Abstract
Aquaculture plays an important role in providing protein and fatty acids to meet human nutritional requirements. The finite supply of marine ingredients has led to increased use of vegetable oils in aquafeed. While these oils can meet energy and growth needs, they lack nutritionally required ω3 long-chain polyunsaturated fatty acids (ω3 LCPUFAs). Development of land-based alternative oil sources to safeguard global aquaculture production, while meeting the nutritional needs of both fish and human consumers, is critical. This review summarizes studies using a new land-based ω3 LCPUFA canola oil in fish feed to support both energy and ω3 LCPUFA requirements of Atlantic salmon during each phase of the life cycle, while producing fish with adequate ω3 LCPUFA content to meet human nutritional needs. In all cases, growth and performance were comparable to fish fed conventional fish-oil-based diets; no adverse effects were attributed to the use of ω3 canola oil. Fatty acid deposition in muscle (fillet) reflected feed composition, resulting in accumulation of alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) and a low ω6:ω3 ratio, making salmon fed ω3 LCPUFA canola oil a sustainable source of these nutrients for human consumption. Additionally, reduced melanin deposits were observed in fish fed ω3 LCPUFA canola oil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As key components of membranes in virtually all cells, ω3 LCPUFAs, especially docosahexaenoic acid (DHA; C22:6ω-3) and eicosapentaenoic acid (EPA; C20:5ω-3), are critical for development and maintenance of health (NIH 2022; Tocher 2015). While EPA and DHA can be synthesized from ⍺-linolenic acid (ALA; 18:3ω-3), vertebrates, including humans and fish, have limited capacity to do so and therefore must rely on dietary sources to meet daily requirements. Fatty fish, such as salmon, tuna, and trout, are common food sources of these two ω3 fatty acids, as are fish oil (FO) supplements derived from fatty fish.
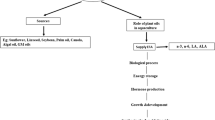
Aquaculture plays a meaningful role in meeting the demand for fish consumption and is a growing food source globally (Hardy & Kaushik 2022; Garlock et al. 2020). The Food and Agriculture Organization (FAO) estimates that aquaculture produces 313,647 tons (live weight) of food every day (Tacon et al. 2022; FAO 2020) currently contributing 49.2% of the world’s supply of seafood and has predicted that cultivated aquatic species will provide an additional 15% of the world’s supply of seafood by 2030 (FAO 2022a). This fast-growing industry requires input of marine-sourced ingredients as a component of the fish diet to support normal growth and development. However, fish, like humans and livestock, require specific dietary nutrients, not a particular feed ingredient such as fish meal or FO. In fact, the industry has been reducing reliance on marine-derived feed components due to pressures imposed by diminishing supplies (Naylor et al. 2021). Whereas 90% of ingredients in Atlantic salmon feed were marine derived in the 1990s in Norway, Chile, Australia, and Canada, current salmon diets contain approximately 25–30% marine ingredients (Aas et al. 2019; Ytrestøyl et al. 2015). Vegetable oils, especially canola and soy, are successfully being used to replace much of the fat and FO used in fish feed, albeit with decreased ω3 LCPUFA content and higher ω6:ω3 ratios in fillets of the harvested fish (Nichols et al. 2014; Sprague et al. 2016)
While plant-based oils provide the fat needed for energy and growth, they lack the nutritionally required ω3 LCPUFAs and cannot completely replace FOs containing the essential fatty acids (EFA) ALA, DHA, and EPA in fish diets. Additionally, replacing ω3 FAs with ω6 FAs in aquafeed further exacerbates the greatly imbalanced ω6:ω3 ratio in the human diet (Simopolous 2010). As recently demonstrated by Lutfi et al. (2022), an adequate supply of ω3 LCPUFA in the feed improves the health of farmed Atlantic salmon, emphasizing the significance of these EFA for welfare, performance, and quality of fish reared in aquaculture. The finite supply of marine-derived ingredients (Naylor et al. 2021) requires land-based alternatives to safeguard global aquaculture production, while meeting nutritional needs (Tocher et al. 2019; FAO, 2020). Thus, sustainable and nutrition-sensitive practices are essential for aquaculture to contribute to global food and nutrition security (Gephart et al. 2021). Here, we provide an overview of the development of alternative, plant-based ω3 sources and review the aquaculture trials conducted to evaluate the efficacy of a novel ω3 LCPUFA canola oil (referred to herein as ω3 canola oil for brevity and commercially available as Aquaterra®) in salmonids.
Oilseed crops as alternative sources of ω3 LCPUFAs
Genetically modified oilseed crops represent a sustainable, land-based alternative source of ω3 LCPUFAs to address aquaculture, livestock, and human needs. The scalability advantages of crop production include an established infrastructure for the cultivation, harvest, processing, and distribution of vegetable oils (Tocher 2015). Oilseed crops deliver a further benefit providing protein meal for use in animal feeds. In recent years, two oilseed crops, canola (Brassica napus L.) and camelina (Camelina sativa), have been engineered to provide useful levels of ω3 LCPUFAs in the seed oil.
Brassica napus (canola)
Early efforts showed that it was possible to produce significant levels of ω3 LCPUFAs in the model species Arabidopsis (Petrie et al. 2012) and in the related crop species Brassica juncea and B. carinata (Wu et al. 2005; Cheng et al. 2010; Petrie et al. 2014). However, canola offers several advantages over other species: it is a widely adapted crop, grown on >44 M ha worldwide, with high grain yield and high seed oil content (ca. 40–45%), and its oil is already well established as an important ingredient in aquaculture feeds.
Two approaches have been used to engineer production of ω3 LCPUFAs in canola. Walsh et al. (2016) transformed canola with a microalgal polyketide synthase-like system to produce DHA and EPA in the seed oil. However, the levels of these FAs were low, at 3.7% and 0.7%, respectively, and there appears to have been no further development of this crop.
The second approach involves transformation with a suite of genes to create an enzymatic pathway leading to production of ω3 LCPUFAs. Petrie et al. (2020) described the development of a genetically engineered canola line capable of biosynthesizing a suite of ω3 LCPUFAs. This canola (OECD Unique Identifier NS-B5ØØ27-4) was modified through the introduction of seven microalgal and yeast genes (Fig. 1) that provide a step-by-step conversion of endogenous oleic acid (OA; 18:1ω9) and ALA to fish oil-like levels of DHA and lower levels of EPA and DPA (docosapentaenoic acid; C22:5ω3) in the seed oil (Petrie et al. 2020; MacIntosh et al. 2021; Table 1).
An attractive feature of this oil is that it contains 19–20% ALA, compared to 10% in conventional canola varieties. This, combined with the significant levels of DHA (≥8%) and other ω3 LCPUFAs, results in a marked change in the ω6:ω3 ratio, from approximately 2 in conventional canola to 0.21 in ω3 canola oil (Table 1). Evaluation of the nutritional content shows that, except for the intended altered fatty acid composition, oil from DHA canola is substantially similar to canola oil (McIntosh et al., 2021). Oil from this crop, referred to as ω3 canola oil in this review, has been approved for use in food and animal feed in Australia, Canada, and the USA. Its use in aquaculture is the subject of this review.
In similar work, biosynthesis of ω3 LCPUFAs in canola through the introduction of multiple genes has been described by Yilmaz et al. (2017). The oil from this transgenic canola line, marketed as Latitude™ by Cargill, contains approximately 7% EPA and 1% DHA (Napier et al. 2019) and, due to a much lower level of ALA than in ω3 canola oil (Aquaterra) (ca. 7%), has a considerably higher ω6:ω3 ratio than ω3 canola oil. Evaluation of Latitude alone or in combination with other oils in feed formulations for farmed shrimp (Litopenaeus vannamei; Gia Vo et al., 2021) and rainbow trout (Oncorhynchus mykiss; Hossain et al. 2021, Hong et al. 2022) suggests that this oil supports shrimp and salmonid growth with no harmful effects.
Camelina sativa
A similar approach has been used to introduce an ω3 LCPUFA pathway into camelina. Using different combinations of elongase and desaturase genes, camelina lines producing high levels of EPA, or combinations of EPA and DHA in the seed oil, have been developed (Ruiz-Lopez et al. 2014; 2015). Studies using these oils as feed ingredients for several fish species (Betancor et al. 2018, 2016a, 2016b; Sprague et al., 2016) demonstrate that partial or full replacement of oils typically used in commercial feed by ω3 LCPUFA camelina oil is not only safe and supports growth but also results in tissue deposition of EPA and DHA at comparable or higher levels than standard feed. However, this has not yet been developed into a commercial product.
Assessment of omega-3 canola oil in Atlantic salmon
Evaluation during the Atlantic salmon aquaculture life cycle
Atlantic salmon are ranked number 9 by quantity (FAO, 2022b) and number 2 by value (FAO, 2019) in world aquaculture, attesting to their importance for the global food supply. Atlantic salmon also represent an appropriate model for studying the utility and safety of novel aquafeed ingredients, including ω3 canola oil, because it is well-studied, sensitive to testing, and commercially relevant. Numerous investigations have evaluated fatty acid requirements in Atlantic salmon, and the consequences of inadequate intake are well documented (Ruyter et al. 2000; Sorenson et al. 2021; Sprague et al. 2016 and 2019), allowing these concerns to be targeted in the design of feeding trials. This review focuses on the safety and efficacy of ω3 canola oil as a viable source of ω3 fatty acids in Atlantic salmon.
To evaluate safety and growth of fish fed ω3 canola oil, trials were conducted during each of three phases of the life cycle, i.e., fry/fingerling, grower, and adult to harvest, in varying water temperatures, with different source fish populations, and in both freshwater (FW) and seawater (SW) environments (Table 2). This allowed for robust assessment of safety and suitability of ω3 canola oil for inclusion in Atlantic salmon feed. The overall objective of the five trials reviewed here was to evaluate the influence of feeds containing different combinations of fish oil, vegetable (canola) oil, and ω3 canola oil on fish growth, survival, and tissue fatty acid deposition at various stages of the salmon life cycle or across the entire life cycle. Additional endpoints were included in the respective publications but are not the subject of this summary.
Trial designs and methods
According to trial objectives, ω3 canola oil replaced all or part of the fish oil or all or part of the vegetable oil in the feed. Details on fish oil, ω3 canola oil, and conventional canola oil content are shown in Tables 3, 4, 5, 6, and 7.
Feeds were formulated such that dry ingredients (protein, carbohydrate, minerals, etc.) were held constant within a particular trial, as was total oil content, the only variation being in the oil composition due to the different sources of oils used. Feeds were manufactured in accredited facilities (CSIRO Aquaculture, Bribie Island, QLD, Australia, or Nofima Feed Technology Centre, Bergen, Norway) or, in the case of the on-farm trials in Chile, by commercial feed manufacturers in their Chilean facilities. Feed pellet size varied according to the size of the fish and increased as required through trial progression. Dry ingredients were assembled first, after which the oil mix was vacuum infiltrated into the pellets. The ω3 canola oil used in the first four trials was produced under permit at various locations in Victoria, Australia; ω3 canola oil for the Chile on-farm trials was produced under permit in the USA. All other ingredients were sourced from typical commercial suppliers.
Fish growth and survival were measured in all trials, with feed intake measured when possible. Common fish growth and performance measures included the following:
-
Specific growth rate (%BW d−1): SGR = ln (W2/W1) × (t2 − t1)−1 × 100, where W1 and W2 are live weights (g) at time (days) t1 and t2, respectively
-
Thermal growth coefficient: TGC = (W21/3 − W11/3) × (T × d)−1 × 1000, where (T × d) is the day-degree sum for the period
-
Feed conversion ratio: FCR = feed intake, g/biomass gain, g, where biomass gain is (final biomass) + (biomass of mortalities) − (start biomass)
Because Atlantic salmon is an important food source for human consumption, fillet quality parameters and fatty acid content were also evaluated in many of the studies and, along with growth, are the focus of this review. Total fat content of tissues was measured by conventional techniques (e.g., Folch et al. 1957). Fatty acid composition was determined by high performance liquid chromatography (HPLC) as in Ruyter et al. (2020) and similar publications (e.g., Betancor et al. 2018). Fillet analysis was conducted on the Norwegian Quality Cut (NQC) in all cases. In some trials, astaxanthin content of fillets was measured, also by GC. For brevity, only the ALA, EPA, DHA, and ω6:ω3 ratios in feed and fillets are shown here; full details on fatty acid composition of the diets and various tissues can be found in the relevant publications. Enzyme activity related to physiological stress was measured according to manufacturers’ kit protocols, and gene expression was measured by standard RT-PCR methods or with Nofima’s 44 K salmon gene microarray. Full details on all methods can be found in the relevant publications (Ruyter et al., 2020; Hatlen et al. 2022; Ruyter et al. 2022). Fillets from one Chilean on-farm trial were evaluated for organoleptic parameters according to ISO 8586 (ISO, 2012) by a qualified 8-member taste panel (Dictuc SA, Santiago, Chile).
Growth and performance trials with ω3 canola oil
Fry/fingerling in freshwater (trials 1 and 2)
Feeding trials were conducted to evaluate the effect of two levels of ω3 canola oil or FO on growth, performance, health, and ω3 LCPUFA content in Atlantic salmon fingerling. Separate feeding trials were conducted with ω3 canola oil at two water temperatures, one in Australia at 16 °C and one in Norway at 12 °C. Whole-body fatty acid composition was determined in both trials, and fatty acid composition in the muscle, liver, and erythrocytes was determined in the 12 °C trial. The key lipid components of the four experimental diets for the trials are shown in Table 3; additional details can be found in the study publication (Ruyter et al., 2020). Note that the FO and FM came from different sources in the two trials, resulting in different EPA and DHA contents in the 12 °C and 16 °C diets. Fish meal comprised 79% of the diets at both temperatures, which resulted in a much higher EPA level in the FO diets than in the ω3 canola oil diets (Table 3). In contrast, ALA content was much higher in the ω3 canola oil diets.
There were no differences in weight gain, specific growth rate, or thermal growth coefficient between the dietary groups within each temperature regime (Table 3). SGR at 16 °C was 40% higher than at 12 °C. At 12 °C, whole-body fatty acid composition varied according to diet, with consistently higher levels of ALA and DHA in fish on the ω3 canola oil diets compared to the FO diets (Table 3, comparing low FO with low ω3 canola oil and high FO with high ω3 canola oil). EPA content was higher in the fish on the FO diets, reflecting the higher EPA content of the FO feeds. Whole-body ALA content at 16 °C was higher with the ω3 canola oil diets, but DHA content was slightly higher with the FO diets. There were no differences in EPA content, comparing low FO with low ω3 canola oil and high FO with high ω3 canola oil, again reflecting the EPA content of the feeds. At both temperatures, the ω6:ω3 ratio was consistently lower in fish raised on the ω3 canola oil diets compared to FO. In general, similar trends were seen in the muscle, liver, and erythrocytes in the 12 °C trial (data not shown here but are available in Ruyter et al. (2020)), although DHA levels tended to be similar from the ω3 canola oil and FO diets.
Salmon health was also assessed in the 12 °C trial by examining intestinal morphology, analyzing expression of genes associated with stress and toxicity as well as enzyme activities related to oxidative stress in organs particularly sensitive to harmful effects, i.e., liver and intestine. No differences were noted between dietary groups for histology, and no significant differences were demonstrated in expression markers for FA oxidation, FA synthesis, stress, inflammation, or oxidative stress between fish consuming FO vs. ω3 canola oil for either the low or high inclusion groups (Ruyter et al. 2020).
Early seawater growth (trial 3)
This trial was designed to investigate the impact of replacing standard canola oil with ω3 canola oil on growth, composition, and fillet quality of Atlantic salmon in the early phase of growth in seawater (Ruyter et al. 2022). The control feed contained 22% conventional canola oil and 5% FO; ω3 canola oil replaced conventional canola oil in stepwise amounts, resulting in 22% ω3 canola oil and no conventional canola oil in the final diet (Table 4 part a). FO content remained constant for all diets, and EPA+DHA levels increased due to increasing levels of ω3 canola oil inclusion in the feed.
Salmon with an average weight of 466 g were distributed among 12 tanks with 25 fish per tank. The weight of individual fish was measured at startup and upon completion when the fish had reached a final weight of approximately 1.5 kg. On completion of the trial, blood samples were collected from 5 fish per tank, after which the liver, intestine, and heart were removed and weighed.
No significant differences in growth and FCR were observed between dietary groups (Table 4 part a). Survival rates were high in all dietary groups (>98%), and no significant differences were noted for condition factor (K), SGR, or TGC.
Fatty acid composition analysis showed that progressively higher ω3 canola oil inclusion in the diet resulted in increased levels of ALA, EPA, and DHA in the fillets (Table 4 part a). ALA and DHA levels increased from 3.9% and 4.8%, respectively, to over 10% of the total fatty acids, which led to an increase in total EPA + DHA from 6.6 to 13.0% (equivalent to approximately 7.5 mg of EPA + DHA per g of fillet in the control group to 13.7 mg per g of fillet in the 100% ω3 canola oil group). As previously described with this oil, EPA and DHA were similarly incorporated whether from ω3 canola oil or FO (Ruyter et al. 2020). Importantly, total ω6 fatty acid levels in the fillets were reduced with increasing ω3 canola oil levels in the diet, resulting in a significant change in the ω6:ω3 ratio, from 1.48 in the control diet to 0.38 in the 100% ω3 canola oil diet (Table 4 part a).
Fillet quality was also evaluated in this study and showed a significantly redder color in groups receiving the three ω3 canola oil-supplemented diets compared with the control diet (Ruyter et al., 2022), although there was no significant difference in astaxanthin and idoxanthin levels in fillets from the different dietary groups.
Twelve-month growth in SW (trial 4)
To complement the above-described outcomes in young fish in freshwater and seawater, a 12-month feeding experiment was conducted with Atlantic salmon growing from 0.7 to 4.7 kg to determine possible effects of long-term feeding with ω3 canola oil (Hatlen et al. 2022). Specifically, growth, feed utilization, tissue fatty acid content, and slaughter quality of the fish were investigated.
Diets varied in fatty acid composition due to the replacement of conventional canola oil with ω3 canola oil (Table 4 part b). Total fat content of the diets increased over the course of the trial, as pellet size increased, from 32–33% in the smallest feed to 35–36% in the final feed; the diet FA composition shown in Table 4 part b represents the content in the final phase of feeding. Atlantic salmon (130 fish per cage, initial average weight 704 g) were distributed in 9 experimental-size (5×5×5 m) net-cages in seawater (Gildeskål Forskningsstasjon AS, Inndyr, Norway). The fish were weighed individually at the start of the trial and selected within a pre-defined size range of 600–800 g. Slight over-feeding was done by hand and spill was collected daily, counted, and weighed to calculate spill mass. Fish were individually weighed at the start and end of the trial and were bulk weighed throughout the trial to ensure appropriately sized feed pellets were provided. Water temperature, salinity, oxygen concentration, and transparency depth were measured and logged daily. Water temperature varied according to the season, averaging 7.7 °C across the entire trial.
Low mortality was observed in this year-long study. Fifty-two fish, representing 4.4% of the starting population died, of which 19 were fed low, 11 med, and 22 high ω3 canola oil diets. These numbers are too low to conclude a dietary effect on mortality. Most of the mortality occurred in mid-winter (Dec–Jan; n = 27).
Growth and performance measures were similar among dietary treatments (Table 4 part b). The fish grew from 0.7 to 4.7 kg during the study with no significant differences observed in weight gain, SGR, TGC, and FCR among diets.
Consistent with previous studies (Ruyter et al. 2020, 2022), changes in fatty acid composition in the muscle (NQC fillet) reflected diet composition. As the level of ALA and DHA in the diet increased, so did the corresponding levels in the muscle (Table 4 part b). In the final sampling, muscle ALA and DHA contents were 7.7 and 5.2%, respectively, in fish on the low ω3 canola oil diet but increased to 10.5 and 8.1% and to 12.3 and 9.8%, respectively, in the muscle of med and high ω3 canola oil diets. Total ω6 fatty acid content of the muscle decreased with increasing dietary ω3 canola oil inclusion level in the diets, and total ω3 increased, resulting in a major change in the ω6:ω3 ratio in the fillets, from 0.9 in the low ω3 canola oil diet group to 0.4 in in the high ω3 canola oil diet group.
Slaughter and fillet yield were similar between dietary groups (data not shown). Muscle pigmentation was similar for all groups; however, the low ω3 canola oil group had significantly larger and more obvious melanin spots and more muscle segments affected by spots than the med and high ω3 canola oil groups (Fig. 2).
Percentage of fillets with melanin deposits in the different fillet segments in fish fed low, medium, (med), or high ω3 canola oil diets (N = 60 fillets examined/cage; data from Hatlen et al. (2022))
Commercial scale trials in Atlantic salmon (trial 5)
To confirm the feasibility of formulating aquaculture feed with ω3 canola oil in a commercial setting, three trials were conducted independently on Atlantic salmon farms in Chile. Five companies (salmon and feed producers) cooperated to conduct three on-farm trials with a total population of 2.65 million salmon over three fish farms (Silva et al., 2020).
Design details for each of the three trials are summarized in Table 5. Trials 1 and 2 included fish with initial weights of 1.6 kg, whereas trial 3 began with fish post-smolt weighing 150 g. The control diet, fed to half of the cages in each trial, was the standard proprietary formulation typically supplied by each feed company. The test diets fed to the other half of the cages incorporated ω3 canola oil at a level of between 4 and 7% of the feed, resulting in a replacement of fish oil with ω3 canola oil between 30 and 60%. EPA+DHA levels ranged between 1.7 and 2% of the feed in all trials and were held constant in the control and ω3 canola oil diets within each trial. Total fat balance was achieved by removing a portion of the fish oil and a portion of the vegetable oil such that the total EPA+DHA concentrations remained the same between the control and experimental diets.
Growth and welfare were monitored by weight gain, FCR, SGR, and survival. Biological feed conversion ratio (FCRb), economic feed conversion factor (FCRe), and TGC were also determined. Fatty acid analysis (gas chromatography) and astaxanthin content (HPLC) of the NQC fillet and color expression in the fillets (SalmoFan™) were determined by validated methods in accredited independent commercial laboratories in Chile (SGS Chile Ltda., Puerto Varas, Chile, and Tracelab, Puerto Montt, Chile).
Sustainability indicators were also calculated:
-
Fish inclusion factor in feed × FCR (FIFO) where fish inclusion factor in feed = [fish meal level in diet (%) + fish oil level in diet (%)]/[wild fish meal yield (%) + wild fish oil yield (%)], and FCR = amount of feed consumed − [total weight of harvested fish − weight of smolts] (all in tons).
-
Forage fish dependency ratio (FFDRo) [(% of fish oil in feed from forage fisheries) × FCR)/(oil yield)]. Fishmeal yield was estimated at 22.5% and fish oil 5.0%.
Productive performance (growth, weight gain, FCR) showed no significant differences between the control and ω3 canola oil diets (Table 6). There was a difference, however, in the level of mortality, which influenced the economic feed conversion factor (FCRe). In trials 1, 2, and 3, there was 1.49%, 1.90%, and 1.87% lower mortality (statistically significant at P < 0.05 in trial 2), respectively, in the cages fed with ω3 canola oil compared to the control diets; in all cases, this led to a reduction in FCRe in fish on the ω3 canola oil diets.
The composition of the main fatty acids of interest (ALA, EPA, DHA) as g/100g NQC are shown in Table 7. In all three trials, ALA and DHA contents were significantly higher in fillets of fish fed the ω3 canola oil diets compared with the control diets. In trials 1 and 2, EPA content was significantly lower in fish on the ω3 canola oil diets. However, in the full cycle trial from 150 g smolt to harvest (trial 3), no statistically significant difference in the EPA level between diets was observed. There were no differences in the sum of EPA+DHA between the two diets in any trial. In all three trials, ω3 FA content was higher in fish fed the ω3 canola oil-containing diet; this was reflected in a statistically significant decrease in the ω6:ω3 ratio in all trials.
There were no differences in the weight of the fillets between diets in trial 2 (the only trial in which this was measured; data not shown). There were no significant differences in astaxanthin content between the control diet and the ω3 canola oil-containing diet in any of the trials. Fillet color was also equal in fish raised on the two diets, although there was a tendency for slightly more fillets to be in the “high” color range in fish fed the ω3 canola oil diets (data not shown).
Finally, sensory panel evaluation revealed no differences in samples of raw salmon between those fed the ω3 canola oil-containing diet and the control diet except for juiciness, which was statistically increased for the ω3 canola oil group (P = 0.01, Figure S1).
Discussion
The objectives of the trials reported here were threefold: first, to evaluate the safety of ω3 canola oil as an ingredient in Atlantic salmon feed; second, to determine efficacy of this oil as an alternative energy source for fish growth; and third, to evaluate it as a source of key fatty acids for tissue deposition. Collectively, the trials encompassed a wide range of fish sizes, from fry to full-weight harvest size, under both controlled conditions (indoor tanks), sea cages, and full-size pens on commercial farms. Some of the diets may not be of immediate commercial relevance but were designed to examine the safety of ω3 canola oil when included at high levels in the diet.
In summary, the studies reviewed here confirm that partial or full replacement of FO with a sustainable, canola-based ω3 oil source is safe and supportive of growth and development of Atlantic salmon and confirms tissue deposition of beneficial long-chain fatty acids essential to fish and human health. Additionally, enhanced quality parameters (i.e., reduced melanin markings) point to a potential consumer acceptance benefit. The confirmation of these results in long-term trials on commercial farms supports the use of ω3 canola oil as an ingredient in Atlantic salmon feed.
No differences were observed in growth of Atlantic salmon raised on ω3 canola oil diets compared to FO-based diets (Tables 3, 4, and 6). This held true for fry undergoing rapid early growth and development (10–20-fold weight increase) and post-smolt seawater growth to full maturity. Even at high inclusion levels of ω3 canola oil, growth was not compromised, and weight gain, SGR, and TGC were consistently equivalent in fish fed the control and ω3 canola oil diets. The results of tank trials were confirmed in a 12-month sea cage trial and on commercial farms in Chile. Similar results were reported from shorter-term (11 or 12 week) trials with post-smolt Atlantic salmon on a diet containing transgenic camelina oils (Betancor et al., 2016a, 2016b, 2018). In a separate trial, no differences in growth and survival were observed in Atlantic salmon fed diets containing ω3 or conventional canola oil during the smoltification process (Hatlen, unpublished).
No detrimental health effects were observed in salmon consuming ω3 canola oil, at any growth stage. Various analyses, including histology, skin strength, enzyme activities associated with stress metabolism, and gene expression, consistently showed no difference between fish fed a diet containing ω3 canola oil and a conventional FO diet. Where there were differences, for example in expression of genes related to lipid metabolism, the differences were associated with the levels of lipid in the diet, not with the source (e.g., Ruyter et al. 2020). Survival was high in all trials, again with no difference between fish fed an ω3 canola oil diet compared to a typical FO diet. Interestingly, survival was slightly higher in ω3 canola oil-fed fish in the three on-farm trials in Chile (Table 6; statistically significant in trial 2), suggesting a possible benefit to fish supplied with a higher level of ω3 fatty acids and/or a reduced ω6:ω3 ratio.
Melanin deposits, thought to be foci of chronic inflammation in muscle cells, have previously been shown to be reduced with increasing dietary EPA&DHA (Lufti et al., 2022; Sissener et al., 2016). In accordance with these findings, melanin spots were reduced in fish supplied high ω3 canola diets in the long-term sea cage trial (Hatlen et al. 2022). Fish raised on the high ω3 canola oil diet in the early SW trial discussed here had a higher level of anti-inflammatory resolvins in the plasma (Ruyter et al. 2022), suggesting an additional benefit of high ω3 LCPUFA inclusion in the diet. The anti-inflammatory effects of these fatty acids could be a plausible explanation for the reduced dark spots observed. These results point to an important fillet quality benefit from higher EPA+DHA diets, beyond the higher levels of ω3 LCPUFAs available to consumers of the fillets.
In agreement with many other comparable reports (e.g., Betancor et al. 2018; Lufti et al. 2022; Bou et al. 2017; Sanden et al. 2011), the key ω3 fatty acids ALA, EPA, and DHA were deposited in fillets, with the level of incorporation correlating well with the level in the feed (Tables 4 and 7). In general, this relationship held true for whole-body and/or tissue FA content across multiple trials with fish of different ages and growing under different conditions. Combined data from two of the trials reported here shows that DHA was incorporated into muscle tissue in a dose-dependent manner (Fig. 3), similar to previously published results (e.g., Bou et al. 2017). Therefore, the fish and consumers of these fish are likely to benefit from higher inclusion levels of oils rich in ω3 FAs in the diet. In all cases, higher inclusion of ω3 canola oil in the feed resulted in fillets (and other tissues) with lower ω6:ω3 ratios, again speaking to the benefit of including an ω3-rich oil in the feed.
The minimum requirement and optimal level of LCPUFAs in Atlantic salmon diets are not well defined and likely vary according to growth stage, growing conditions, and other lipid components in the diet (Glencross, 2009). Sissener et al. (2016) found no benefit from 8% EPA+DHA of total lipid in the diet of Atlantic salmon growing in sea cages compared to 5% (ca. 2.1 vs. 1.3 EPA+DHA in the diet). Another study found no growth benefit to including EPA and DHA in the diets of Atlantic salmon growing in tanks from ca. 50 to 190 g (Emery et al. 2016). In contrast, other studies have shown a benefit to higher levels of EPA+DHA, on both growth and health markers (Hixson et al., 2017; Rosenlund et al. 2016; Lufti et al. 2022; Bou et al. 2017). For example, Lutfi et al. (2022) reported improved growth and fillet quality in Atlantic salmon on a 3.5% EPA+DHA diet compared to lower levels (1.0 to 1.6%). Feed inclusion rates of EPA+DHA at 3.5% resulted in higher final weights, improved internal organ health and external welfare indicators, better fillet quality (higher visual color score), and higher EPA and DHA contents in tissues at the end of the trial. It is likely that the requirement for LCPUFAs may be reduced in smaller fish growing under ideal conditions but becomes higher when the entire life cycle is considered, and fish are exposed to more challenging growing conditions.
The results summarized here suggest that the relatively low level of EPA in ω3 canola oil (ca. 0.5%) did not compromise fish health or growth compared to a standard FO diet. Previous reports have indicated that under certain conditions, EPA may not be required in salmonid diets (Emery et al., 2016; Hixson et al. 2017; Bou et al. 2017), and evidence has been presented showing that ALA can be forward converted to EPA and DHA when either of these ω3 LCPUFAs is in short supply. Bou et al. (2017) also reported some conversion of DHA to EPA in Atlantic salmon fed a diet containing high DHA but no EPA, suggesting an ability to alter FA metabolism (via β-oxidation in this case) to compensate for an imbalance in ω3 LCPUFAs in the diet. Ruyter et al. (2022) reported forward conversion of ALA to EPA in hepatocytes of Atlantic salmon raised on an ω3 canola oil diet, suggesting that the low EPA content can in part be overcome by forward conversion of ALA. The high ALA content of ω3 canola oil may therefore contribute to a higher EPA content in the fillets than would be predicted from the dietary inclusion level alone.
The fillets produced in these trials constitute an important source of EPA and DHA for human consumption. Current global recommendations for EPA+DHA range from 250 to 1000 mg/day (Panchal and Brown, 2021). The European Food Safety Authority (EFSA, 2009) has proposed a 250 mg/day intake of ω3 LCPUFAs (EPA+DHA), in accordance with the available evidence on the relationship between intake of these fatty acids and cardiovascular health in healthy populations. Other agencies have recommended higher levels, e.g., 500 mg/day (ISSFAL, 2004) or 1000 mg/day (Ministry of Health, Labor and Welfare of Japan, 2014). At the level of 250 mg/day, a 100-g portion of fish from the three on-farm trials described here would cover the EPA+DHA requirement for 2.3, 4.2, and 5.4 days (trials 1, 2, and 3, respectively). The levels of EPA+DHA in the feed in these trials were between 1.7 and 2.0% of the diet. Higher inclusion rates of ω3 canola oil or other sources of these FAs to increase EPA+DHA levels in feed could benefit fish health and support human health by increasing the amount of these key fatty acids in the fillets.
A recent report (Rocker et al. 2022) suggests that the current supply of FO is sufficient to sustain the continued growth of Atlantic salmon aquaculture at 2% per year until the end of the century. However, this is based on relatively low FO inclusion levels (3% FO, equivalent to ca. 0.75% LCPUFA content), which would require slightly over three 100 g portions of salmon per week to supply consumers with the recommended intake levels. Such a low level of LCPUFA in the feed is almost certainly sub-optimal for fish health and productivity and compromises the value of salmon as good source of nutritional fatty acids for human consumption. Maintaining FO content at 10% of the feed would support 3% annual growth only until 2031, suggesting a shortfall in marine derived LCPUFAs could occur within the next decade.
Tocher et al. (2019) calculated that EPA+DHA intake of 500 g/person/day would require ~1.27 million ton/year of ω3 LCPUFA for a population of approximately 7 billion. This is much more than is currently available and would result in a deficit, based on various estimates, between 0.4 and >1 M tons. This shortfall requires alternative solutions, and it has been argued that new sources of LCPUFAs can play an important role in increasing the total supply available to consumers (Tocher et al. 2019; Napier et al. 2019). Others have suggested that novel feed sources are required to reduce pressure on forage fish typically relied on as a source of ω3-LCPUFAs for aquaculture (Cottrell et al., 2020). Given the finite supply of FO and the possibility of further reductions in the allowable catch of forage fish to prevent over-fishing, the potential effects of climate change on the oceanic supply of microalgal LCPUFA (Hixson and Arts, 2016; Kang, 2011) and the unlikely scenario that salmon consumption will increase to three servings per week, the importance of alternative sources of ω3 LCPUFAs for fish and human nutrition should not be underestimated. Because salmon feed supplemented with ω3 canola oil has been consistently demonstrated to result in tissue deposition of ω3 fatty acids in fillets, this oil represents an important means of ensuring one of the most highly cultivated and consumed fish in the human diet remains a meaningful source of these FAs.
Regarding safety of this oil to human consumers, a placebo-controlled, 16-week clinical trial in adults demonstrated high availability of DHA from encapsulated ω3 canola oil and improved blood LC-ω3 profiles with no negative effects (Lin et al. 2022). Similar results were reported for EPA and DHA incorporation into blood lipids from a short-term (8 h) trial using transgenic camelina oil containing EPA and DHA (West et al. 2020). Together, these results indicate that plant-derived oils can be an important source of ω3 LCPUFAs beneficial for human health. To this end, ω3 canola oil has been approved for use in human food in Australia, Canada, and the USA (MacIntosh et al. 2021).
Challenges exist in providing adequate ω3 fatty acids to support the growing global population. Terrestrial systems offer a sustainable alternative to our dependence on marine sources. For example, assuming a canola seed yield of 1 ton/ha, 40% oil content, and 10% DHA in the oil, 1 ha can yield approximately 100 kg DHA. In comparison, assuming oily fish contain 10% oil, at 12% DHA, 10,000 kg of such fish would yield approximately 120-kg DHA. Even at conservative estimates of a crop yield of 0.75 tons/ha and lower fish oil content, as is sometimes observed, 1 ha of the ω3 canola oil crop can produce as much DHA as 5000-kg (5 tons) harvested fish. Continued investment in the development of plant-based sources of ω3 serves as an essential contribution to food security.
References
Aas TS, Ytrestøyl T, Åsgård T (2019) Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: an update for 2016. Aquac Rep 5100216. https://doi.org/10.1016/j.aqrep.2019.100216
Betancor MB, Sprague M, Montero D, Usher S, Sayanova O, Campbell PJ, Napier JA, Caballero MJ, Izquierdo M, Tocher DR (2016a) Replacement of marine fish oil with de novo omega-3 oils from transgenic Camelina sativa in feeds for Gilthead Sea bream (Sparus aurata L.). Lipids 51(10):1171–1191. https://doi.org/10.1007/s11745-016-4191-4
Betancor MB, Sprague M, Sayanova O, Usher S, Metochis C, Campbell PJ, Napier JA, Tocher DR (2016b) Nutritional evaluation of and EPA-DHA oil from transgenic Camelina sativa in feeds for post-smolt Atlantic salmon (Salmo salar L.). PLoS One 11:e0159934. https://doi.org/10.1371/journal.pone.0159934
Betancor MB, Li K, Bucerzan VS, Sprague M, Sayanova O, Usher S, Han L, Norambuena F, Torrissen O, Napier JA, Tocher DR, Olsen RE (2018) Oil from transgenic Camelina sativa containing over 25% n-3 long-chain PUFA as the major lipid source in feed for Atlantic salmon (Salmo salar). Br J Nutr 119:1378–1392. https://doi.org/10.1017/S0007114518001125
Bou M, Berge GM, Baeverfjord G, Sigholt T, Østbye TK, Ruyter B (2017) Low levels of very long-chain n-3 PUFA in Atlantic salmon (Salmo salar) diet reduce fish robustness under challenging conditions in sea cages. J Nutr Sci 6:1–14. https://doi.org/10.1017/jns.2017.28
Cheng B, Wu G, Vrinten P, Falk K, Bauer J, Qiu X (2010) Towards the production of high levels of eicosapentaenoic acid in transgenic plants: the effects of different host species, genes and promoters. Transgenic Res. 19:221–229. https://doi.org/10.1007/s11248-009-9302-z
Cottrell RS, Blanchard JL, Halpern BS, Metian M, Froehlich HE (2020) Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nature Food 1:301–308. https://doi.org/10.1038/s43016-020-0078-x
EFSA (2009) Scientific opinion of the Panel on Dietetic products, Nutrition and Allergies on a request from European Commission related to labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J 1176:1–11. https://doi.org/10.2903/j.efsa.2009.1176
Emery JA, Norambuena F, Trushenski J, Turchini GM (2016) Uncoupling EPA and DHA in fish nutrition: dietary demand is limited in Atlantic salmon and effectively met by DHA alone. Lipids 51:399–412. https://doi.org/10.1007/s11745-016-4136-y
FAO (2019) Top 10 species groups in global aquaculture 2017. Food and Agriculture Organization of the United Nations, Rome. https://www.fao.org/publications/card/en/c/CA5224EN [Accessed 16 November 2022]
FAO (2020) FishStatJ, a tool for fishery statistics analysis. Release: 4.00.10. Universal Software for Fishery Statistical Time Series. Global aquaculture production: Quantity 1950–2018; Value 1950–2018. Global capture production: Quantity 1950–2018; Rome, Italy: FAO. https://www.fao.org/fishery/statistics/software/fishstatj/en [Accessed 16 November 2022]
FAO (2022a) The State of World Fisheries and Aquaculture 2022. Towards blue transformation, Rome, FAO. https://doi.org/10.4060/cc0461en
FAO (2022b) Top 10 species groups in global aquaculture 2020 Factsheet. Food and Agriculture Organization of the United Nations, Rome. https://www.fao.org/fishery/en/publication/295292 [Accessed 16 November 2022]
Folch J, Lees M, Stanley GHS (1957) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Garlock T, Asche F, Anderson J et al (2020) A global blue revolution: aquaculture growth across regions, species, and countries. Rev Fish Sci Aquac 28:107–116. https://doi.org/10.1080/23308249.2019.1678111
Gephart JA, Golden CD, Asche F et al (2021) Scenarios for global aquaculture and its role in human nutrition. Rev Fish Sci Aquac 29:122–138. https://doi.org/10.1080/23308249.2020.1782342
Gia Vo LL, Galkanda-Arachchige HS, Iassonova DR, Davis DA (2021) Efficacy of modified canola oil to replace fish oil in practical diets of Pacific white shrimp Litopenaeus vannamei. Aquac Res 52:2446–2459. https://doi.org/10.1111/are.15094
Glencross BD (2009) Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aquac 1:71–124. https://doi.org/10.1111/j.1753-5131.2009.01006.x
Hatlen B, Larsson T, Østbye T-K, Romarheim OH, Rubio LM, Ruyter B (2022) Improved fillet quality in harvest-size Atlantic salmon fed high n-3 canola oil as a DHA-source. Aquaculture 560:738555. https://doi.org/10.1016/j.aquaculture.2022.738555
Hardy RW & Kaushik SJ eds. 2022 Fish nutrition, 4th edition published by Academic Press. https://doi.org/10.1016/C2018-0-03211-9
Hixson SM, Arts MT (2016) Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Global Change Biol 22:2744–2755. https://doi.org/10.1111/gcb.13295
Hixson SM, Parrish CC, Xue X, Wells JS, Collins SA, Anderson DM, Rise ML (2017) Growth performance, tissue composition, and gene expression responses in Atlantic salmon (Salmo salar) fed varying levels of different lipid sources. Aquaculture 467:76–88. https://doi.org/10.1016/j.aquaculture.2016.04.011
Hong J, Bledsoe JW, Overturf KE, Lee S, Iassonova D, Small BC (2022) LatitudeTM Oil as a sustainable alternative to dietary fish oil in rainbow trout (Oncorhynchus mykiss): effects on filet fatty acid profiles, intestinal histology, and plasma biochemistry. Front Sustain Food Syst 6:837628. https://doi.org/10.3389/fsufs.2022.837628
Hossain MS, Peng M, Small BC (2021) Optimizing the fatty acid profile of novel terrestrial oil blends in low fishmeal diets of rainbow trout (Oncorhynchus mykiss) yields comparable fish growth, total fillet n-3 LC-PUFA content, and health performance relative to fish oil. Aquaculture 545:737230. https://doi.org/10.1016/j.aquaculture.2021.737230
International Organization for Standardization. 2012. Sensory analysis — general guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors (ISO/DIS Standard No. 8586). https://www.iso.org/standard/45352.html [Accessed 5 January 2023]
International Society for the Study of Fatty Acids and Lipids (2004). Report of the sub-committee on recommendations for intake of polyunsaturated fatty acids in healthy adults. [online] Available at: https://www.issfal.org/statement-3 [Accessed December 14, 2022]
Kang JX (2011) Omega-3: a link between global climate change and human health. Biotechnol Adv 29:388–390. https://doi.org/10.1016/j.biotechadv.2011.02.003
Lin XL, Baisley J, Bier A, Vora D, Holub B (2022) Transgenic canola oil improved blood Omega-3 profiles: a randomized placebo-controlled trial in healthy adults. Front Nutr 9:847114. https://doi.org/10.3389/fnut.2022847114
Lutfi E, Berge G, Bæverfjord G, Sigholt T, Bou M, Larsson T, Ruyter B (2022) Increasing dietary levels of the n-3 long-chain PUFA, EPA and DHA, improves the growth, welfare, robustness and fillet quality of Atlantic salmon in sea cages. Brit J Nutr 1-19. https://doi.org/10.1017/S0007114522000642
MacIntosh SC, Shaw M, Connelly M, Yao ZJ (2021) Food and feed safety of NS-B5ØØ27-4 omega-3 canola (Brassica napus): a new source of long-chain omega-3 fatty acids. Front Nutr. 8:716659. https://doi.org/10.3389/fnut.2021.716659
Ministry of Health, Labour and Welfare of Japan (2014) Dietary reference intakes for Japanese (2015 version). Development Review Committee Report excerpt. 2014. [online] Available at: http://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000114399.pdf [Accessed May 27, 2016]
National Institutes of Health (NIH) (2022) Omega 3 fatty acids. Fact Sheets for Health Professionals. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/#en5 [Accessed 16 November 2022]
Napier JA, Olsen RE, Tocher DR (2019) Update on GM canola crops as novel sources of omega-3 fish oils. Plant Biotechnol J 17(4):703–705. https://doi.org/10.1111/pbi.13045
Naylor RL, Hardy RW, Buschmann AH et al (2021) A 20-year retrospective review of global aquaculture. Nature 591:551–563. https://doi.org/10.1038/s41586-021-03308-6
Nichols PD, Glencross B, Petrie JR, Singh SP (2014) Readily available sources of long-chain omega-3 oils: is farmed salmon a better source of the good oil than wild-caught seafood? Nutrients 6:1063–1079. https://doi.org/10.3390/nu6031063
Panchal SK, Brown L (2021) Addressing the insufficient availability of EPA and DHA to meet current and future nutritional demands. Nutrients 13:2855. https://doi.org/10.3390/nu13082855
Petrie JR, Shrestha P, Zhou XR, Mansour MP, Liu Q, Belide S, Nichols PD, Singh SP (2012) Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS One 7:e49165. https://doi.org/10.1371/journal.pone.0049165
Petrie JR, Shrestha P, Belide S, Kennedy Y, Lester G, Liu Q, Divi UK, Mulder RJ, Mansour MP, Nichols PD, Singh SP (2014) Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS One 9(1):e85061. https://doi.org/10.1371/journal.pone.0085061
Petrie JR, Zhou X-R, Leonforte A, McAllister J, Shrestha P, Kennedy Y, Belide S, Buzza G, Gororo N, Gas W, Lester GL, Mansour MP, Mulder R, Liu Q, Tian L, Silva C, Cogan N, Nichols PD, Green A et al (2020) Development of a Brassica napus (canola) crop containing fish oil-like levels of DHA in the seed oil. Front Plant Sci 11:727–741. https://doi.org/10.3389/fpls.2020.00727
Rocker MM, Mock TS, Turchini GM, Francis DS (2022) The judicious use of finite marine sources can sustain Atlantic salmon (salmo salar) aquaculture to 2100 and beyond. Nat Food 3:644–649
Rosenlund G, Torstensen BE, Subhaug I, Usman N, Sissener NH (2016) Atlantic salmon require long-chain n-3 fatty acids for optimal growth throughout the seawater period. J Nutr Sci. https://doi.org/10.1017/jns.2016.10
Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77(2):198–208. https://doi.org/10.1111/tpj.12378
Ruiz-Lopez N, Haslam RP, Usher S, Napier JA, Sayanova O (2015) An alternative pathway for the effective production of the omega-3 long-chain polyunsaturates EPA and ETA in transgenic oilseeds. Plant Biotech J 13:1264–1275. https://doi.org/10.1111/pbi.12328
Ruyter B, Røsjø C, Einen O, Thomassen MS (2000) Essential fatty acids in Atlantic salmon: time course of changes in fatty acid composition of liver, blood and carcass induced by a diet deficient in n-3 and n-6 fatty acids. Aquac Nutr 6:109–117. https://doi.org/10.1046/j.1365-2095.2000.00137.x
Ruyter B, Sissener NH, Østbye T-K, Simon CJ, Krasnov A, Bou M, Sanden M, Nichols PD, Lutfi E, Berge GM (2020) n-3 canola oil effectively replaces fish oil as a new safe dietary source of DHA in feed for juvenile Atlantic salmon. Br J Nutr 122:1329–1345. https://doi.org/10.1017/S0007114519002356
Ruyter B, Bour M, Berge GM, Mørkøre S, NH, Sanden M, Lutfi, E, Romarheim O-H, Krasnov A, and Østbye T-K K. (2022) A dose-response study with omega-3 rich canola oil as a novel source of docosahexaenoic acid (DHA) in feed for Atlantic salmon (Salmo salar) in seawater; effects on performance, tissue fatty acid composition, and fillet quality. Aquaculture 561:738733. https://doi.org/10.1016/j.aquaculture.2015.01.010
Sanden M, Stubhaug I, Berntssen MHG, Lie Ø, Torstensen BE (2011) Atlantic salmon (Salmo salar L.) as a net producer of long-chain marine ω3 fatty acids. J Agr Food Chem 59:12697–12706
Silva S, Berner P, Devine M, Boettner B. 2020. Applied research on the use of Aquaterra® a new source of omega-3 for use in salmon feed. Salmonexpert 2020/85 p.58-67. https://www.salmonexpert.cl/edicin-85-revista-salmonexpert/1223832 [accessed 13 March 2023, in Spanish]
Simopoulos AP (2010) The omega-6/omega-3 fatty acid ratio: health implications. Oléagineux Corps Gras Lipides 17:267–275. https://doi.org/10.1051/ocl.2010.0325
Sissener NH, Torstensen BE, Stubhaug I, Rosenlund G (2016) Long-term feeding of Atlantic salmon in seawater with low dietary long-chain n-3 fatty acids affects tissue status of the brain, retina and erythrocytes. Br J Nutr 115:1919–1929. https://doi.org/10.1017/S0007114516000945
Sprague M, Dick JR, Tocher R (2016) Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006-2015. Sci Rep 6:21892. https://doi.org/10.1038/srep21892
Sprague M, Xu G, Betancor MB, Olsen RE, Torrisen O, Glencross BD, Tocher DR (2019) Endogenous production of n-3 long-chain PUFA from first feeding and the influence of dietary linoleic acid and the α-linolenic:linoleic ratio in Atlantic salmon (Salmo salar). Br J Nutr 122:1091–1102. https://doi.org/10.1017/S0007114519001946
Sørensen SL, Park Y, Gong Y, Vasanth GK, Dahle D, Korsnes K, Phuong TH, Kiron V, Øyen S, Pittman K, Sørensen M (2021) Nutrient digestibility, growth, mucosal barrier status, and activity of leucocytes from head kidney of Atlantic Salmon fed marine- or plant- derived protein and lipid sources. Front Immunol 11:623726. https://doi.org/10.3389/fimmu.2020.62372
Tocher DR, Betancor MB, Sprague M, Olsen RE, Napier JA (2019) Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: bridging the gap between supply and demand. Nutrients 11(1):89. https://doi.org/10.3390/nu11010089
Tocher DR (2015) Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449:94–107. https://doi.org/10.1016/j.aquaculture.2015.01.010
Tacon AGJ, Metian M, McNevin AA (2022) Future feeds: suggested guidelines for sustainable development. Rev Fish Sci Aquac 30(2):135–142. https://doi.org/10.1080/23308249.2020.1860474
Walsh TA, Bevan SA, Gachotte DJ, Larsen CM, Moskal WA, Merlo PA, Sidorenko LV, Hampton RE, Stoltz V, Pareddy D, Anthony GI, Bhaskar PB, Marri PR, Clark LM, Chen W, Adu-Peasah PS, Wensing ST, Zirkle R, Metz JG (2016) Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat Biotechnol 34(8):881–887. https://doi.org/10.1038/nbt.3585
West AL, Miles EA, Lillycrop KA, Napier JA, Calder PC, Burdge GC (2020) Genetically modified plants are an alternative to oily fish for providing n-3 polyunsaturated fatty acids in the human diet: a summary of the findings of a Biotechnology and Biological Sciences Research Council funded project. Nutr Bull. https://doi.org/10.1111/nbu.12478
Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol 23:1013–1017. https://doi.org/10.1038/nbt1107
Yilmaz JL, Lim ZL, Beganovic M, Breazeale S, Andre C, Stymne S, Vrinten P, Senger T (2017) Determination of substrate preferences for desaturases and elongases for production of docosahexaenoic acid from oleic acid in engineered canola. Lipids 52:207–222. https://doi.org/10.1007/s11745-017-4235-4
Ytrestøyl T, Aas T, Åsgård T (2015) Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 228:365–374. https://doi.org/10.1016/j.aquaculture.2015.06.023
Funding
Details regarding financial considerations for each study may be found in the corresponding publications. The commercial trials were funded by the companies involved with an in-kind donation of ω3 canola oil by Nuseed Nutritional US, Inc.
Author information
Authors and Affiliations
Contributions
BAD and MDD wrote the main manuscript. BAD prepared the figures and tables. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
MDD was a former consultant to and BAD is a current employee of Nuseed Nutritional US, Inc.
Additional information
Handling editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davis, B.A., Devine, M.D. Evaluation of long-chain omega-3 canola oil on Atlantic salmon growth, performance, and essential fatty acid tissue accretion across the life cycle: a review. Aquacult Int 31, 2559–2579 (2023). https://doi.org/10.1007/s10499-023-01099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01099-3