enhanced digestive enzyme activities compared to the control group. Likewise, it was reported that B. licheniformis could boost the nutritional digestibility of aquatic animals by increasing enzymatic production, such as amylase, protease, and cellulase. Ziaei-Nejad et al. (2006) found that probiotic Bacillus strains increased such enzymes in Indian shrimp. Here, the increase of protease, amylase, and lipase enzymes may be the primary factor that led to improved growth performance. It seems that B. licheniformis could enhance the absorption, digestion, and availability of certain nutrients (Yaqub et al. 2022).
Enhancing the functioning of digestive enzymes may improve digestion and absorption of food, hence enhancing growth performance and feed efficiency (Xie et al. 2019). The current research findings indicate that adding a probiotic Bacillus mixture to rearing water increased the growth rate of shrimp. This may be due to their positive growth-promoting role (Yanbo and Zirong 2006). Chen et al. (2020) found that B. licheniformis considerably enhanced the weight gain and specific growth rates of prawns. Moreover, it was also found that Bacillus improved the growth performance of L. vannamei (Cao et al. 2022) and Indian shrimp (Ziaei-Nejad et al. 2006). On the other hand, Kewcharoen and Srisapoome (2019) found that the probiotic B. subtilis AQAHBS001 could enhance the histomorphology of the midgut of white shrimp, increasing intestinal villi height/width, which increased nutrient uptake and shrimp development. Verschuere et al. (2000) and Zokaeifar et al. (2012) demonstrated that the Bacillus secretes a vast array of enzymes that help in the nutritional improvement of the host, hence promoting growth. The improved feed digestion may be linked to higher digestive enzyme activity (Zokaeifar et al. 2012).
The present research showed that probiotic Bacillus–added water increases crude protein and ash of shrimp compared to the control. These findings were consistent with those reported by Cao et al. (2022), who found that applying a particular concentration of probiotics may increase the crude muscle protein content of whiteleg shrimp compared to the control group. Seenivasan et al. (2014) declared that oral administration of a combination of Lactobacillus sporogenes, B. subtilis, and Saccharomyces cerevisiae enhanced the crude protein and ash contents of chicken carcasses. In contrast to our findings, Yu et al. (2009) and Heizhao et al. (2008) confirmed that dietary probiotics did not affect the body composition of whiteleg shrimp.
The oxidative stress–related enzymes (SOD, CAT, and GPx) and lipid peroxidation (represented by MDA) are bioindicators of oxidative cell injury, which are implicated in pathological processes and the etiology of numerous fish diseases (Abdel-Tawwab and Wafeek 2017; Hermes-Lima 2004; Kehrer 1993; Storey 1996). In the current investigation, probiotic application enhanced the antioxidative properties, as evidenced by increased SOD, CAT, and GPx and decreased MDA levels in shrimp hepatopancreas. Such findings might be associated with the elevated Bacillus sp. counts in the water of the rearing ponds. Shen et al. (2010) concluded that probiotics added to water improved the antioxidant enzyme activity of L. vannamei (Shen et al. 2010). In comparison with the control group, Amoah et al. (2019) observed a substantial increase in SOD (serum) and GPx (serum and liver) activities in whiteleg shrimp treated with B. coagulans ATCC 7050. Such an antioxidant impact has been found in investigations on different commercial probiotics (Abdel-Tawwab et al. 2020; Župan et al. 2015).
In shrimp, lysozyme and polyphenol oxidase (PO) activities are essential immunological enzymes contributing to innate immune responses (Magnadóttir 2006; Whyte 2007). In the present investigation, lysozyme and PO activities were increased in shrimps reared in ponds treated with probiotics than in the control group. These results may be attributable to the B. subtilis probiotic in water, which helps to enhance the innate immunity of L. vannamei (Amoah et al. 2019; Wongsasak et al. 2015; Zokaeifar et al. 2012). Moreover, Kewcharoen and Srisapoome (2019) observed that probiotic B. subtilis AQAHBS001 induced lysozyme activity in L. vannamei.
In the current research, the probiotic administered to shrimp ponds increased phagocytosis, THC, and respiratory burst activities. Phagocytes are responsible for attacking foreign pathogens, activating T cells (Parham 2014), and producing antibody signals linked to innate immune system activation. Shrimps have three kinds of hemocyte cells: hemocytes, hyalinocytes, granulocytes, and semi-granulocytes (Martin and Graves 1985). Hemocytes are protective cells in the hemolymph, shared defense systems versus foreign particles. Hemocyte cells function in phagocytosis, encapsulation, nodule formation, wound healing, and coagulation (Aguirre-Guzman et al. 2015; Martínez 2007). These hemocytes are concerned with phagocytosis and engulfing pathogens and foreign substances. During phagocytosis, a molecular mechanism recognized as respiratory leukocyte burst increases oxygen uptake, producing oxygen reduction and superoxide anion (Biller-Takahashi and Urbinati 2014). The present investigation showed a substantial improvement in immunological markers compared to the control group, suggesting that a probiotic mixture may positively affect immunity in L. vannamei hemolymph.
Probiotics are crucial in developing innate immunity in aquatic species, enabling them to combat pathogenic microorganisms and environmental stressors (Abdel-Latif et al. 2022; Abdel-Tawwab et al. 2022; El-Saadony et al. 2021; Rahman et al. 2021; Yilmaz et al. 2022). In the current investigation, F. solani infection resulted in significant mortality rates in the control group, but animals raised in ponds supplemented with probiotics had decreased mortality rates. B subtilis and B. licheniformis regulated the immune response when added to the water as probiotics, resulting in enhanced resistance to fungal infection. Hence, probiotic bacteria may compete with other invading microorganisms, reducing susceptibility and mortality rates (Yaqub et al. 2022). Similar findings were previously reported by Balcázar et al. (2006), who demonstrated the efficacy of dietary B. subtilis UTM 126 in preventing vibriosis in white shrimp. Furthermore, Zokaeifar et al. (2012) demonstrated that B. subtilis supplementation decreased mortalities following experimental challenge with Vibrio harveyi.
Conclusion
In summary, the current research indicated that water application of Bacillus species probiotic composed of B. subtilis and B. licheniformis had a positive influence on growth performance, chemical composition, digestive enzymes, antioxidant status, immunological indices, and disease resistance in whiteleg shrimp, with the greatest results achieved in T2 and T3, which received 0.02 and 0.03 g/m3 respectively.
Data availability
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Abdel-Tawwab M, Adeshina I, Issa ZA (2020) Antioxidants and immune responses, resistance to Aspergilus flavus infection, and growth performance of Nile tilapia, Oreochromis niloticus, fed diets supplemented with yeast, Saccharomyces Serevisiae. Anim Feed Sci Technol 263:114484
Abdel-Tawwab M, Khalil RH, Nour AM, Elkhayat BK, Khalifa E, Abdel-Latif HMR (2022) Effects of Bacillus subtilis-fermented rice bran on water quality, performance, antioxidants/oxidants, and immunity biomarkers of white leg shrimp (Litopenaeus vannamei) reared at different salinities with zero water exchange. J Appl Aquac 34(2):332–357. https://doi.org/10.1080/10454438.2020.1844110
Abdel-Tawwab M, Wafeek M (2017) Fluctuations in water temperature affected waterborne cadmium toxicity: hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 477:106–111. https://doi.org/10.1016/j.aquaculture.2017.05.007
Abdel-Latif HMR, Yilmaz E, Dawood MAO, Ringø E, Ahmadifar E, Yilmaz S (2022) Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: a review. Aquaculture 737951. https://doi.org/10.1016/j.aquaculture.2022.737951
Aftabuddin S, Kashem MA, Kader MA, Sikder MNA, Hakim MA (2013) Use of Streptomyces fradiae and Bacillus megaterium as probiotics in the experimental culture of tiger shrimp Penaeus monodon (Crustacea, Penaeidae). Aquac. Aquarium. Conserv Legis 6:253–267
Aguirre-Guzman G, Sanchez-Martinez JG, Campa-Cordova AI, Luna-Gonzalez A, Ascencio F (2015) Penaeid shrimp immune system. Thai J Vet Med 39:205–215
Amoah K, Huang Q-C, Tan B-P, Zhang S, Chi S-Y, Yang Q-H, Liu H-Y, Dong X-H (2019) Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus Vannamei. Fish Shellfish Immunol 87:796–808. https://doi.org/10.1016/j.fsi.2019.02.029
Anderson DP, Siwicki AK (1995) Basic hematology and serology for fish health programs. In: Symposium; 2nd, Diseases in Asian aquaculture. Fish Health Section, Asian Fisheries Society, Phuket; Thailand, pp 185–202
AOAC (1995) Association of official methods of analytical chemist. Official Method Analysis, 16th edn. Arlington, VA
APHA (1998) Standard methods for the examination of the water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Balcázar JL, De Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114:173–186
Banerjee S, Khatoon H, Shariff M, Yusoff FM (2010) Enhancement of Penaeus monodon shrimp postlarvae growth and survival without water exchange using marine Bacillus pumilus and periphytic microalgae. Fish Sci 76:481–487. https://doi.org/10.1007/s12562-010-0230-x
Barman P, Bandyopadhyay P, Kati A, Paul T, Mandal AK, Mondal KC, Mohapatra PKD (2018) Characterization and strain improvement of aerobic denitrifying EPS producing bacterium Bacillus cereus PB88 for shrimp water quality management. Waste Biomass Valorization 9:1319–1330. https://doi.org/10.1007/s12649-017-9912-2
Biller-Takahashi JD, Urbinati EC (2014) Fish immunology. The modification and manipulation of the innate immune system: Brazilian studies. An Acad Bras Cienc 86:1484–1506
Boyd CE (1982) Water quality management for pond fish culture, 1st edn. Elsevier Scientific Publishing Co., Amsterdam, Netherlands
Boyd CE, Gross A (1998) Use of probiotics for improving soil and water quality in aquaculture ponds. In: Flegel TW (ed) Advances in shrimp biotechnology. Citeseer, Chiengmai, Thailand, pp 101–105
Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Springer Sci Busi Media. https://doi.org/10.1007/978-1-4615-5407-3
Byers JE (2021) Marine parasites and disease in the era of global climate change. Ann Rev Mar Sci 13:397–420
Cao H, Chen D, Guo L, Jv R, Xin Y, Mo W, Wang C, Li P, Wang H (2022) Effects of Bacillus subtilis on growth performance and intestinal flora of Penaeus vannamei. Aquac Reports 23:101070. https://doi.org/10.1016/j.aqrep.2022.101070
Cha J-H, Rahimnejad S, Yang S-Y, Kim K-W, Lee K-J (2013) Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 402–403:50–57. https://doi.org/10.1016/j.aquaculture.2013.03.030
Chen M, Chen X-Q, Tian L-X, Liu Y-J, Niu J (2020) Beneficial impacts on growth, intestinal health, immune responses and ammonia resistance of pacific white shrimp (Litopenaeus vannamei) fed dietary synbiotic (mannan oligosaccharide and Bacillus licheniformis). Aquac Reports 17:100408
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Das S, Ward LR, Burke C (2008) Prospects of using marine actinobacteria as probiotics in aquaculture. Appl Microbiol Biotechnol 81:419–429. https://doi.org/10.1007/s00253-008-1731-8
dos Santos Simoes F, Moreira AB, Bisinoti MC, Gimenez SMN, Yabe MJS (2008) Water quality index as a simple indicator of aquaculture effects on aquatic bodies. Ecol Indic 8:476–484
Draper HH, Hadley M (1990) [43] Malondialdehyde determination as index of lipid Peroxidation. In: Methods in Enzymology. Elsevier, pp. 421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Dytham C (2011) Choosing and using statistics: a biologist’s guide. John Wiley & Sons
Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MAO, Eltholth M (2018) Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac Nutr 24:1613–1622. https://doi.org/10.1111/anu.12797
El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MA, Dhama K, Abdel-Latif HM (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52. https://doi.org/10.1016/j.fsi.2021.07.007
FAO (2020) Fishery and Aquaculture Statistics 2018. FAO Rome, Italy. https://doi.org/10.4060/cb1213t
Figueroa M, Hammond-Kosack KE, Solomon PS (2018) A review of wheat diseases—a field perspective. Mol Plant Pathol 19:1523–1536. https://doi.org/10.1111/mpp.12618
Geiger R (1984) Chymotrypsin, pp 99–109. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol V, enzymes 3: peptidases, proteinases and their inhibitors, 3rd edn. Verlag Chemie, Weinheim – Deerfield Beach, Florida – Basel, pp 598
Geiger R, Fritz H (1981) [39] Human urinary kallikrein. Methods Enzymol 80:466–492
Ghosh S, Sinha A, Sahu C (2008) Bioaugmentation in the growth and water quality of livebearing ornamental fishes. Aquac Int 16:393–403. https://doi.org/10.1007/s10499-007-9152-8
Gomes LC, Brinn RP, Marcon JL, Dantas LA, Brandão FR, Sampaio de Abreu J, McComb DM, Baldisserotto B (2008) Using Efinol®L during transportation of marbled hatchet fish, Carnegiella strigata (Günther). Aquac Res 39:1292–1298. https://doi.org/10.1111/j.1365-2109.2008.01993.x
Gunzler W, Flohe L (1985) Handbook methods for oxygen radical research (1st ed.) CRC Press. https://doi.org/10.1201/9781351072922
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119:917–935. https://doi.org/10.1111/jam.12886
Hainfellner P, Cardozo MV, Borzi MM, Almeida CC, José L, Pizauro L, Schocken-Iturrino RP, Costa GN, de Ávila FA (2018) Commercial probiotic increases survival rate and water quality in aquariums with high density of Nile tilapia larvae (Oreochromis niloticus). Int J Probiotics & Prebiotics 13(4):193–142
Hatai K, Egusa S (1978) Studies on the pathogenic fungus associated with black gill disease of kuruma prawn, Penaeus Japonicus-II. Fish Pathol 12:225–231. https://doi.org/10.3147/jsfp.12.225
Heizhao LIN, Zhuojia LI, Zhixun GUO, Juan F, Guoliang WEN, Xian D (2008) Effects of dietary probiotics on growth and biochemical composition of whole body of juvenile shrimp, Litopenaeus Vannamei. South China Fish Sci 4(6):95–100
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. Funct Metab Regul Adapt 1:319–366
Hose JE, Lightner DV, Redman RM, Danald DA (1984) Observations on the pathogenesis of the imperfect fungus, Fusarium solani, in the California brown shrimp, Penaeus californiensis. J Invertebr Pathol 44:292–303
Hui C, Wei R, Jiang H, Zhao Y, Xu L (2019) Characterization of the ammonification, the relevant protease production and activity in a high-efficiency ammonifier Bacillus amyloliquefaciens DT. Int Biodeterior Biodegradation 142:11–17. https://doi.org/10.1016/j.ibiod.2019.04.009
Hura MUD, Zafar T, Borana K, Prasad JR, Iqbal J (2018) Effect of commercial probiotic Bacillus megaterium on water quality in composite culture of major carps. Int J Curr Agric Sci 8:268–273
Ishikawa Y (1968) Observations on the gill blackening of cultured kuruma-prawn, Penaeus japonicus BATE, parasitized by a fungus. Fish Pathol 3:34–38. https://doi.org/10.3147/jsfp.3.34
Islam T, Hossain MI, Alam MM, Khalil SMI, Rahman MM, Abdullah-Al-Mamun M (2020) Status of shrimp diseases and their management practices at Satkhira in Bangladesh. J Entomol Zool Stud 8:1017–1026
Jahangiri L, Esteban MÁ (2018) Administration of probiotics in the water in finfish aquaculture systems: a review. Fishes 3(3):33. https://doi.org/10.3390/fishes3030033
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48. https://doi.org/10.3109/10408449309104073
Kewcharoen W, Srisapoome P (2019) Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol 94:175–189. https://doi.org/10.1016/j.fsi.2019.09.013
Khoa LV, Hatai K, Aoki T (2004) Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J Fish Dis 27:507–515. https://doi.org/10.1111/j.1365-2761.2004.00562.x
Khoa LV, Hatai K, Yuasa A, Sawada K (2005) Morphology and molecular phylogeny of Fusarium solani isolated from kuruma prawn Penaeus japonicus with black gills. Fish Pathol 40:103–109. https://doi.org/10.3147/jsfp.40.103
Kibenge FSB (2019) Emerging viruses in aquaculture. Curr Opin Virol 34:97–103. https://doi.org/10.1016/j.coviro.2018.12.008
Koops H-P, Pommerening-Röser A (2001) Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol Ecol 37:1–9. https://doi.org/10.1016/S0168-6496(01)00137-4
LaPatra SE, Fehringer TR, Cain KD (2014) A probiotic Enterobacter sp. provides significant protection against Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) after injection by two different routes. Aquaculture 433:361–366. https://doi.org/10.1016/j.aquaculture.2014.06.022
Lara-Flores M, Olivera-Castillo L, Olvera-Novoa MA (2010) Effect of the inclusion of a bacterial mix (Streptococcus faecium and Lactobacillus acidophilus), and the yeast (Saccharomyces cerevisiae) on growth, feed utilization and intestinal enzymatic activity of Nile tilapia (Oreochromis niloticus). Int J Fish Aquac 2:93–101
Lightner DV (1996) A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp. World Aquaculture Society, Baton Rouge, LA
Liu C-H, Chiu C-H, Wang S-W, Cheng W (2012) Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper. Epinephelus Coioides Fish Shellfish Immunol 33:699–706
Liu C-H, Yeh S-T, Cheng S-Y, Chen J-C (2004) The immune response of the white shrimp Litopenaeus vannamei and its susceptibility to Vibrio infection in relation with the moult cycle. Fish Shellfish Immunol 16:151–161
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151. https://doi.org/10.1016/j.fsi.2004.09.006
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Martin GG, Graves BL (1985) Fine structure and classification of shrimp hemocytes. J Morphol 185:339–348. https://doi.org/10.1002/jmor.1051850306
Martínez FS (2007) The immune system of shrimp. In Boletines Nicovita; Nicovita-ALICORP SAA Technical Service; SAA Technical: Johannesburg, South Africa, pp 1–6
Mohapatra S, Chakraborty T, Kumar V, Deboeck G, Mohanta KN (2013) Aquaculture and stress management: a review of probiotic intervention. J Anim Physiol Anim Nutr (berl). https://doi.org/10.1111/j.1439-0396.2012.01301.x
Moretti A, Logrieco AF, Susca A (2017) Mycotoxins: an underhand food problem BT - mycotoxigenic fungi: methods and protocols. In: Moretti A, Susca A (eds) Springer. New York, NY, New York, pp 3–12. https://doi.org/10.1007/978-1-4939-6707-0_1
Nimrat S, Suksawat S, Boonthai T, Vuthiphandchai V (2012) Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet Microbiol 159:443–450. https://doi.org/10.1016/j.vetmic.2012.04.029
Palmero D, Iglesias C, De Cara M, Lomas T, Santos M, Tello JC (2009) Species of Fusarium isolated from river and sea water of southeastern Spain and pathogenicity on four plant species. Plant Dis 93:377–385. https://doi.org/10.1094/PDIS-93-4-0377
Parham P (2014) The immune system, 4th edn. Garland Science, New York
Pazir MK, Pourmozaffar S, Mena IG, Shengjie R, Ahmadi A, Sharifpour I (2022) Black gill disease in Litopenaeus vannamei made by various agents. Aquaculture and Fisheries (in press)
Rahman A, Shefat SHT, Chowdhury MA (2021) Effects of probiotic Bacillus on growth performance, immune response and disease resistance in aquaculture. Preprints.org 2021:2021030075. https://doi.org/10.20944/preprints202103.0075.v1
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 191:271–288. https://doi.org/10.1016/S0044-8486(00)00440-3
Rick W, Stegbauer HP (1974) α-Amylase measurement of reducing groups. In Methods of enzymatic analysis. Bergmeyer HU (2nd edn, vol 2). Academic Press, pp 885–890
Rout PR, Bhunia P, Dash RR (2017) Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour Technol 244:484–495. https://doi.org/10.1016/j.biortech.2017.07.186
Salminen S, Ouwehand A, Benno Y, Lee YK (1999) Probiotics: how should they be defined? Trends food Sci. Technol 10:107–110
Seenivasan C, Radhakrishnan S, Shanthi R, Muralisankar T, Saravana Bhavan P (2014) Effect of Lactobacillus sporogenes on survival, growth, biochemical constituents and energy utilization of freshwater prawn Macrobrachium rosenbergii post larvae. J Basic Appl Zool 67:19–24. https://doi.org/10.1016/j.jobaz.2013.12.002
Shen W-Y, Fu L-L, Li W-F, Zhu Y-R (2010) Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aquac Res 41:1691–1698. https://doi.org/10.1111/j.1365-2109.2010.02554.x
Soltani M, Ghosh K, Hoseinifar SH, Kumar V, Lymbery AJ, Roy S, Ringø E (2019) Genus Bacillus, promising probiotics in aquaculture: aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev Fish Sci Aquac 27:331–379
Storey KB (1996) Oxidative stress: animal adaptations in nature. Brazilian J Med Biol Res 29:1715–1733
Summerell BA (2019) Resolving Fusarium: current status of the genus. Annu Rev Phytopathol 57:323–339. https://doi.org/10.1146/annurev-phyto-082718-100204
Supamattaya K, Ruangsri J, Kiriratnikom S, Songsrichan N (2000) Immune system in black tiger shrimp, Penaeus monodon, Fabricius: IV. Normal immuno-physiological values in black tiger shrimp, Penaeus monodon, Fabricius. Songklanakarin J Sci Technol 22(Suppl.):597–603
Svåsand T, Crosetti D, García-Vázquez E, Verspoor E (2007) Genimpact- evaluation of genetic impact of aquaculture activities on native populations. A European network (EU contract n. RICA-CT-2005-022802). Final scientific report
Tseng HC, Grendell JH, Rothman SS (1982) Food, duodenal extracts, and enzyme secretion by the pancreas. Am J Physiol Liver Physiol 243:G304–G312
Vargas-Albores F, Guzmán M-A, Ochoa J-L (1993) An anticoagulant solution for haemolymph collection and prophenoloxidase studies of penaeid shrimp (Penaeus californiensis). Comp Biochem Physiol Part A Physiol 106:299–303. https://doi.org/10.1016/0300-9629(93)90516-7
Velmurugan S, Rajagopal S (2009) Beneficial uses of probiotics in mass scale production of marine ornamental fish. African J Microbiol Res 3:185–190
Verbaendert I, Boon N, De Vos P, Heylen K (2011) Denitrification is a common feature among members of the genus Bacillus. Syst Appl Microbiol 34:385–391. https://doi.org/10.1016/j.syapm.2011.02.003
Versaw WK, Cuppett SL, Winters DD, Williams LE (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:1557–1558
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671
Whyte SK (2007) The innate immune response of finfish – a review of current knowledge. Fish Shellfish Immunol 23:1127–1151. https://doi.org/10.1016/j.fsi.2007.06.005
Wongsasak U, Chaijamrus S, Kumkhong S, Boonanuntanasarn S (2015) Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture 436:179–187. https://doi.org/10.1016/j.aquaculture.2014.10.028
Wu DX, Zhao SM, Peng N, Xu CP, Wang J, Liang YX (2016) Effects of a probiotic (Bacillus subtilis FY99-01) on the bacterial community structure and composition of shrimp (Litopenaeus vannamei, Boone) culture water assessed by denaturing gradient gel electrophoresis and high-throughput sequencing. Aquac Res 47:857–869. https://doi.org/10.1111/are.12545
Xie J-J, Liu Q, Liao S, Fang H-H, Yin P, Xie S-W, Tian L-X, Liu Y-J, Niu J (2019) Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus Vannamei. Fish Shellfish Immunol 90:456–465
Yanbo W, Zirong X (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 127:283–292
Yao L, Wang C, Li G, Xie G, Jia Y, Wang W, Liu S, Xu T, Luo K, Zhang Q, Kong J (2022) Identification of Fusarium solani as a causal agent of black spot disease (BSD) of Pacific white shrimp, Penaeus Vannamei. Aquaculture 548:737602
Yaqub A, Awan MN, Kamran M, Majeed I (2022) Evaluation of potential applications of dietary probiotic (Bacillus licheniformis SB3086): effect on growth, digestive enzyme activity, hematological, biochemical, and immune response of Tilapia (Oreochromis mossambicus). Turkish J Fish Aquat Sci 22:TRJFAS19882. https://doi.org/10.4194/TRJFAS19882
Yousuf J, Thajudeen J, Rahiman M, Krishnankutty S, Alikunj AP, Abdulla MHA (2017) Nitrogen fixing potential of various heterotrophic Bacillus strains from a tropical estuary and adjacent coastal regions. J. Basic Microbiol. 57:922–932. https://doi.org/10.1002/jobm.201700072
Yu M-C, Li Z-J, Lin H-Z, Wen G-L, Ma S (2009) Effects of dietary medicinal herbs and Bacillus on survival, growth, body composition, and digestive enzyme activity of the white shrimp Litopenaeus vannamei. Aquac Int 17:377–384
Zanuzzo FS, Sabioni RE, Montoya LNF, Favero G, Urbinati EC (2017) Aloe vera enhances the innate immune response of pacu (Piaractus mesopotamicus) after transport stress and combined heat killed Aeromonas hydrophila infection. Fish Shellfish Immunol 65:198–205. https://doi.org/10.1016/j.fsi.2017.04.013
Zhang Q, Liu Q, Liu S, Yang H, Liu S, Zhu L, Yang B, Jin J, Ding L, Wang X, Liang Y, Wang Q, Huang J (2014) A new nodavirus is associated with covert mortality disease of shrimp. J Gen Virol 95:2700–2709. https://doi.org/10.1099/vir.0.070078-0
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi A-R, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Zink IC, Benetti DD, Douillet PA, Margulies D, Scholey VP (2011) Improvement of water chemistry with Bacillus probiotics inclusion during simulated transport of yellowfin tuna yolk sac larvae. N Am J Aquac 73:42–48. https://doi.org/10.1080/15222055.2011.544622
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus Vannamei. Fish Shellfish Immunol 33:683–689. https://doi.org/10.1016/j.fsi.2012.05.027
Zorriehzahra MJ, Delshad ST, Adel M, Tiwari R, Karthik K, Dhama K, Lazado CC (2016) Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet Quart 36:228–241. https://doi.org/10.1080/01652176.2016.1172132
Zou Y, Xie G, Jia T, Xu T, Wang C, Wan X, Li Y, Luo K, Bian X, Wang X, Kong J, Zhang Q (2020) Determination of the infectious agent of translucent post-larva disease (TPD) in Penaeus vannamei. Pathogens 9(9):741. https://doi.org/10.3390/pathogens9090741
Župan I, Tkalčić S, Šarić T, Čož-Rakovac R, Strunjak-Perović I, Topić-Popović N, Kardum M, Kanski D, Ljubić BB, Matijatko V, Poljičak-Milas N (2015) Supplementation with imuno-2865® in gilthead sea bream (Sparus aurata Linnaeus, 1758): Effects on hematological and antioxidant parameters. Fish Shellfish Immunol 47:590–594. https://doi.org/10.1016/j.fsi.2015.09.049
Yilmaz S, Yilmaz E, Dawood MAO, Ringø E, Ahmadifar E, Abdel-Latif HMR (2022) Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: a review. Aquaculture 547:737514. https://doi.org/10.1016/j.aquaculture.2021.737514
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed N. Monier: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Software. Hoda Kabary: Visualization, Investigation, Microbiology, Software, Validation. Amal Elfeky: Visualization, Investigation, Software, Validation. Saadea Saadony: Visualization, Investigation, Physiology. Nadia N.B. Abd El-Hamed: Visualization, Investigation, Water quality measurements. Moaheda E.H. Eissa: Visualization, Investigation. El-Sayed Hemdan Eissa: Conceptualization, The experimental design, Methodology, Visualization, Investigation.
Corresponding author
Ethics declarations
Ethical approval
All practical steps were carried out according to ARRIVE 2.0 guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) in the Faculty of Agriculture, Suez Canal University (Code: 71/2022).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monier, M.N., Kabary, H., Elfeky, A. et al. The effects of Bacillus species probiotics (Bacillus subtilis and B. licheniformis) on the water quality, immune responses, and resistance of whiteleg shrimp (Litopenaeus vannamei) against Fusarium solani infection. Aquacult Int 31, 3437–3455 (2023). https://doi.org/10.1007/s10499-023-01136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01136-1