Abstract
The present study tested the symbiotic effects of dietary multi-strain Bacillus probiotics (MSB) (Bacillus licheniformis, B. pumilus, and B. subtilis) in Nile tilapia (Oreochromis niloticus) exposed to Aspergillus flavus infection. Furthermore, this study investigated water quality, growth performance, blood metabolites, histological morphology, immune regulatory genes, and resistance to A. flavus infection. For 70 days, fish (n = 240) were divided into four groups in triplicate: T0 (control group; MSB0), T1 (1 g/kg, MSB1), T2 (2 g/kg, MSB2), and T3 (3 g/kg, MSB3). The immune response was then assessed by challenging all fish groups with the A. flavus pathogen. The results showed that the rearing water quality, fish growth, and blood parameters, as well as total proteins, albumin, globulins, and amylase activity were significantly (P < 0.05) increased in all MSB-treated groups with the best results in MSB2 and MSB3 groups. Meanwhile, the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, and glucose levels were significantly (P < 0.05) modulated, particularly at higher concentrations of the probiotic mixture (MSB3 group). Fish fed with various levels of MSB showed a maintained histological structure of the hepatopancreas, intestine, and spleen tissues. The mRNA expression of growth hormone (GH), insulin-like growth factor-1 (IGF-1), insulin-like growth factor receptor-1 (IGF-1R), and interleukin-8 (IL-8) were increased in a dose-dependent manner due to MSB dietary inclusion (P < 0.05). Conversely, the mRNA expression of interleukin-1β (IL-1β) gene was significantly decreased in MSB groups compared to untreated group (P < 0.05). Surprisingly, supplemented groups in Bacillus spp. probiotics exhibited significant modulations in all computed parameters. MSB supplementation improved the pathogenic tolerance of tilapia after change with A. flavus. The integration of growth performance, biochemical, and transcriptomic results confirms that the dietary intervention of multi-strain Bacillus spp. is symbiotic and enhances the benefits for the maintenance of O. niloticus’ health, growth, and digestion. This is achieved by supporting growth genes, reducing inflammatory genes, and enhancing immune-antioxidant resistance to combat A. flavus infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nile tilapia is one of the most widely cultivated species in aquaculture, and the industry is expanding rapidly around the world (Magbanua and Ragaza 2022). With increased fish production, many diseases emerge and cause mortality (Mahboub et al. 2022a, b; Eissa et al. 2024), particularly bacterial diseases (Alzahrani et al. 2022). Recent studies have attempted to use natural antimicrobial products (Ashour et al. 2020; Abdelnour et al. 2020; Mahboub et al. 2022a, b; Rahman et al. 2022; Abd El-Hack et al. 2022). Among these products, probiotics are safe, eco-friendly, live microorganisms that, when supplied in sufficient amounts, improve the health status of the fish (Eissa et al. 2022a, b) by elevating favorable bacteria, augmenting metabolism, and strengthening the immune system against diseases (Ringø et al. 2022). There are numerous benefits to using probiotics in the fish industry, including disrupting pathogens’ stroke by releasing inhibitory materials, improving enzymatic activity, feed utilization and digestibility, and performance (Dawood 2021; Mamun et al. 2019; Monier et al. 2023; Moaheda et al. 2023). Probiotics play an important role in aquaculture as immunostimulants, maintaining gastrointestinal health, stabilizing pathogenic strains, and reducing toxicity (Ahmed et al. 2022; El-Bouhy et al. 2021; Puri et al. 2022; Munir et al., 2016a, b; 2018a, b).
Bacillus spp. is a proven probiotic that has a promising effect on the growth, hematological, and defense mechanisms, intestinal microbiota, biomass gain, and intestinal absorption in several fish species (Aftabgard et al. 2019; Azevedo et al., 2016; Chelladurai et al. 2023; do Veiga et al. 2020). This bacterium could also be the most effective substitute as dietary probiotic for disease control and prevention in animals. Bacillus subtilis, B. licheniformis, B. pumilus, and B. amyloliquefaciens are among the most commonly used Bacillus species as probiotics in aquaculture (Yi et al. 2018). Bacillus supplementation has been associated with increased resistance to V. parahaemolyticus, Edwardsiella tarda (Santos et al. 2021), Aeromona salmonicida, Lactococcus garvieae, Streptococcus iniae (Cha et al. 2013), A. hydrophila (Ramesh and Souissi 2018), and Acinetobacter spp. (Kavitha et al., 2018). Furthermore, improved disease resistance through dietary Bacillus administration has been reported in various aquatic species, such as rainbow trout (Newaj-Fyzul et al. 2007), tilapia (Aly et al. 2008), and white prawns (Tseng et al. 2009). Furthermore, Bacillus subtilis spores work well as an oral vaccination against Streptococcus agalactiae infection in O. niloticus (Yao et al. 2019). As a result, the current study investigated the efficacy of dietary multistrain Bacillus spp. bacteria as antimicrobials against A. flavus infection in O. niloticus. Thus, the growth pattern, digestive enzyme activity, haemato-biochemical parameters, blood metabolites, tissue assessment, and gene expression were all investigated.
Materials and methods
Fish rearing and experimental design
Nile tilapia healthy fingerlings (20.3 ± 0.6 g) were obtained from a private fish farm in Ismailia, Egypt. The farm has a routing health checks in their stock. They were kept in a 3 m3 fiberglass tank for 2 weeks to adjust to indoor laboratory conditions. Following the assimilation period, 240 healthy Nile tilapia (O. niloticus) were used in this experiment. The fish were divided into four groups (20 fish per tank). Each treatment consists of three tanks, each with 60 fish.
A pure lyophilized culture of Bacillus spp. probiotic strains, including B. pumillis, B. licheniformis, and B. subtilis, was obtained from the Microbiological Resource Centre at Ain Shams University in Cairo, Egypt. The colony forming unit (CFU) was determined using the dilution method described by Munir et al. (2016a) and was 108/gm. Multi-strain Bacillus probiotics (MSB) were made with equal amounts of each strain (a combination of Bacillus licheniformis, B. pumillis, and B. subtilis). Probiotics were added to the diets during manufacturing in accordance with the study’s protocol. The fish in the tanks were divided into four groups for treatment. Experimental fish were fed basal diets containing varying levels of MSB: 0 (MSB0), 1 (MSB1), 2 (MSB2), and 3 (MSB3) g/kg. The MSB was well mixed with wheat bran and supplemented to the diets according to the protocol of this study. For 70 days, fish were fed experimental diets three times a day at 9:00, 12:00 and 15:00. with 4-h intervals until apparent satiety was achieved. The diet was offered based on body weight, with the fish being fed 6% of their body weight for the first month and 4% for the remainder of the experiment. Every day, the water in the tanks was changed by 25%, along with the fish feces, and replaced with fresh, well-aerated water from a storage tank. Fish were raised in natural light with no artificial lighting. Table 1 contains information on fish dietary ingredients and chemical analysis.
Water quality parameters
Every day at 15:00, water quality parameters including pH, temperature, dissolved oxygen, salinity, ammonia, and nitrite were measured. Temperature, dissolved oxygen, pH, and salinity of the water were measured using a SensoDirect150 MultiMeter (Lovibond, Tintometer Limited, Amesbury, UK). Total ammonia nitrogen (TAN) was measured using the HANNA HI 96715-11 Ammonia Medium Range photometer (HANNA, Nusfalau, Romania). Unionized ammonia was calculated using the pre-estimated TAN, temperature, and pH values (NH3). Nitrate was measured using the HANNA HI 708 (HANNA, Nusfalau, Romania).
Growth efficacy and survival percentage
Every 2 weeks, fish samples were collected at random to assess weight growth. Final body weight (FW), average daily gain (ADG), weight gain (WG), specific growth rate (SGR), and rate of survival were calculated using the following equations (Fath El-Bab et al. 2022):
-
Weight gain (WG; g/fish) = final body weight (FBW, g) − initial body weight (IBW, g)
-
ADG (g/fish /day) = (FBW- IBW) / duration (day)
-
Specific growth rate (%/day): SGR = 100 × [(ln FBW − ln IBW) / days].
-
Survival (%) = 100 × (final number of fish / initial number of fish).
-
Feed utilization: The fish feed utilization was computed depending on the equation illustrated by (Farliana et al. 2022; Wu et al. 2021).
-
Feed intake (g/fish): Quantity of feed offered or supplied throughout the experimental period /fish (g).
-
Feed conversion ratio (FCR) = feed intake (g) / weight gain (g)
Analytical protocols for fish and feed
Feed samples were analyzed at the beginning of experiment, and fish samples were collected at the end of each study to evaluate the proximate analyses of commercial diets and fish bodies, such as protein, moisture, lipid, and ash components. On stocking day, five fish (n = 5) were selected at random for chemical analysis. The crude protein, moisture, and crude fat compositions of entire fish bodies were calculated on a dry matter basis using (AOAC 1997).
Blood sampling
Blood was extracted from the caudal vertebral vein of six fish from each group (Clark et al. 2011). Whole blood was drawn into sterile tubes and mixed with an anticoagulant before being analyzed. The hemocytometer was used to count leukocytes and erythrocytes and measure hemoglobin concentrations using the cyanomet hemoglobin protocol, Drabkin’s solution, and Natt-Herrik solution (Stoskopf 1993). The packed cell volume (PCV) and differential leukocyte count values were calculated in accordance with Lamas et al. (1991). This formula was used to calculate the total differential leukocytic count (DLC), according to Thrall (2004).
Blood samples were drained into sterile Eppendorf tubes without anticoagulant, then centrifuged at 1500 × g/15 min to collect clear serum for biochemical analysis. To calculate the content of globulins in the blood, albumin and total proteins were estimated at 540 and 550 nm, respectively. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were colorimetrically measured at 540 nm (Reitman and Frankel 1957). The CHOD-PAP (commercial clinical kit) protocol was used to measure cholesterol (CHL) levels. Glucose concentration (mg/100 ml) was determined using glucose enzymatic PAP (Trinder 1969) kits (Bio-Merieux, France). The amylase activity was calculated three times.
Challenge assays with Aspergillus flavus
The pathogen, Aspergillus flavus strain was previously isolated from O. niloticus and obtained from the Microbiological Unit of the Fish Diseases Department, Animal Health Institute, Dokki, Giza, Egypt. Sabourauds dextrose agar (SDA, Difco) with streptomycin (100 ug/ml) and penicillin (100 UI/ml) was used to culture A. flavus for spore suspension, which was then incubated at 25 °C for a week (Eissa et al. 2023b). To collect the mass of conidia, each plate was filled with 20 mL of sterile distilled water, and the suspension was harvested in sterile tubes. The suspension was filtered through two layers of sterilized gauze.
The erythrocyte counting chamber of the hemocytometer was used to calculate and adjust the conidial suspension to 4 × 103 conidia/ml in sterile distilled water. After that, 20 fish from each group were injected with a sterilized needle carrying nearly 0.2 ml of A. flavus (4 × 103 conidia/ml) and monitored daily for the next 15 days. Injected fish were given a commercial diet. At the end of the experimental challenge, the cumulative mortality percentage was calculated.
The histological examination
At the end of the study, from the four experimental groups, 3 samples of hepatopancreas, intestine, and spleen were removed, cleaned in saline solution, and then fixed in 10% neutral formalin for 48 h. Next, the tissues were passed through increasing grades of ethyl alcohol and xylene, and finally paraplast blocks were created and cut using a rotating microtome. The processed tissues were then stained with hematoxylin and eosin stains, and finally photographs of the various groups’ tissues were taken. In accordance with the protocols described by Eissa et al. (2023a, b, c; Jastaniah et al. (2023), the Nile tilapia’s intestinal villi length and width are measured in each experimental group, the means ± standard errors are computed, and a chart graph is created to show the variations between the groups fed MSB and their resistance to A. flavus infection.
The total RNA extraction and real-time quantitative PCR (RTqPCR)
Using the RNA purification kit (Thermo Fisher Scientific, USA), total RNA was extracted from 50 mg of each experimental group’s hepatic tissues in accordance with the manufacturer’s instructions. The O.D. 260 nm/O.D. 280 nm ratio was measured using a nanodrop lite spectrophotometer (Thermo Scientific, USA) to assess the purity of the extracted RNA. Complementary DNAs (cDNAs) were generated from 1 µg of RNA using oligo-dT primers and the SuperScript TM III First-Strand Synthesis System (Invitrogen, USA) in accordance with manufacturer instructions. Following that, the cDNA samples were stored at −20 °C until needed again. The primers used in the current study are listed in Table 2.
In order to quantify the folds of gene transcription, qPCR (SensiFast SYBR Lo-Rox kit, Bioline, London, UK) was used to determine the mRNA expressions of genes associated with growth (growth hormone (GH), insulin-like growth factor-1 (IGF-1), insulin-like growth factor receptor-1 (IGF-1R), inflammatory genes including interleukin one beta (IL-1β), and interleukin 8 (IL-8). Following were the conditions of the reaction’s heat cycle that were observed: 10 min at 95 °C, 40 brief cycles at 95 °C, 30 min at 60 °C, and finally 5 min at 85 °C. The 2 − ΔΔCT protocol was used to standardize the transcription levels to the β-actin gene (Livak and Schmittgen 2001).
Data analysis
Bartlett and Kolmogorov-Smirnov tests were used to verify that the results were homogeneous and normal. Then, using the SPSS software (Version 21.0, IBM Corp., Armonk, NY, USA), the data was analyzed using a one-way ANOVA. To find the significant mean differences at a probability level of P < 0.05, Tucky’s B test was employed. We present the data as means ± standard error.
Results
Effects on water quality
Table 3 illustrates how dietary inclusion of multi-strain Bacillus (MSB) probiotics affected the quality of the water. All groups that added MSB showed no significant effect (P > 0.05) in both salinity temperature compared to control. When compared to other groups, the pH was slightly lower in the MSB2 and MSB1 groups. In comparison to the other groups, the MSB1 group had the lowest dissolved oxygen (DO) values (P < 0.05). The inclusion of dietary MSB did not significantly affect the values of NH4, NO2, or NO3 (P > 0.05).
Effects of MSB on growth and feed utilization
Supplementing with dietary probiotics (Table 4) significantly increased fish final weight, weight gain, SGR, feed intake, ADG, survival, fish biomass, and fish final number (P < 0.05). The fish fed with MSB3 had the highest values for all growth performance and feed utilization (P < 0.05). Fish final number and biomass per 1 m3 were higher in the MSB2 and MSB3 groups than in the other groups (P < 0.05). Regarding survival, feed intake, and final body weight, all MSB-treated groups showed comparable results (P > 0.05), with a notable difference compared to the untreated group (P < 0.05).
Effects on blood hematology
The findings showed that after adding MSB, hematocrit, RBCs, WBCs, heterophils, and Hb were all significantly (P < 0.05) elevated in a dose-dependent manner (Table 5). The MSB3 group had the highest values of MCHC, MCH, and eosinophils. Additionally, the MSB2 and MSB3 groups had significantly higher levels of monocytes and basophils than the other groups (p < 0.05). The lymphocyte, MCHC, and MCH values of fish fed probiotics at 1–2 g/kg were comparable to those of the control group. Compared to other groups, fish in the MSB3 group had fewer monocytes (P < 0.05). Overall, the hematological variables showed better results for fish fed on MSB3.
Effects on blood metabolites
Table 6 shows the impact of different MSB dosages on Nile tilapia, O. niloticus, serum blood metabolites after 70 days. The blood protein fraction (total protein, albumin, and globulins) improved significantly (P < 0.05) with dietary MSB inclusion following MSB addition in a dose-dependent manner. In contrast, fish fed diets containing MSB (1, 2, and 3 g/kg diet) showed significantly lower serum levels of triglycerides, uric acid, and liver enzymes (AST, ALT, and ALP) (P < 0.05). Serum digestive enzyme levels were considerably higher, and creatinine levels were significantly lower across all MSB treatments, with the MSB3 group showing the best results. When comparing the cholesterol values of the MSB2 group to those of the MSB1 and MSB0 groups, there was a significant decrease (P < 0.05). Compared to the MSB1 and control groups, MSB2 and MSB3 had significantly lower serum levels of urea and glucose (P < 0.05).
Effects on body composition
Figure 1 illustrates the effects of multi-strain Bacillus probiotics on the body composition of Nile tilapia, O. niloticus, after 70 days. MSB1 and MSB2 had higher moisture content; MSB-fed fish had higher protein content compared to the untreated group (P < 0.05). The percentage of lipid in the body composition decreases gradually with an increase in MSB level (P < 0.05). MSB2 and MSB3 had higher Ash percentage compared to the other groups.
Effect of various levels of multi-strains Bacillus (MSB) probiotics on body composition including moisture %, protein %, lipid %, and ash % of Nile tilapia, O. niloticus, after 70 days. Fish given multi-strains Bacillus probiotics (MSB) at various levels 0 (MSB0), 1 (MSB1), 2 (MSB2), and 3 (MSB3) g /kg diet for 70 days
Histological results
The histological interpretations revealed that the hepatopancreas tissues in the control group (MSB0) exhibited degraded hepatic tissue cells scattered throughout the hepatic strands surrounding the hepatic central vein engorged with blood, and Kuffer immunocytes were present within the hepatic sinusoids in a swollen form and in large numbers, as well as the presence of lymphatic immunocytes in the hepatic pancreas’ ducts and erosion of certain parts of the hepatopancreas tissues and the activity of fibroblasts to restore the tissue’s shape and give the appearance of hepatic cirrhosis caused by Aspergillus flavus infection. Hepatic structures, including the hepatic pancreatic ducts and the hepatic cells inside the hepatic strands, were seen to be improving. Furthermore, there was a noticeable improvement in the quantities of immunocytes, similar to those found in the hepatic sinusoids, in groups MSB3 (Fig. 2D), MSB2 (Fig. 2C), and MSB1 (Fig. 2B), respectively. These cells have a reduced number and a normal oval shape.
Photomicrograph sections of hepatopancreatic slices (A–D) for different experimental groups. Nile tilapia given multi-strains Bacillus probiotics (MSB) g /kg diet for 70 days at various levels 0 (MSB0): A, 1 (MSB1): B, 2 (MSB2): C, and 3 (MSB3): D. HC, hepatocytes; S, hepatic sinusoids; CV, hepatic central vein; C, hepatic cirrhosis. Asterisks: pancreatic duct. Green stars: inflammatory lymphocytes aggregations. Red arrows: blood accumulation. Blue stars: Kuffer macrophage cells [H&E and scale bar: 50 μm]
The impact of varying MSB dosages on the intestinal tissues of Nile tilapia (O. niloticus) after 70 days revealed that, in comparison to the other groups, the addition of dietary MSB greatly improved (P ≤ 0.01) the average length of the intestinal tissue layers and significantly improved (P < 0.05) the width of the intestinal villi. The third experimental group (MSB3) exhibited the best results for bacillus on intestinal tissues, as depicted in Figs. 3D and 4.
Photomicrograph sections of intestinal slices (A–D) for different experimental groups. Nile tilapia given multi- strains Bacillus probiotics (MSB) g /kg diet for 70 days at various levels 0 (MSB0): Fig. 2A, 1 (MSB1): Fig. 2B, 2 (MSB2): Fig. 2C, and 3 (MSB3): Fig. 2D. Four intestinal layers; S, serosa; Mus, muscularis; sM, mucosa; M, mucosa; Asterisks: lympho-immunocytes infiltrations. Green arrows: erosion of tips for some intestinal villi. Red arrows: macrophagocytes scattered on the fish villi which infects with A. flavus [H&E, and scale bar: 50 μm]
The impact charts of various levels of multi-strains Bacillus probiotics (MSB): 0 (MSB0), 1 (MSB1), 2 (MSB2), and 3 (MSB3) g /kg diet on the intestinal villi parameters including villi length (P ≤ 0.01) and villi width (P < 0.05) of Nile tilapia, O. niloticus. Means ± standards errors for each column, the different letters (a-b) which represented the significant between the experimental four groups by Tukey B test after 70 days
Figure 5A–D shows tilapia infected with A. flavus and fed varying amounts of MSB. Figure 5D, C, and B illustrate how the histological morphology of the spleen improved in all MSB-treated groups for MSB3, MSB2, and MSB1. The MSB3 group’s lymphocyte immunocytes and the splenic histological structure, including the red and white pulp, showed the greatest enhancement (Fig. 5D).
Photomicrograph sections of splenic slices (A–D) for different experimental groups. Nile tilapia given multi-strains Bacillus probiotics (MSB) g /kg diet for 70 days at various levels 0 (MSB0): Fig. 2A, 1 (MSB1): Fig. 2B, 2 (MSB2): Fig. 2C, and 3 (MSB3): Fig. 2D. Red pulp (rp) and white pulp (wp), they are distributed in the splenic tissues; C, capsule; MMC,, centers of the melanomacrophage which infects with A. flavus [H&E and scale bar: 50 μm]
Growth and pro-inflammatory related genes
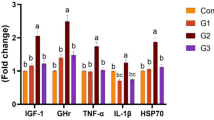
The results of relative mRNA expression revealed that dietary supplementation of Bacillus probiotics significantly upregulated the expression of growth-related genes, such as GH (, IGF-1 (Fig. 6B), and IGF-1 (Fig. 6C) in a dose-dependent manner, meaning that MSB3 shows better progress in growth performance than MSB2 and MSB1 when the levels of MSB in diets were increased (P < 0.05). Conversely, the effect of MSB on immunity genes was contradictory, meaning that fish given MSB showed a dose-dependent reduction in the IL-1β gene, with the MSB3 group showing the lowest expression, followed by the MSB2 and MSB1 groups (Fig. 6D). Furthermore, the expression of the IL-8 gene was different from IL-1β in all MSB-treated groups. The expression of IL-1β showed the lowest significant expression (P < 0.05) in the MSB2 group, followed by the MSB3 and MSB1 groups, with the highest expression noticed in the MSB0 group (control).
Survival during challenge with pathogen
Figure 7 illustrates the infection of tilapia fish with the pathogen A. flavus at different levels of MSB. The control group had a 40% infection survival rate, which was lower than that of the other groups. More fish survived in all MSB-treated groups; for MSB0, MSB1, MSB2, and MSB3, the corresponding survival rates were 40%, 55%, 65%, and 70%. It was found that MSB3 had the highest survival rate.
Discussion
Probiotics, according to FAO/WHO, can facilitate the growth and activity of beneficial bacteria in hosts, allowing them to remain alive and active in the gut (Zendeboodi et al. 2020). This article explains the importance of combining a probiotic mixture of Bacillus species (B. pumilus, B. licheniformis, and B. subtilis) to determine its effectiveness in treating A. flavus infection in O. niloticus. Therefore, an analysis was conducted on the blood picture, growth profile, digestion, biochemical indices, and gene expression.
This study was conducted to assess the immune-stimulating and growth-promoting effects of adding a Bacillus probiotic mix to the water in Nile tilapia rearing systems, as well as the fish’s vulnerability to A. flavus infection. The effects of this probiotic blend were examined by analyzing a variety of factors, such as the expression of genes linked to growth and immunity, growth performance, blood biochemical parameters, and water quality. Healthy water quality is essential for aquaculture production. To achieve optimal growth, survival, and productivity, a thorough understanding of the relationship between aquatic productivity and water quality is necessary (Dalia et al. 2023; Soundarapandian and Babu 2010). The current investigation demonstrated a definite improvement in water quality, as evidenced by a significant decrease in both total and toxic ammonia levels in the probiotic-treated groups. These findings are consistent with those of Basma et al. (2023), who found that adding probiotics to fish rearing water reduced the levels of harmful compounds (NH3, NO2, and NO3). Probiotics have also been shown to reduce unionized ammonia (NH3) and other nitrogenous waste in fish rearing water.
Probiotics can significantly alter gut microbiota, drive metabolic processes, improve digestion, activate the immune system, and protect against illnesses and exogenous bacteria, according to earlier studies (Falcinelli et al. 2018; Li et al. 2019; Munir et al. 2016a, 2018a, b). These benefits have been linked to probiotics’ critical roles in digestion, metabolism, and growth performance. The results of this study made it clear that the probiotic mixture significantly increased growth parameters, growth hormone (GH), insulin-like growth factor-1 (IGF-1), and insulin-like growth factor receptor-1 (IGF-1R) genes. The capacity of the probiotics to colonies and adhere to the intestinal wall (Munir et al. 2018a) may account for this, as it increases the secretion of digestive enzymes, which in turn increases metabolism and growth rate. A recent study (Zhao et al. 2020) confirmed that Bacillus spp. have the ability to adhere to fish intestines, improving digestion, which lends support to this finding. Furthermore, as partially accepted in comparison with our data, B. coagulans, either by itself or in conjunction with β-glucan added to the tilapia diets, could decrease the expression of the IL-1β gene while upregulating the expression of the GH and IL-8 genes (Fath El-Bab et al. 2022).
Furthermore, according to Ringø et al. (2022), probiotic microorganisms can colonize the gastrointestinal tract by releasing a variety of digestive and degrading enzymes that allow the body to use a wide range of nutritional components, thereby improving digestion. Comparably, Zibiene and Zibas (2019) showed that commercial probiotics can unquestionably enhance European catfish (Silurus glanis) growth performance. According to a recent study, Bacillus subtilis can enhance the expression of genes related to appetite and somatic growth in zebrafish (Santos et al. 2020). In addition, Zou et al. (2016) discovered that common carp (Cyprinus carpio) had increased intestinal cytokine gene expression in response to probiotic powder containing Agaricus bisporus. Furthermore, Fath El-Bab et al. (2022) discovered that adding Bacillus coagulants to Nile tilapia diets greatly enhanced the fish’s growth and feed utilization.
In terms of evaluating hepato-renal function, the current data showed a significant alteration in creatinine levels and liver enzymes. Consistent with earlier research, different fish species’ AST, ALT, and creatinine levels can be regulated by adding Bacillus sp. to their diets (Hassaan et al. 2018; Yu et al. 2018). This may be explained by the immune-modulatory effects of the Bacillus spp. mixture on the hepatic cells, which promote hepatocyte anabolism and the release of blood proteins along with a positive outcome regarding the preservation of liver cell integrity (Eladawy 2019).
Probiotics have been shown in earlier studies to play a critical role in growth, metabolism, digestion, and absorption as well as to boost immune system function, improve digestion and absorption, and protect against infectious diseases (Munir et al. 2016a). Additionally, the present study demonstrated that the probiotic mixture greatly accelerated the process of enhancing the tissue structures of the intestines and hepatopancreas, which are home to Bacillus probiotic bacteria like Bacillus subtilis and Bacillus cereus. It lessens the absorption of aluminum, which lessens tissue damage brought on by the buildup of aluminum in tilapia fish tissues (Arun et al. 2021). Furthermore, Lazado et al. (2014) demonstrated that the bacteria produce mucous secretions that function to shield the body’s tissues from infectious diseases by boosting the activity of immune lymphocytes that are supportive of the mucous tissue. In 2023, Shija et al. observed that including Bacillus probiotics in the diet of various fish species strengthens fish immunity and prevents the need for antibiotics by protecting fish against a variety of infectious diseases, enhancing autoimmunity, and promoting improved growth in farmed fish. Recently, Gao et al. (2024) reported that the immune function and hepatic health of flounder (Paralichthys olivaceus) were improved by dietary supplementation with Bacillus in their diets. Probiotic mixture supplements have a number of advantages over (A) flavus, as evidenced by their strong antibacterial activity, reduced mortality, improved blood picture, and immune-promoting effects. Elevations in blood parameters, immune-related indices (total protein, albumin, phagocytosis), and immune (interleukin 8 (IL-8) and interleukin one beta (IL-1β) associated genes were indicative of these results. This may be primarily due to the interaction between probiotic bacteria (Bacillus spp.) and various immune system cell types (monocytes, lymphocytes, macrophages, granulocytes, neutrophils, and natural killer cells), as reported by Mazziotta et al. (2023). A recent study by Tachibana et al. (2021) that fed Nile tilapia enriched diets containing Bacillus species revealed increased granulocyte and intraepithelial lymphocyte proliferation, supporting our results. It is possible that recent reports have indicated that adding Bacillus probiotics, such as (B) amyloliquefaciens, B. licheniformis, B. subtilis, and B. pumilus, to the diet of Nile tilapia improves phagocytosis (Eissa et al. 2023a; Ghalwash et al. 2022). According to Wu et al. (2021), feeding Nile tilapia with Bacillus (NPUST1) for 8 weeks improved immune biomarkers in head kidney leukocytes (e.g., phagocytic activity and respiratory burst activity), lysozymes, and expression of immune-related genes in the head kidney and spleen. This is in line with previous reports (Chien et al. 2020) that found that B. subtilis E20 boosted the immune response in white shrimp by improving the metabolism of glutamine and the hexosamine biosynthesis pathway. In line with Dutta and Ghosh’s (2021) findings, this study reported that immune genes (TNF-1α and TNF-2α) were up-regulated in goldfish (Carassius auratus gibelio) when exposed to dietary Bacillus spp. Tang et al. (2017) provided support for these results by observing that juvenile tilapia challenged with virulent (A) hydrophila exhibited enhanced antibacterial and anti-inflammatory activities following dietary intervention with (B) subtilis. Supplementation of B. subtilis as a probiotic improved growth pattern, antioxidant response, protein content, and immunocompetency against S. aureus in striped catfish (Liaqat et al. 2024). Moreover, a recent study of Neissi et al. (2024) found that adding B. subtilis to the diet can enhance the health and blood profile of rainbow trout (Oncorhynchus mykiss) under acute hypoxia stress.
Conclusion
This current research confirmed the synergistic effects of probiotics (Bacillus subtilis, B. pumilus, and B. licheniformis) as immune stimulants, growth promoters, enhancers of histological structures, and antifungal agents against A. flavus infection in tilapia fish. In O. niloticus, this probiotic mixture resulted in improved growth genes expression and helped combat anemia. Combining probiotics from the Bacillus species can work together to maximize their benefits, especially at higher concentrations. Further research is needed to explore the additional antimicrobial effects of the Bacillus species mixture and their impact on other fish species.
Data availability
No datasets were generated or analysed during the current study.
References
Abd El-Hack ME, El-Saadony MT, Elbestawy AR, Nahed A, Saad AM, Salem HM, El-Tarabily KA (2022) Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult Sci 101(2):101590
Abdelnour SA, El-Saadony MT, Saghir SAM, El-Hack A, Al-Shargi ME, Al-Gabri OYA, Salama N, A (2020) Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest Sci 240:104220
Aftabgard M, Salarzadeh A, Mohseni M, Bahri Shabanipour AH, Zorriehzahra MEJ (2019) The combined efficiency of dietary isomaltooligosaccharides and Bacillus spp on the growth, hemato-serological, and intestinal microbiota indices of Caspian brown trout (Salmo trutta caspius Kessler, 1877). Probiotics and antimicrobial proteins 11, 198–206
Ahmed SA, Nada HS, Elsheshtawy HM, Ibrahim SM, Fahmy EM, Khedr MH, Moustafa SM, Ismail TA, Gesriha S, Assayed ME (2022) Comparative antitoxic potency of honey and natamycin-supplemented diets against aflatoxicosis and their influences on growth, serum biochemistry, immunohistochemistry, and residual deposition in Nile tilapia (Oreochromis niloticus). Aquaculture 551, 737934
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136
Alzahrani OM, Elumalai P, Nada HS, Ahmed SA, Zaglool AW, Shawky SM, Alkafafy M, Mahboub HH (2022) Pseudomonas putida: sensitivity to various antibiotics, genetic diversity, virulence, and role of formic acid to modulate the immune-antioxidant status of the challenged nile tilapia compared to carvacrol oil. Fishes 8(1):6
AOAC (1997) Association of Official Analytical Chemists International Official Methods of Analysis. 16th Edition, AOAC, Arlington
Arun K, Madhavan A, Sindhu R, Emmanual S, Binod P, Pugazhendhi A, Sirohi R, Reshmy R, Awasthi MK, Gnansounou E et al (2021) Probiotics and gut microbiome—prospects and challenges in remediating heavy metal toxicity. J Hazard Mater 420:126676
Ashour EA, El-Hack MEA, Shafi ME, Alghamdi WY, Taha AE, Swelum AA, El-Saadony MT (2020) Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture 10(10):457
Azevedo RV, Filho F, Pereira JC, Cardoso SL, Andrade LD, Vidal DR, Júnior MV (eds) (2016) Dietary mannan oligosaccharide and Bacillus subtilis in diets for Nile tilapia (Oreochromis niloticus). Acta Scientiarum. Anim Sci 38, 347–353
Basma M, Hendam MB, Munir, Moaheda EH, Eissa E, El-Haroun H, van Doan TH, Chung E-SH, Eissa (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against aspergillus flavus of Nile tilapia (Oreochromis niloticus). 303:115696. Anim Feed Sci Technol. https://doi.org/10.1016/j.anifeedsci.2023.115696
Cha J-H, Rahimnejad S, Yang S-Y, Kim K-W, Lee K-J (2013) Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 402:50–57
Chelladurai K, Ayyash M, Turner MS, Kamal-Eldin A (2023) Lactobacillus helveticus: Health effects, current applications, and future trends in dairy fermentation. Trends Food Sci Technol
Chien CC, Lin TY, Chi CC, Liu CH (2020) Probiotic, Bacillus subtilis E20 alters the immunity of white shrimp, Litopenaeus vannamei via glutamine metabolism and hexosamine biosynthetic pathway. Fish Shellfish Immunol 98:176-185L. https://doi.org/10.1016/j.fsi.2020.01.014
Clark T, Donaldson M, Drenner S, Hinch S, Patterson D, Hills J, Ives V, Carter J, Cooke S, Farrell A (2011) The efficacy of field techniques for obtaining and storing blood samples from fishes. J Fish Biol 79(5):1322–1333
Dalia A, Abuljadayel DAA, Bukhari, Moaheda EH, Eissa (2023) Mohammad Bodrul Munir, Ahmed Jalal Khan Chowdhury, El-Sayed Hemdan Eissa, Utilization of probiotic bacteria Pediococcus acidilactici to enhance water quality, growth performance, body composition, hematological indices, biochemical parameters, histopathology and resistance of red tilapia (Oreochromis sp.) against Aeromonas sobria Desalination and Water Treatment. 315 (2023) 469–478
Dawood MA (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquacult 13(1):642–663
do Veiga N, Owatari PT, Nunes MS, Rodrigues AL, Dichoff Kasai RA, Fernandes RY, de Campos CE (2020) C.M., Bacillus subtilis C-3102 improves biomass gain, innate defense, and intestinal absorption surface of native Brazilian hybrid Surubim (Pseudoplatystoma corruscans x P. reticulatum). Aquacult Int 28, 1183–1193
Dutta D, Ghosh K (2021) Improvement of growth, nutrient utilization and haemato-immunological parameters in rohu, Labeo rohita (Hamilton) using Bacillus tequilensis (KF623287) through diets or as water additive. Aquacult Nutr 27:29–47
Eissa EH, Elsayed SB, Alkhateib Y, Gaafar, Ashraf A, El-Badawi, Bazina WK, Al-Kareem OM, Abd El-Hamed NNB (2022a) Assessing the influence of dietary Pediococcus acidilactici probiotic supplementation in the feed of European sea bass (Dicentrarchus labrax L) (Linnaeus, 1758) on farm water quality, growth, feed utilization, survival rate, body composition, blood biochemical parameters, and intestinal histology, Aquaculture Nutrition, vol. 2022, Article ID 5841220, 11 pages. https://doi.org/10.1155/2022/5841220
Eissa ESH, Ahmed NH, El-Badawi AA, Munir MB, Abd Al‐Kareem OM, Eissa ME, Hussien EH, Sakr SES (2022b) Assessing the influence of the inclusion of Bacillus subtilis AQUA‐GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac Res 53(18):6606–6615
Eissa E-SH, Ahmed RA, Abd Elghany NA, Elfeky A, Saadony S, Ahmed NH, Sakr SE-S, Dayrit GB, Tolenada CPS, Atienza AAC (2023a) a Potential symbiotic effects of β-1, 3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani Infection. Fishes 8(2), 105
Eissa E-SH, Alaidaroos BA, Jastaniah SD, Munir MB, Shafi ME, El-Aziz A, Bazina YM, Ibrahim WK, Eissa Sb, Paolucci ME, M (2023b) Dietary effects of Nano Curcumin on Growth performances, body composition, blood parameters and histopathological alternation in Red Tilapia (Oreochromis Sp) challenged with aspergillus flavus. Fishes 8(4):208
Eissa E-SH, Bazina WK, Abd El-Aziz YM, Abd Elghany NA, Tawfik WA, Mossa MI, Megeed AE, El-Hamed OHA, El-Saeed NNB, El-Haroun AF, Davies E, Hasimuna SJ, Eissa OJ, Khalil MEH, H.S (2023c) Nano-selenium impacts on growth performance, digestive enzymes, antioxidant, immune resistance and histopathological scores of Nile tilapia, Oreochromis niloticus against Aspergillus flavus infection. Aquacult Int. https://doi.org/10.1007/s10499-023-01230-4
Eissa ESH, El-Sayed AM, Ghanem SF, Dighiesh HS, Abd Elnabi HE, Hendam BM, Elleithy AA, Eissa MEH, Abd El-Aziz YM (2024) Dietary mannan-oligosaccharides enhance hematological and biochemical parameters, reproductive physiology, and gene expression of hybrid red tilapia (Oreochromis niloticus x O. mossambicus). 740453:0044–8486. https://doi.org/10.1016/j.aquaculture.2023.740453
El-Bouhy ZM, Reda RM, Mahboub HH, Gomaa FN (2021) Chelation of mercury intoxication and testing different protective aspects of Lactococcus lactis probiotic in African catfish. Aquac Res 52(8):3815–3828
Eladawy MM (2019) Characterization of probiotic Bacillus subtilis isolated from Nile tilapia (Oreochromis niloticus) digestive tract and evaluation its positive impact on health and immunity. Egypt J Aquaculture 9(2):1–26
Falcinelli S, Rodiles A, Hatef A, Picchietti S, Cossignani L, Merrifield DL, Unniappan S, Carnevali O (2018) Influence of probiotics administration on gut microbiota core: a review on the effects on appetite control, glucose, and lipid metabolism. J Clin Gastroenterol 52:S50–S56
Farliana W, Alias SL, Munir MB, Asdari R, Hannan A, Hasan J (2022) Dietary lacto-sacc improved growth performance, food acceptability, body indices, and basic hematological parameters in empurau (Tor tambroides) fries reared in the aquaponics system. J Appl Aquac 1–23. https://doi.org/10.1080/10454438.2022.2095239
Fath El-Bab AF, Majrashi KA, Sheikh HM, Shafi ME, El-Ratel IT, Neamat-Allah AN, El-Raghi AA, Elazem AYA, Abd-Elghany MF, Abdelnour SA (2022) Dietary supplementation of Nile tilapia (Oreochromis niloticus) with β-glucan and/or Bacillus coagulans: synergistic impacts on performance, immune responses, redox status and expression of some related genes. Front Veterinary Sci 9:1011715
Gao Y, Tan R, Wang Z, Qiang L, Yao H (2024) The effects of Bacillus subtilis on the immunity, mucosal tissue morphology, immune-related gene transcriptions, and intestinal microbiota in flounder (Paralichthys olivaceus) with two feeding methods: continuous versus discontinuous feeding. Vet Immunol Immunopathol 110742. https://doi.org/10.1016/j.vetimm.2024.110742
Ghalwash HR, Salah AS, El-Nokrashy AM, Abozeid AM, Zaki VH, Mohamed RA (2022) Dietary supplementation with Bacillus species improves growth, intestinal histomorphology, innate immunity, antioxidative status and expression of growth and appetite‐regulating genes of Nile tilapia fingerlings. Aquac Res 53(4):1378–1394
Hassaan MS, Soltan MA, Jarmołowicz S, Abdo HS (2018) Combined effects of dietary malic acid and Bacillus subtilis on growth, gut microbiota and blood parameters of Nile tilapia (Oreochromis niloticus). Aquacult Nutr 24(1):83–93. https://doi.org/10.1111/anu.12536
Jastaniah SD, Alaidaroos BA, Shafi ME, Aljarari RM, El-Aziz A, Munir YM, Eissa MB, AL-Farga MEH, Eissa A, Said E-SH, R.M (2023) Dietary Pediococcus acidilactici improved the growth performance, feed utilization, gut microbiota, and disease resistance against Fusarium solani in Pacific white shrimp, Litopenaeus vannamei. Aquacult Int. https://doi.org/10.1007/s10499-023-01318-x
Kavitha M, Raja M, Perumal P (2018) Evaluation of probiotic potential of Bacillus spp isolated from the digestive tract of freshwater fish Labeo calbasu. Aquac Rep 11:59–69 (Hamilton 1822)
Lamas J, Bruno DW, Santos Y, Anadón R, Ellis AE (1991) Eosinophilic granular cell response to intraperitoneal injection with Vibrio anguillarum and its extracellular products in rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol 1(3):187–194
Lazado CC, Marlowe C, Caipang A (2014) Mucosal immunity and probiotics in fish. Fish Shellfish Immunol 39:78–89
Li X, Ringø E, Hoseinifar SH, Lauzon HL, Birkbeck H, Yang D (2019) The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev Aquacult 11:603–618. https://doi.org/10.1111/raq.12248
Liaqat R, Fatima S, Komal W, Minahal Q, Kanwal Z, Suleman M, Carter CG (2024) Effects of Bacillus subtilis as a single strain probiotic on growth, disease resistance and immune response of striped catfish (Pangasius hypophthalmus). PLoS ONE 19(1):e0294949
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25(4), 402–408
Magbanua TO, Ragaza JA (2022) Systematic review and meta-analysis of the growth performance and carcass composition of Nile tilapia (Oreochromis niloticus) fed dietary copra meal. Front Sustainable Food Syst 6:1025538
Mahboub HH, Faggio C, Hendam BM, Algharib SA, Alkafafy M, Hashem MA, Mahmoud YK, Khamis T, Abdel-Ghany HM, Masoud SR (2022a) Immune-antioxidant trait, Aeromonas veronii resistance, growth, intestinal architecture, and splenic cytokines expression of Cyprinus carpio fed Prunus armeniaca kernel-enriched diets. Fish Shellfish Immunol 124:182–191
Mahboub HH, Nada HS, Abdel-Ghany HM, Ghanem R, Ahmed Ismail T, Abdel Rahman AN (2022b) Detection, diagnosis, Koch’s postulate, hepatorenal and antioxidant indicators for some systemic pathogenic fungi invading the liver and kidneys of African catfish (Clarias gariepinus) in Egypt with a histopathological approach. Aquac Res 53(7):2670–2685
Mamun M, Nasren S, Rathore S, Sidiq M, Dharmakar P, Anjusha K (2019) Assessment of probiotic in aquaculture: functional changes and impact on fish gut. Microbiol Res J Int 29(1):1–10
Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC (2023) Probiotics mechanism of action on Immune cells and Beneficial effects on Human Health. Cells 12(1). https://doi.org/10.3390/cells12010184
Moaheda EH, Eissa FS, Alaryani S, Elbahnaswy MS, Khattab A, Elfeky KY, AbouelFadl E-SH, Eissa RA, Ahmed H, Van Doan, Ehab El-Haroun (2023) Dietary inclusion of Pediococcus acidilactici probiotic promoted the growth indices, hemato-biochemical indices, enzymatic profile, intestinal and liver histomorphology, and resistance of Nile Tilapia against Aspergillus flavus, Animal Feed Science and Technology,Volume 306,2023,115814,ISSN 0377–8401. https://doi.org/10.1016/j.anifeedsci.2023.115814
Monier MN, Kabary H, Elfeky A, Saadony S, El-Hamed NNA, Eissa ME, Eissa E-SH (2023) The effects of Bacillus species probiotics (Bacillus subtilis and B. Licheniformis) on the water quality, immune responses, and resistance of whiteleg shrimp (Litopenaeus vannamei) against Fusarium solani infection. Aquac Int 1–19. https://doi.org/10.1007/s10499-023-01136-1
Munir MB, Roshada H, Yam HC, Terence LM, Azizah S (2016a) Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquaculture. Volume 460, 1 July 2016, Pages 59–68
Munir MB, Roshada H, Abdul-Manaf MS, Azizah S (2016b) Dietary prebiotics and probiotics influence the growth performance, feed utilisation, and body indices of snakehead (Channa striata) fingerlings. Trop Life Sci Res 27(2):111–125
Munir MB, Roshada H, Azizah S, Terence LM (2018a) Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: haematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol. 75 (2018), pp 99–108
Munir MB, Terence L, Blaud A, Roshada H, Wizilla J, Azizah S (2018b) Analysing the effect of dietary prebiotics and probiotics ongut bacterial richness and diversity of Asian snakeheadfingerlings using T-RFLP method. Aquac Res 49(10):3350–3361
Neissi A, Zahed M, H., Roshan R (2024) Probiotic performance of B. subtilis MS. 45 improves aquaculture of rainbow trout Oncorhynchus mykiss during acute hypoxia stress. Sci Rep 14(1):3720
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt J, Austin B (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum. J Appl Microbiol 103:1699–1706
Puri P, Sharma JG, Singh R (2022) Biotherapeutic microbial supplementation for ameliorating fish health: developing trends in probiotics, prebiotics, and synbiotics use in finfish aquaculture. Anim Health Res Reviews, 1–23
Rahman ANA, Van Doan H, Elsheshtawy HM, Dawood A, Salem SM, Sheraiba NI, Masoud SR, Abdelnaeim NS, Khamis T, Alkafafy M (2022) Dietary Salvia officinalis leaves enhances antioxidant-immune-capacity, resistance to Aeromonas sobria challenge, and growth of Cyprinus carpio. Fish Shellfish Immunol 127:340–348
Ramesh D, Souissi S (2018) Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquac Res 49:367–377
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Ringø E, Harikrishnan R, Soltani M, Ghosh K (2022) The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 12(21):3016
Santos KO, Costa-Filho J, Spagnol KL, Nornberg BF, Lopes FM, Tesser MB, Marins LF (2020) The inclusion of a transgenic probiotic expressing recombinant phytase in a diet with a high content of vegetable matter markedly improves growth performance and the expression of growth-related genes and other selected genes in zebrafish. Aquaculture 519:734878
Santos RA, Monteiro M, Rangel F, Jerusik R, Saavedra MJ, Carvalho AP, Oliva-Teles A, Serra CR (2021) Bacillus spp. Inhibit Edwardsiella tarda Quorum-Sensing and Fish infection. Mar Drugs 19:602. https://doi.org/10.3390/md19110602
Shija VM, Amoah K, Cai J (2023) Effect of Bacillus probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus). Rev Fishes 8(7):366. https://doi.org/10.3390/fishes8070366
Soundarapandian P, Babu R (2010) Effect of probiotics on the hatchery seed production of black tiger shrimp, Penaeus monodon (Fabricius). Int J Anim Veterinary Adv 2(1):9–15
Stoskopf MK (1993) Fish medicine. No Title)
Tachibana L, Telli GS, Dias DdC, Goncalves GS, Guimaraes MC, Ishikawa CM, Cavalcante RB, Natori MM, Alarcon F, Tapia-Paniagua MF (2021) S., Bacillus subtilis and Bacillus licheniformis in diets for Nile tilapia (Oreochromis niloticus): effects on growth performance, gut microbiota modulation and innate immunology. Aquac Res 52(4), 1630–1642
Tang L, Huang K, Xie J, Yu D, Sun L, Huang Q, Bi Y (2017) 1-Deoxynojirimycin from Bacillus subtilis improves antioxidant and antibacterial activities of juvenile Yoshitomi tilapia. Electron J Biotechnol 30:39–47
Thrall JH (2004) Nanotechnology and medicine. Radiology 230(2):315–318
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6(1):24–27
Tseng DY, Ho PL, Huang SY, Cheng SC, Shiu YL, Chiu CS, Liu CH (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol. 2009;26:339–344
Wu P-S, Liu C-H, Hu S-Y (2021) Probiotic Bacillus safensis NPUST1 administration improves growth performance, gut microbiota, and innate immunity against Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Microorganisms 9(12):2494
Yao Y-Y, Chen D-D, Cui Z-W, Zhang X-Y, Zhou Y-Y, Guo X, Li A-H, Zhang Y-A (2019) Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing sip. Fish Shellfish Immunol 86:999–1008
Yi Y, Zhang Z, Zhao F, Liu H, Yu L, Zha J, Wang G (2018) Probiotic potential of Bacillus velezensis JW: antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol 78:322–330
Yu Y, Wang C, Wang A, Yang W, Lv F, Liu F, Liu B, Sun C (2018) Effects of various feeding patterns of Bacillus coagulans on growth performance, antioxidant response and Nrf2-Keap1 signaling pathway in juvenile gibel carp (Carassius auratus Gibelio). Fish Shellfish Immunol 73:75–83
Zendeboodi F, Khorshidian N, Mortazavian AM, da Cruz AG (2020) Probiotic: conceptualization from a new approach. Curr Opin Food Sci 32:103–123
Zhao D, Wu S, Feng W, Jakovlić I, Tran NT, Xiong F (2020) Adhesion and colonization properties of potentially probiotic Bacillus paralicheniformis strain FA6 isolated from grass carp intestine. Fish Sci 86:153–161
Zibiene G, Zibas A (2019) Impact of commercial probiotics on growth parameters of European catfish (Silurus glanis) and water quality in recirculating aquaculture systems. Aquac Int 27(6):1751–1766
Zou HK, Hoseinifar SH, Miandare HK, Hajimoradloo A (2016) Agaricus Bisporus powder improved cutaneous mucosal and serum immune parameters and up-regulated intestinal cytokines gene expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol 58:380–386
Acknowledgements
All authors extend their appreciation to their universities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by the Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
H.S.D., S.E.S., Conceptualization, visualization, methodology, E.N.A.A., O.F.A., W.E.A., S.A.H., N.M.A. and Y.M.A.El-A., software, validation, formal analysis, N.A., H.H.M., M.B.M., E.H.E., A.A.E. and Y.M.A.El-A.; investigation, data curation, S.A.H., N.M.A., N.A. and Y.M.A.El-A.; histological processing, examination, and histological data interpretation; M.B.M., Y.M.A.El-A. writing—original draft preparation, H.H.M., E.H.E., A.A.E., M.B.M, and Y.M.A.El-A.; writing—review and editing, W.E.A., S.A.H., N.M.A., N.A., and Y.M.A.El-A.; supervision, E.H.E.; project administration, E.H.E. All authors have read and approved the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Ethical approval was granted by the Research ethical of Arish university, with research code: Agri-11.
Consent to participate
Not applicable.
Consent for publication
All authors review and approve the manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dighiesh, H.S., Alharbi, N.A., Awlya, O.F. et al. Dietary multi-strains Bacillus spp. enhanced growth performance, blood metabolites, digestive tissues histology, gene expression of Oreochromis niloticus, and resistance to Aspergillus flavus infection. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01502-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01502-7