Abstract
Omega-3 (n-3) long-chain polyunsaturated fatty acids (LC-PUFA) are essential components of the diet of all vertebrates. The major dietary source of n-3 LC-PUFA for humans has been fish and seafood but, paradoxically, farmed fish are also reliant on marine fisheries for fish meal and fish oil (FO), traditionally major ingredients of aquafeeds. Currently, the only sustainable alternatives to FO are vegetable oils, which are rich in C18 PUFA, but devoid of the eicosapentaenoic (EPA) and docosahexaenoic acids (DHA) abundant in FO. Two new n-3 LC-PUFA sources obtained from genetically modified (GM) Camelina sativa containing either EPA alone (ECO) or EPA and DHA (DCO) were compared to FO and wild-type camelina oil (WCO) in juvenile sea bream. Neither ECO nor DCO had any detrimental effects on fish performance, although final weight of ECO-fed fish (117 g) was slightly lower than that of FO- and DCO-fed fish (130 and 127 g, respectively). Inclusion of the GM-derived oils enhanced the n-3 LC-PUFA content in fish tissues compared to WCO, although limited biosynthesis was observed indicating accumulation of dietary fatty acids. The expression of genes involved in several lipid metabolic processes, as well as fish health and immune response, in both liver and anterior intestine were altered in fish fed the GM-derived oils. This showed a similar pattern to that observed in WCO-fed fish reflecting the hybrid fatty acid profile of the new oils. Overall the data indicated that the GM-derived oils could be suitable alternatives to dietary FO in sea bream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish is considered as the main source of the beneficial omega-3 (n-3) long-chain polyunsaturated fatty acids (LC-PUFA) eicosapentaenoic and docosahexaenoic acids, EPA (20:5n-3) and DHA (22:6n-3), respectively. These LC-PUFA play important roles in neural development, immune and inflammatory responses as well as having beneficial effects in certain pathologies such as those affecting the cardiovascular and neurological systems or some types of cancers [1–5]. According to estimations of the International Society for the Study of Fatty acids and Lipids (ISSFAL), a daily intake of 500 mg of EPA and DHA is recommended for optimum cardiovascular health [6]. In a world where the global population is expected to grow to reach 9.6 billion people by 2050 [7], more than 1.7 million metric tonnes of EPA + DHA would be necessary to cover annual human requirements. This quantity is not met by the actual total global supply of n-3 LC-PUFA and thus there is a large gap between supply and demand [8, 9].
Farmed fish and seafood accounted for 44.1 % of total production (including for non-food uses) from capture fisheries and aquaculture in 2014, up from 42.1 % in 2012 and 31.1 % in 2004 [7]. Although fish farming contributes to the global n-3 LC-PUFA production in order to meet human dietary requirements, the marine ingredients, fish meal and oil (FM and FO, respectively) are still, almost exclusively, the only raw materials in aquafeeds that, in turn, can supply n-3 LC-PUFA to farmed fish. The use of high levels of FM and FO to maintain n-3 LC-PUFA levels in farmed fish is not a sustainable practice as they are finite (on an annual basis) and limited resources [9]. Sustainable alternatives to FO used at present are vegetable oils (VO), which are rich in C18 but lack n-3 LC-PUFA, which in turn reduces the proportions of EPA and DHA in farmed fish, and does not significantly increase global production of n-3 LC-PUFA [9]. Obtaining alternative sources of n-3 LC-PUFA from other marine organisms such as microalgae or zooplankton (krill or copepods) poses significant technological and economic challenges and there are no currently feasible alternatives for mass supply [9]. Therefore, it is clear that completely new, de novo sources of EPA and DHA are required. In this respect, metabolic engineering of oilseed crops such as Camelina sativa or false flaxseed provides a currently viable option to deliver n-3 LC-PUFA in the place of fish oil [10–12]. By the insertion of cassettes containing five or seven fatty acyl desaturase and elongase genes from several algae species, genetically modified (GM) Camelina is capable of producing EPA or both EPA and DHA in their seeds [10]. Recent studies have successfully demonstrated the feasibility of using both the high-EPA and EPA + DHA oils as substitutes for FO in feeds for post-smolt Atlantic salmon (Salmo salar) without compromising fish growth or health [13–15].
Total replacement of FO in salmonids has been shown to be feasible without compromising fish performance, although a reduction in tissue n-3 LC-PUFA was observed due to reduced dietary intake and limited biosynthesis [16–18]. In contrast, total substitution of FO by VO in gilthead sea bream (Sparus aurata) feeds significantly reduced fish performance [19] and greatly altered fatty acid profile of bream tissues [20] given their incomplete LC-PUFA biosynthesis pathway [21, 22]. In this context, the aim of the present study was to evaluate the new GM Camelina-derived oils that contain features of both marine fish (n-3 LC-PUFA) and terrestrial plant (high levels of C18 fatty acids) oils as substitutes for FO in feeds for a marine teleost species that have a very limited LC-PUFA biosynthesis capacity from C18 PUFA [22]. The specific objectives were to evaluate the efficacy of the high-EPA and EPA + DHA oils as replacements for dietary FO in feeds for gilthead sea bream juveniles in terms of growth, feed efficiency, health and welfare, and nutritional quality of the fish focussing particularly on tissue levels of EPA and DHA. Additionally, assessment of the expression of genes of lipid metabolism as well as molecular markers of health and immune function was also performed.
Materials and Methods
Diets and Feeding Trial
Four isonitrogenous and isoenergetic diets were formulated to contain 50 g/kg crude protein and 11 g/kg crude lipid, and manufactured at BioMar Tech-Centre (Brande, Denmark). The four feeds were produced by vacuum coating identical dry basal extruded pellets with either fish oil (FO), wild-type Camelina oil (WCO), EPA-Camelina oil (ECO) or EPA + DHA-Camelina oil (DCO) and were named according to the oils used (Table 1). ECO and DCO oils were produced as previously described [10, 13, 15]. In line with commercial practice, non-defatted fishmeal was employed as the major protein source to ensure EFA requirements were met [23], and yttrium oxide was added (0.5 g/kg) as an inert marker for calculation of lipid and fatty acid digestibility.
A total of 420 juvenile gilthead sea bream with an average body weight of 55.5 ± 0.4 g (mean ± SD) were distributed into 12 seawater tanks (35 per tank) and fed one of the four experimental feeds in triplicate for 11 weeks. The experimental system comprised 500 L tanks supplied by flow-through seawater (9 L/min) at ambient temperature that averaged 20.3 ± 0.6 °C. Experimental feeds were delivered until apparent satiation by hand-feeding three times a day with uneaten feed collected 30 min later in order to determine accurate feed efficiency. Collected feed was placed in an oven (110 °C) until constant weight was achieved in order to calculate feed intake based on initial moisture content. All procedures were conducted in accordance with the regulations set forward by the Spanish RD 53/2013 (BOE 8th February 2013) and the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. The experiment was subjected to ethical review by the Animal Welfare and Bioethical Committee at the University of Las Palmas de Gran Canaria (Ref 007/202 CEBA ULPGC).
Sample Collection and Digestibility
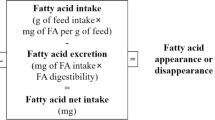
After 11 weeks of feeding, fish were not fed for 48 h prior to being sampled. All fish were anaesthetised with clove oil and weighed and their length measured. Ten fish for biometric measurements (hepato-somatic and viscero-somatic indices) and tissue analyses were killed by overdose with clove oil. Samples of anterior intestine, liver, gills, flesh and brain from 3 fish per tank were immediately frozen and stored at −70 °C prior to total lipid extraction and fatty acid analyses. Further samples of liver and anterior intestine were collected from six fish per treatment (two per tank) and stabilised in RNAlater® (Sigma, Poole, UK) prior to RNA extraction. All remaining fish were fed for a further week prior to faeces being collected according to the method described by [24]. Briefly, fish were killed by overdose with clove oil 7 h after being fed and faecal samples collected after dissecting the rectum of the fish. Faecal samples were pooled by tank and stored −20 °C prior to lipid and fatty acid analysis. The apparent digestibility coefficient (ADC) of lipid and selected fatty acids was calculated as: 100 − [100 × (Y2O3 concentration in feed/Y2O3 concentration in faeces) × (lipid or fatty acid concentration in faeces/lipid or fatty acid concentration in feed)]. The concentration of individual fatty acids in diets and faeces were calculated based on the relative proportion of each fatty acid compared with a known amount of the internal standard (17:0) added and the total lipid content determined in the samples. Yttrium was estimated after acid digestion of the samples via Inductively Coupled Plasma Mass Spectrometry (Thermo Scientific, XSeries2 ICP-MS, USA) using argon and hydrogen as carrier gas.
Proximate Composition
Diets and whole fish were ground before determination of proximate composition according to standard procedures [25]. Fish were pooled per tank (n = 3) and freeze-dried until further analysis whereas three technical replicates of feeds (single batch production) were analysed. Moisture contents were obtained after drying in an oven at 110 °C for 24 h and ash content determined after incineration at 600 °C for 16 h. Crude protein was measured by determining nitrogen content (N × 6.25) using automated Kjeldahl analysis (Tecator Kjeltec Auto 1030 analyser, Foss, Warrington, UK) and crude lipid content determined gravimetrically after Soxhlet lipid extraction (Tecator Soxtec system 2050 Auto Extraction apparatus).
Faeces and Tissue Lipid Content and Fatty Acid Composition
Samples of faeces, muscle (flesh), liver, gills, anterior intestine and brain from three fish per tank were prepared as pooled homogenates (n = 3 per treatment) and total lipid extracted by homogenising in chloroform/methanol (2:1, v/v) using an Ultra-Turrax tissue disrupter (Fisher Scientific, Loughborough, UK), with content determined gravimetrically [26]. Fatty acid methyl esters (FAME) were prepared from total lipid by acid-catalysed transesterification at 50 °C for 16 h [27], and FAME extracted and purified as described previously [28]. FAME were separated and quantified by gas-liquid chromatography using a Fisons GC-8160 (Thermo Scientific, Milan, Italy) equipped with a 30 m × 0.32 mm i.d. × 0.25 μm ZB-wax column (Phenomenex, Cheshire, UK), on-column injector and a flame ionisation detector. Data were collected and processed using Chromcard for Windows (version 2.01; Thermoquest Italia S.p.A., Milan, Italy). Individual FAME were identified by comparison to known standards and published data [28] and results expressed as mole percentage.
Histological Analysis
Samples of liver and intestine from 2 fish per tank (n = 6 per treatment) were fixed in 4 % buffered formalin dehydrated through graded alcohol, then xylene, and finally embedded in paraffin wax. The paraffin blocks were sectioned at 3 μm and stained with haematoxylin and eosin [29] before blind examination under a light microscope. Stained sections of liver were assessed for cytoplasmic lipid vacuolization and peripancreatic fat infiltration using a four graded examination scheme: 0, not observed; 1, few; 2, medium; 3, severe. Posterior intestine sections were examined for integrity of the intestinal mucosa and the presence of any inflammatory response.
RNA Extraction
Liver and anterior intestine from six individual fish per dietary treatment were homogenised in 1 ml of TriReagent® (Sigma-Aldrich, Dorset, UK) RNA extraction buffer using a bead tissue disruptor (Bio Spec, Bartlesville, Oklahoma, USA). Total RNA was isolated following the manufacturer’s instructions and quantity and quality determined by spectrophotometry using a Nanodrop ND-1000 (Labtech Int., East Sussex, UK) and electrophoresis using 250 ng of total RNA in a 1 % agarose gel. cDNA was synthesised using 2 μg of total RNA and random primers in 20 μl reactions and the High capacity reverse transcription kit without RNase inhibiter according to the manufacturer’s protocol (Applied Biosystems, Warrington, UK). The resulting cDNA was diluted 20-fold with milliQ water. Expression of genes of interest was determined by quantitative PCR (qPCR) from fish fed all diets (Table 2). Results were normalised using reference genes, elongation factor 1α (elf1α) and beta-actin (actb), as their expression did not vary among treatments. The efficiency of the primers for each gene was previously evaluated by serial dilutions to ensure that it was close to 100 %. qPCR was performed using a Biometra TOptical Thermocycler (Analytik Jena, Goettingen, Germany) in 96-well plates in duplicate 20-μl reaction volumes containing 10 μl of Luminaris Color HiGreen qPCR Master Mix (Thermo Scientific, Hemel Hempstead, UK), 1 μl of the primer corresponding to the analysed gene (10 pmol), 3 μl of molecular biology grade water and 5 μl of cDNA, with the exception of the reference genes, which were determined using 2 μl of cDNA. In addition amplifications were carried out with a systematic negative control (NTC-non template control) containing no cDNA. Standard amplification parameters contained a UDG pre-treatment at 50 °C for 2 min, an initial denaturation step at 95 °C for 10 min, followed by 35 cycles: 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C.
Tracking of the nptII Gene in Gilthead Sea Bream Liver, Intestine and Muscle
Genomic DNA was extracted from fish flesh, pyloric caeca and liver using REALPURE extraction kit (Valencia, Spain) according to the manufacturer’s instructions. Briefly, tissue samples were incubated in 300 μl of lysis solution overnight at 55 °C with 3 μl of Proteinase K. Following the incubation, samples were cooled down and RNase treatment performed (37 °C for 60 min). After protein precipitation, DNA was precipitated by adding 600 μl of isopropanol and hydrated with 5 mM Tris. Total DNA was quantified by spectrophotometry and quality determined by electrophoresis as described above. Two primers pairs targeting an endogenous sea bream gene (cytochrome b; cytb) and a transgene marker for ECO – plants (Kanamycin resistance gene, nptII) were used (Table 2). Fifty ng of extracted DNA was used in PCR amplifications that were performed in a final volume of 10 μl, containing 5 μl of MyTaq™ HS Mix (Bioline, London, UK). Each set of PCR included a positive control (DNA from genetically modified-Camelina) and a non-template control (NTC).
Statistical Analysis
All data are mean ± SE (n = 3) unless otherwise specified. Percentage data were subjected to arcs in square-root transformation prior to statistical analyses. Data were tested for normality and homogeneity of variances with Levene’s test prior to one-way analysis of variance followed by a Tukey–Kramer HSD multiple comparisons of means. A non-metric multidimensional scaling plot (NMDS) was performed in order to separate the fatty acid profile of the five evaluated tissues. Stress values <0.05 indicated an excellent representation of the clusters and <0.1 and <0.2 indicated good and potentially useful plots respectively. All statistical analyses were performed using SPSS software (IBM SPSS Statistics 19; SPSS Inc., Chicago, IL, USA), excepting the NMDS analysis (PAST) [30].
Results
Fish Performance
Sea bream fed all the experimental diets more than doubled in weight after 11 weeks feeding, and no mortalities were recorded (Table 3). Fish fed ECO displayed the lowest final weight and total length among the dietary treatments, although not different to fish fed WCO, with highest growth achieved in fish fed the FO and DCO feeds (Table 3). Final weights reflected feed intake, which was highest in fish fed FO and DCO, lowest in fish fed ECO and intermediate in fish fed WCO. There were no significant differences in weight gain or specific growth rate (SGR) other than it being slightly lower in fish fed ECO compared to fish fed FO (Table 3). There were no differences in the hepatosomatic or viscerosomatic index (HSI and VSI, respectively), although values tended to be lowest in fish fed DCO and ECO (p = 0.576 and 0.869 respectively). No differences were observed in other fish performance parameters including feed conversion ratio (FCR) or k condition factor (Table 3).
Lipid and Fatty Acid Digestibility
The apparent digestibility coefficients (ADC) were calculated for lipids and fatty acids using yttrium oxide as an inert marker. There were no significant differences in crude lipid ADC among the dietary treatments (Table 4). Figure 1 represents the analysed fatty acid composition of feeds and faeces in order to compare which fatty acid groups were preferentially digested and absorbed (i.e. those found in lower amounts in faeces). There was a trend for higher proportions of saturated fatty acids (SAFA) in faeces relative to the feeds, with the ADC for SAFA varying between around 81 and 87 %, generally slightly lower than the ADCs for the other fatty acids (Table 4). In contrast, proportions of PUFA in the faeces were generally lower compared to proportions in feeds with ADCs ranging from 93 to almost 97 %, whereas proportions of monounsaturated fatty acid (MUFA) were similar in diet and faeces with ADC ranging from around 83 to 92 % (Fig. 1). Some variations were found between feeds, with ADC of SAFA being highest in fish fed FO and lowest in fish fed ECO, with ADC of MUFA being higher in fish fed ECO and DCO compared to fish fed FO (Table 4). Similarly, ADC of 18:2n-6 and n-6 PUFA were higher in fish fed DCO compared to fish fed FO. Digestibility was highest with n-3 PUFA generally although ADC for EPA was lowest in WCO, although not different to that of ECO. Regarding DHA, FO and DCO showed the highest ADC, being over 96 % (Table 4).
Whole Fish Composition
No differences were found in any of the analysed components among fish fed the four dietary treatments (Table 3). However, there were trends for higher lipid content in fish fed the vegetable-based feeds (p = 0.186), and increased protein in fish fed the ECO and DCO feeds containing oil from transgenic Camelina (p = 0.202).
Tissue Lipid Content
The lipid content of flesh (muscle) varied between around 3 and 4.5 % but diet had no significant effect (Table 5). In contrast, the lipid content of liver was higher in fish fed WCO than in fish fed FO with fish fed the oils derived from transgenic Camelina showing intermediate values (Table 6). Diet had no significant effect on the lipid content of other tissues including gill (Table 6), anterior intestine and brain (Table 7).
Tissue Fatty Acid Compositions
Muscle (Flesh)
No differences were observed in the proportions of total n-3 PUFA in muscle (p = 0.508), although clear differences were found in individual fatty acids (Table 5). The mole percentages of EPA were highest in fish fed FO and ECO, lowest in fish fed WCO and intermediate in fish fed DCO. Proportions of DHA were similar in fish fed FO and DCO and higher than in fish fed WCO, with fish fed ECO showing intermediate values. Fish fed the FO and ECO diets displayed similar proportions of n-3 docosapentaenoic acid (DPA, 22:5n-3) in flesh, which were significantly higher than those found in fish fed WCO or DCO. The totals of EPA + DHA and EPA + DPA + DHA in fish fed all feeds varied in the rank order FO > ECO > DCO > WCO. The proportions of 18:2n-6 and total n-6 PUFA were higher in flesh of sea bream fed all the diets containing VO (WCO, ECO and DCO) compared to fish fed FO (Table 5). No differences were observed in total SAFA and total MUFA were higher in fish fed FO and WCO compared to fish fed the oils from transgenic Camelina.
Liver
The fatty acid profile of liver was similar to that of muscle and mainly reflected dietary compositions. No differences were found in total n-3 PUFA among the four dietary treatments as low levels of n-3 LC-PUFA were associated with high levels of short chain precursors (Table 6). The percentages of EPA were similar and highest in fish fed FO and ECO, lowest in fish fed WCO and intermediate in fish fed DCO. Proportions of DHA were similar in fish fed FO and DCO and higher than in fish fed WCO, with fish fed ECO showing intermediate values. Again, the totals of EPA + DHA and EPA + DPA + DHA in fish fed all feeds were in the rank order FO > ECO > DCO > WCO. Proportions of total n-6 PUFA were higher in liver of fish-fed ECO and DCO reflecting the higher dietary n-6 contents, particularly 18:2n-6 and arachidonic acid (ARA, 20:4n-6).
Gills
In general terms, gill fatty acid compositions mirrored dietary input, although some small differences were found (Table 6). Proportions of EPA varied in the rank order FO = ECO > DCO > WCO, DHA in the rank order FO > DCO > ECO = WCO, and EPA + DHA and EPA + DPA + DHA in the rank order FO > ECO > DCO > WCO. 18:2n-6 was higher in gills of fish fed the VO diets compared to fish fed FO while ARA varied in the rank order ECO > DCO = FO > WCO (Table 6).
Anterior Intestine
Proportions of EPA, DHA and total EPA + DPA + DHA varied in anterior intestine with essentially the same pattern as described for gills (Table 7). However, total n-6 PUFA contents were higher in anterior intestine than in the other tissues analysed, particularly in fish fed the diets containing oil from transgenic Camelina due to higher levels of 18:2n-6 and ARA.
Brain
The fatty acid composition of brain was least influenced by diet, with fewer individual fatty acids showing significant differences and the magnitude of differences being lower (Table 7). Specifically and importantly, DHA, EPA + DHA, EPA + DPA + DHA and EPA/DHA ratio did not vary between fish fed the different diets, and neither did 18:2n-6 or the totals of n-3 PUFA, PUFA, SAFA and MUFA.
The non-metric multidimensional scaling (NMDS) plot clearly showed that tissue fatty acid compositions were affected by the dietary input rather than by tissue type with the exception of brain, which clustered in a different group (Fig. 2, stress 0.07). This was confirmed by the global R value and low p obtained (R = 0.481; p < 0.001) when comparing the feeds. Pair-wise R indicated that the segregation was strongest between fish fed FO and WCO (R = 0.711; p = 0.005), whereas the weakest separation was observed between fish fed the ECO and DCO diets (R = 0.233; p = 0.030). The tissue fatty acid profiles of fish fed the two transgenic-derived oils did not show high separation with WCO (R = 0.431 and 0.404, and p = 0.003 and 0.004 for ECO and DCO, respectively). Tissue-wise, a weak segregation was observed (global R = 0.261; p = 0.008), although brain displayed a strong separation from other tissues (i.e. R = 1 for muscle and 0.958 for gill).
Tissue Histology
Fish fed the FO diet showed regular hepatocyte morphology with large centrally located nuclei with few cytoplasmic vacuoles that did not alter hepatocyte shape or size (Table 8). Fish fed wild-type Camelina oil (WCO) displayed a higher degree of vacuolisation, although no structural changes, such as inflammation, necrosis or perivascular cuffing were observed. Fish fed the feeds containing the GM-derived oils showed intermediate levels of vacuolisation, with no differences between FO and DCO-fed fish or between WCO and ECO-fed fish (Table 8). No significant differences were observed in the infiltration of peripancreatic fat although fish fed FO showed the lowest scores and DCO-fed fish the highest (p = 0.293; Table 8). With intestinal tissue, good integrity of the absorptive membrane was observed in the sections from fish fed all the dietary treatments. However, cellular infiltration, mainly represented by acidophilic granulocytes and some lymphocytes was observed, mainly in fish fed ECO (data not shown). Most of the acidophilic granulocytes were located in the lamina propria although a few could be found in submucosa and were present both in mid and hindgut.
Liver and Anterior Intestine Gene Expression
LC-PUFA Biosynthetic Genes
In liver, higher expression of fatty acyl desaturase 2 (fads2) was observed in fish fed all three diets containing VO (WCO, ECO and DCO), with expression significantly greater in liver of WCO-fed fish compared to FO-fed fish (Fig. 3a). Similarly higher expression in liver of fish fed all VO was observed in fatty acid elongase 4 (elovl4), significantly so in fish fed both GM-derived oils compared to fish fed FO (Fig. 3a). There was also a non-significant trend for increased expression of fatty acid elongase 5 (elovl5) in fish fed VO diets compared to fish fed FO (Fig. 3a). In contrast, anterior intestine showed different nutritional regulation of these genes. Firstly, only elovl4 expression showed a similar pattern of expression to that observed in liver with higher expression, albeit not significant, in fish fed the VO compared to fish fed FO (Fig. 3b). Secondly, fold changes (FC) were less with the highest being 1.7 FC for elovl4 for ECO-fed fish (Fig. 3b) compared to 4.6 FC in liver for this gene in fish fed ECO (Fig. 3a). Only fads2 showed significant regulation by dietary oil source, being down-regulated in intestine in VO-fed fish compared to fish fed FO, and a similar, non significant, trend was found in elovl5 in intestine (Fig. 3b).
Expression, measured by qPCR of LC-PUFA biosynthesis pathway genes in sea bream liver (a) and anterior intestine (b) after eleven weeks of feeding. Different superscript letters denote differences in gene expression among the treatments according to one-way ANOVA (p < 0.05). Results are normalized expression ratios (average ± SEM; n = 6) of the expression of these genes in fish fed the different diets in relation to fish fed FO feed. Diets contain either fish oil (FO), wild-type Camelina oil (WCO), high-EPA Camelina oil (ECO) or EPA + DHA Camelina oil (DCO). fads2, delta-6-fatty acyl desaturase; elovl4, fatty acid elongase 4; elovl5, fatty acyl elongase 5
Lipid Metabolism Genes
As above, the nutritional regulation of this group of genes was more marked in liver (Fig. 4a) than in anterior intestine (Fig. 4b). Lysophosphatidylcholine acyltransferase (lpcat) was up-regulated in VO-fed fish in both tissues, although the highest expression was found in liver of ECO-fed fish, whereas fish fed WCO showed the highest significant FC in intestine. Fatty acid binding protein 2 (FABP2) gene expression was also regulated in liver, with highest expression in fish fed ECO, whereas no dietary regulation of this gene was observed in anterior intestine. Both lipoprotein lipase (lpl) and hepatic lipase (hl) genes showed the same pattern in liver, with highest levels of expression in fish fed both diets with GM-derived oils, with WCO showing intermediate levels between those of ECO/DCO and fish fed FO (Fig. 4a). In contrast, no regulation was observed in the anterior intestine for lpl, although its expression showed a downward trend in VO-fed fish, and hl could not be detected in intestinal tissue (Fig. 4b).
Expression, measured by qPCR of lipid metabolism genes in sea bream liver (a) and anterior intestine (b) after 11 weeks of feeding. Different superscript letters denote differences in gene expression among the treatments according to one-way ANOVA (p < 0.05). Results are normalized expression ratios (average ± SEM; n = 6) of the expression of these genes in fish fed the different diets in relation to fish fed FO feed. Diets contain either fish oil (FO), wild-type Camelina oil (WCO), high-EPA Camelina oil (ECO) or EPA + DHA Camelina oil (DCO). lpcat1, lysophosphatidylcholine acyltransferase 1; FABP2, fatty acid binding protein 2; hl, hepatic lipase; lpl, lipoprotein lipase
Fatty Acid Catabolism Genes
Gene expression of two isoforms of carnitine palmitoyltransferase 1, cpt1a and cpt1b, were evaluated in liver and anterior intestine of sea bream (Fig. 5). In general terms, expression of both cpt1 in liver was higher in fish fed the VO compared to fish fed FO. However, WCO-fed fish showed intermediate values of cpt1a expression, lower FC than fish fed ECO, but in the same range as DCO-fed fish (Fig. 5a). Fish fed all three VO diets showed similar and higher levels of expression of cpt1b in liver than fish fed FO. No significant differences in expression of either cpt1a or cpt1b were observed in anterior intestine of sea bream fed the dietary treatments (Fig. 5b).
Expression, measured by qPCR of energy metabolism genes in sea bream liver (a) and anterior intestine (b) after 11 weeks of feeding. Different superscript letters denote differences in gene expression among the treatments according to one-way ANOVA (p < 0.05). Results are normalized expression ratios (average ± SEM; n = 6) of the expression of these genes in fish fed the different diets in relation to fish fed FO feed. Diets contain either fish oil (FO), wild-type Camelina oil (WCO), high-EPA Camelina oil (ECO) or EPA + DHA Camelina oil (DCO). cpt1a, carnitine palmitoyltransferase, isoform 1a; cpt1b, carnitine palmitoyltransferase, isoform 1b
Nuclear Receptors
Liver expression of the three evaluated nuclear receptors was generally higher in fish fed all of the VO diets compared to fish fed FO, although differences were not significant for peroxisome proliferator-activated receptor α (PPARα) (Fig. 6a). Expression in liver of PPARγ was highest in ECO- and DCO-fed fish with WCO-fed fish showing intermediate values, whereas fish fed all the VO diets showed higher expression of sterol regulatory element binding protein 1 (srebp1) (Fig. 6a). Although there were no significant differences in the expression of these genes among the dietary treatments in anterior intestine, a consistent pattern of lower expression in fish fed the VO diets was observed for all three nuclear receptors (Fig. 6b).
Expression, measured by qPCR of transcription factor genes in sea bream liver (a) and anterior intestine (b) after 11 weeks of feeding. Different superscript letters denote differences in gene expression among the treatments according to one-way ANOVA (p < 0.05). Results are normalized expression ratios (average ± SEM; n = 6) of the expression of these genes in fish fed the different diets in relation to fish fed FO feed. Diets contain either fish oil (FO), wild-type Camelina oil (WCO), high-EPA Camelina oil (ECO) or EPA + DHA Camelina oil (DCO). PPARα, peroxisome proliferator-activated receptor alpha; PPARγ, peroxisome proliferator-activated receptor gamma; srebp1, sterol regulatory element-binding protein 1
Fish Health and Immune System Genes
Again the three evaluated genes all showed higher expression in liver of fish fed the VO diets compared to fish fed FO (Fig. 7a). In the case of caspase 3 (casp3), ECO-fed fish showed the highest expression levels, although no differences were found with WCO and DCO-fed fish which also showed similar levels of expression to FO-fed fish (Fig. 7a). No statistical differences were observed for proliferating cell nuclear antigen (pcna) expression in liver, although DCO, and particularly ECO-fed fish, tended to have higher levels of expression. DCO-fed fish showed a clear up-regulation in hepatic interleukin 8 (il8) expression, with WCO and ECO-fed fish displaying intermediate levels (Fig. 7a). In contrast, although il8 was also differentially regulated by dietary oil source in intestine, the pattern was opposite to that observed in liver, with VO-fed fish showing lower expression compared to FO-fed fish (Fig. 7b).
Expression, measured by qPCR of fish health and immune system genes in sea bream liver (a) and anterior intestine (b) after 11 weeks of feeding. Different superscript letters denote differences in gene expression among the treatments according to one-way ANOVA (p < 0.05). Results are normalized expression ratios (average ± SEM; n = 6) of the expression of these genes in fish fed the different diets in relation to fish fed FO feed. Diets contain either fish oil (FO), wild-type Camelina oil (WCO), high-EPA Camelina oil (ECO) or EPA + DHA Camelina oil (DCO). casp3, caspase 3; pcna, proliferating cell nuclear antigen; il8, interleukin 8
Transgenes
All analysed sea bream tissues (muscle, liver and anterior intestine) tested negative for the presence of the Camelina T-DNA gene construct as monitored by the use of npt-II primers (data not shown).
Discussion
The replacement of FO in aquafeeds depends on finding alternative, sustainable sources of EPA and DHA, with this currently being one of the main issues in aquaculture nutrition, particularly in marine species that have limited ability for endogenous synthesis of LC-PUFA. Previous trials completely replacing FO by VO in feeds for gilthead sea bream resulted in reduced growth probably related to a reduced intake of essential LC-PUFA, which are not found in VO [31–33]. In the present study we evaluated the complete substitution of FO by two different oils obtained from GM-oilseed crops rich in either EPA (ECO) or containing both EPA and DHA (DCO) in feeds for sea bream juveniles. Both oils proved to be effective substitutes of FO, displaying growth rates that were similar to those achieved by fish fed FO (in the case of DCO) or WCO (for ECO). Similarly, recent studies employing both oil iterations as substitutes for FO in Atlantic salmon feeds showed that fish fed these oils were as successful as those fed FO [13, 15]. The lack of effect on growth in WCO-fed fish in the present trial is explained by the inclusion of relatively high levels of FM which ensured that n-3 LC-PUFA requirements were fully satisfied (1.9 % n-3 LC-PUFA in WCO-feed), estimated to be 0.9 % of dry feed [34].
It was surprising that the ECO-fed fish showed slightly reduced performance, albeit no different to WCO-fed fish, given that n-3 LC-PUFA requirements, including DHA, were satisfied. One reason for this could be related to the balance between the different dietary LC-PUFA including the dietary EPA/DHA ratio, which differed among the feeds. An EPA/DHA ratio of 2:1 was reported to be optimal for sea bream juveniles [35] and in the present trial ECO feed presented a ratio of approximately 3:1, perhaps suggesting an imbalance in these essential fatty acids. However, in previous trials in Atlantic salmon, where the EPA/DHA ratio was even higher (around 9:1), given the higher oil inclusion and lower FM level, no adverse effect was observed on growth [13]. However, it should be noted that the optimal dietary EPA/DHA ratio is likely to be species-specific. Another fatty acid that differed between ECO and the other feeds was ARA, with the ECO diet having more than double the ARA content of the FO diet. Increased dietary ARA levels have been associated with enhanced stress resistance, survival and improved growth in sea bream juveniles and larvae [36–40] as occurs in other marine warm water species such as European sea bass (Dicentrarchus labrax) [41]. However, ARA produces pro-inflammatory effects due to production of prostaglandin E2 (PGE2), leukotriene B4 (LTB4) and lipoxins [42], and so diets rich in n-6 PUFA, primarily ARA, could lead to overproduction of PGE2 although that can, in turn, have an immunosuppressant effect [43]. Although no major or obvious alteration in fish health was shown by the histology and qPCR results in the present study, increased ARA levels or even imbalanced proportions of n-3 and n-6 PUFA may partly explain the slightly lower growth of ECO-fed sea bream. On the other hand, the ratios of ARA, EPA and DHA to each other are also known to be of importance for marine finfish nutrition. For instance, a diet with high EPA and low ARA (9:1) significantly reduced performance of Atlantic salmon when compared to fish fed a more balanced ratio of EPA and ARA (1.5:1) [44], similar to what was observed in the present trial. Additionally, multiple regression analysis demonstrated meaningful relationships between ARA and DHA in California yellowtail (Seriola dorsalis) with ARA and DHA contributing positively to weight gain whereas EPA contributed negatively [45]. Thus, the lower growth observed in ECO-fed sea bream could be due to imbalanced proportions between these three essential LC-PUFA.
Despite the slightly reduced performance of ECO-fed fish compared to FO and DCO-fed fish, no marked differences were observed in lipid or individual fatty acid digestibility, which is consistent with previous studies in salmon using the same oil [14]. However, ECO-fed fish consumed less feed (g/tank) than fish fed FO or WCO diets, which may suggest a palatability issue with this oil. However, it must be noted that both oils were extracted using the same process and stabilized using the same concentration of antioxidant (ethoxyquin). In addition, exactly the same batch of ECO was used in the earlier trial in salmon where no differences in performance were observed between treatments [13]. Moreover, the sea bream feeds were formulated with higher FM levels than the earlier salmon feeds, which would in turn be expected to increase palatability. Thus, it appears that the slightly reduced performance of ECO-fed fish is more likely to be related to a species-specific sensitivity to high dietary ARA/n-6 levels that affected feed intake rather than a problem with palatability.
Complete substitution of dietary FO by VO is also associated with increased deposition of C18 fatty acids and reduced proportions of LC-PUFA in fish tissues. The two-GM derived oils investigated in the present trial can be considered as “hybrid” oils given that they contain features of both vegetable and marine oils, and the tissue fatty acid profiles reflected this characteristic. Thus, in general terms tissues of ECO and DCO fed fish had higher proportions of n-3 LC-PUFA than fish fed WCO, which in the case of flesh enhanced the nutritional value of the product. Some limited biosynthetic activity was observed particularly in fish fed ECO with high EPA (and ARA), where higher levels of DPA (22:5n-3) and 22:4n-6 were observed in liver, muscle and gills compared to fish fed either WCO or DCO. This likely reflected the higher hepatic expression of elovl4, an enzyme that participates in the elongation of ARA and EPA to 22:4n-6 and 22:5n-3, respectively [46], observed in ECO and DCO-fed fish. However, the increased expression of fads2 was not statistically significant in ECO fed fish, which showed intermediate values between FO and WCO-fed fish. However, increased expression of fads2 has been reported previously in sea bream fed VO compared to fish fed FO [21, 22]. The involvement of elovl4 in only later stages of the biosynthetic pathway (predominantly elongation of C22), particularly the synthesis of very long chain fatty acids (VLC-FA), explains why sea bream tissue fatty acid compositions largely reflected dietary fatty acid compositions. This was clear in the PCA (NMDS Plot) analysis where all tissues, except brain, grouped according to the feeds, with ECO and DCO clustering in the same group. This indicated that the fatty acid composition of brain was more conserved and less affected by diet than the other tissues, consistent with other studies in the same species [20] and other teleost species [47, 48].
Inclusion of high levels of VO and reduction of FO in feeds has been associated with increased tissue lipid deposition in several fish species [13, 14, 33, 49–51]. In the present study increased lipid deposition was observed only in liver, being highest in WCO-fed fish with ECO and DCO-fed fish showing intermediate values, with liver histology following the same trend. Similar results were found in Atlantic salmon fed high ECO, reflecting the hybrid nature of the GM-derived oil [13, 14]. The mechanism for increased lipid deposition in fish fed VO is not clear although high n-3 LC-PUFA levels found in FO can suppress triacylglycerol (TAG) accumulation in mammalian pre-adipocytes [52] and lipid accumulation in Atlantic salmon adipocytes [53]. Another explanation for the enhanced lipid deposition could be the high levels of both C18 fatty acids, 18:2n-6 and 18:3n-3, together with the limited LC-PUFA biosynthetic capacity of this species [22]. In the present study we evaluated the expression of genes involved in lipid and energy metabolism, as well as transcription factors such as SREBP1, which plays a key role in lipid metabolism participating in fatty acid metabolism and de novo lipogenesis [54]. Up-regulation in srebp1 expression in liver was observed in fish fed the three VO in agreement with studies carried out in elovl5−/− mice that indicated low levels of EPA and DHA lead to activation of this transcription factor [55]. Similar results have been obtained in other fish species when fed low levels of n-3 LC-PUFA or VO [50, 56–58]. Up-regulation of srebp1 in VO-fed fish could lead to the regulation of srebp1 target genes such as fads2, hl, lpl, cpt1a and cpt1b. Hydrolysis of TAG in lipoproteins is mediated by lpl, and hl converts intermediate density lipoprotein to low density lipoprotein, mediating uptake of fatty acids by tissues. Thus, enhanced expression of these two lipase genes would enhance lipid deposition in liver in agreement with a previous study in turbot (Scophtalmus maximus) fed soybean oil [50]. On the other hand, cpt1a, a gene involved in fatty acid oxidation was up-regulated in fish fed the GM-derived oils and, although it is a srebp1 target gene, down-regulation of this gene was expected [50]. However, in comparison to the clear effects on liver cpt expression in the present study, published data show only marginal effects of replacing FO by VO on β-oxidation [59, 60]. In addition, there are other nutrients that can affect the regulation of these genes such as carbohydrate that can affect lipid metabolism, enhancing the expression of srebp1 and cpt1a and cpt1b in rainbow trout (Oncorhynchus mykiss) when fed VO [18].
Two other transcription factors, PPARα and PPARγ, that regulate lipid metabolism and energy homeostasis [61] were evaluated in the present trial, but no regulation of pparα was noted in either liver or anterior intestine. PPARα activation by n-3 LC-PUFA may induce the expression of lipolytic genes, such as cpt, enhancing fatty acid oxidation [62]. However, in the present study and others, no regulation of pparα expression has been observed in fish fed more sustainable diets based on VO [50, 63–65]. In contrast, feeding VO, particularly the GM-derived oils, increased pparγ expression. PPARγ activation in mice is known to increase glycolysis, fat storage, fatty acid desaturation and elongation [66]. Thus, up-regulation of pparγ may be a compensatory mechanism to attenuate the increased lipid deposition found in fish fed high levels of VO, although it did not have a direct effect on lipolytic genes. Consistent with this, pparγ expression was up-regulated in mice fed the high-EPA oil (ECO) compared to mice fed a FO-based diet [67].
Acyltransferases such as LPCAT play a major role in phospholipid remodelling as they alter the fatty acid composition of phospholipids at the sn-2 position [68]. The expression of this gene tended to increase in VO-fed fish compared to FO-fed fish in both liver and intestine. This could be reflected in enhanced conversion of lysophosphatidylcholine to phosphatidylcholine in VO-fed fish. Although there is only limited information regarding the regulation of this gene in teleosts, n-3 PUFA have been found to regulate lcpat1 in larvae of Senegalese sole (Solea senegalensis), where gene expression was up-regulated in larvae fed diets containing linseed oil compared to other sources of VO, although no differences were observed with fish fed FO [69]. It is difficult to make comparisons with this study, as fast growing larvae have a high requirement for dietary phospholipids [70] but it is clear that nutritional regulation by n-3 PUFA or LC-PUFA occurs regardless of the life stage. In fact, lpcat1 regulation has also been found in sea bream [71] and sea bass [72] subjected to fasting, although the direction of regulation varied among species. Also fabp2, a gene related to fatty acid transport and uptake, was regulated in liver, being clearly up-regulated in fish fed ECO. Similarly, Atlantic cod (Gadus morhua) fed plant meal diets showed a trend towards up-regulation of fabp2 compared to fish fed fishmeal, in this case associated with lower fat digestibility of the plant-based feeds [73]. Although we cannot fully explain this result at present, we suggest that the up-regulation may be associated with the lower growth of these fish, which in turn could elicit compensatory mechanisms such as fatty acid mobilization.
Some genes were analysed in order to evaluate the immune system and fish health in response to dietary oils. Caspases are central regulators of apoptosis, while PCNA is a marker of cell proliferation, also expressed in non-dividing cells undergoing DNA synthesis and repair [74]. In previous studies, expression of caspases was affected by replacement of dietary FO by VO in fish [63, 64, 75]. Apoptosis is particularly important in tissues with high cell turnover rate such as liver [76] but, in addition, their expression can be altered by factors such as pathological or toxic conditions [77]. Certain VO have been found to enhance oxidative stress and cellular damage [78], and DHA can suppress caspase 3 activation and cell death in neurons [79], which suggests FO diets could have a cellular protective effect in fish. In contrast, the lack of nutritional regulation in anterior intestine was consistent with the results observed in sea bass fed diets containing soybean oil [80]. Expression of pcna was not altered when fish were fed the VO-feeds consistent with a previous study in Atlantic salmon [75]. Interleukin 8 (il8), a cytokine that serves as a chemical messenger in innate and adaptive immune systems, showed differential regulation in liver and intestine of fish fed VO, probably related to the specific functions of each tissue and their response to nutritional challenge. In this respect, fish gut epithelium acts and reacts as the first line of protection against potentially harmful substances in the diet, with VO causing changes in fish that could favour intestinal dysfunction, including alterations in the gut associated immune system [81]. Thus, down-regulation of il8 in intestine of VO-fed fish confirmed the importance of dietary fatty acid profile on the immune system of marine fish in agreement with previous studies [80, 82]. In contrast, liver of DCO-fed fish showed higher expression of il8, albeit not different to expression in liver of bream fed the other VO diets, which may indicate that the VO fatty acid profile may enhance il8 activity in this tissue.
In summary, both genetically engineered Camelina oils (EPA only or EPA + DHA) were shown to be viable sources of n-3 LC-PUFA and potential candidates to replace FO in feeds for sea bream, with the growth of fish fed DCO similar to that of fish fed FO. Both oils improved the nutritional quality of the fish fillet, enhancing the n-3 LC-PUFA levels compared to the fish fed the regular (wild-type) VO. Limited LC-PUFA biosynthesis was observed, specifically in liver of fish fed ECO, reflected in higher levels of n-3 DPA, consistent with increased expression of elovl4 elongase. In general, gene expression reflected the “hybrid” fatty acid composition of the GM-derived oils, eliciting responses that were between the levels of expression in FO and WCO-fed fish, although often more similar to WCO. This may suggest the importance of not only EPA and DHA, but also C18 PUFA levels in marine fish lipid metabolism. Based on histology and gene expression, no adverse effects on fish health were observed and thus the cause for the slight reduction in feed intake and consequent fish growth observed in fish fed ECO was not clear but may be related to dietary ARA levels and/or LC-PUFA ratios. Longer term trials with fish up to market size are required to further validate the feasibility of oils from transgenic oilseed crops in marine fish aquaculture.
Change history
18 April 2017
An erratum to this article has been published.
Abbreviations
- ACTB:
-
β Actin
- ADC:
-
Apparent digestibility coefficient
- ARA:
-
Arachidonic acid
- CASP:
-
Caspase
- CPT:
-
Carnitine palmitoyltransferase 1
- CYTB:
-
Cytochrome b
- DCO:
-
EPA + DHA Camelina oil
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- ECO:
-
EPA-Camelina oil
- ELF1α:
-
Elongation factor 1α
- ELOVL:
-
Fatty acid elongase
- EPA:
-
Eicosapentaenoic acid
- FABP2:
-
Fatty acid binding protein 2
- FADS2:
-
Fatty acyl desaturase 2
- FAME:
-
Fatty acid methyl esters
- FCR:
-
Feed conversion ratio
- FM:
-
Fish meal
- FO:
-
Fish oil
- GC:
-
Gas–liquid chromatography
- GM:
-
Genetically modified
- HL:
-
Hepatic lipase
- HSI:
-
Hepatosomatic index
- IL:
-
Interleukin
- K:
-
Condition factor
- LC-PUFA:
-
Long chain polyunsaturated fatty acid(s)
- LPCAT:
-
Lysophosphatidylcholine acyltransferase
- LPL:
-
Lipoprotein lipase
- LTB4:
-
Leukotriene B4
- MUFA:
-
Monounsaturated fatty acid(s)
- n-3:
-
Omega 3
- n-6:
-
Omega 6
- n.d.:
-
Not determined
- NMDS:
-
Non-metric multidimensional scaling plot
- NPTII:
-
Kanamycin resistance gene
- NTC:
-
Non-template control
- PCA:
-
Principal component analysis
- PCNA:
-
Proliferating cell nuclear antigen
- PGE2:
-
Prostaglandin E2
- PPARα:
-
Peroxisome proliferator-activated receptor α
- PUFA:
-
Polyunsaturated fatty acid(s)
- SAFA:
-
Saturated fatty acid(s)
- SGR:
-
Specific growth rate
- SREBP:
-
Sterol regulatory element binding protein 1
- TAG:
-
Triacylglycerol(s)
- VLC-FA:
-
Very long chain fatty acid(s)
- VO:
-
Vegetable oil(s)
- VSI:
-
Viscerosomatic index
- WCO:
-
Wild-type Camelina oil
References
Gil A, Serra-Majem L, Calder PC, Uauy R (2012) Systematic reviews of the role of omega-3 fatty acids in the prevention and treatment of disease. Br J Nutr 107:S1–S2
Campoy C, Escolano-Margarit V, Anjos T, Szajewska H, Uauy R (2012) Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr 107:S85–S106
Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F (2012) Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr 107:S201–S213
Miles EA, Calder PC (2012) Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr 107:S171–S184
Laviano A, Rianda S, Molfino A, Rossi Fanelli F (2013) Omega-3 fatty acids in cancer. Curr Opin Clin Nutr Metab Care 16:156–161
International Society for the Study of Fatty Acids and Lipids (ISSFAL) (2004) Report of the sub-committee on: recommendations for intake of polyunsaturated fatty acids in healthy adults. ISSFAL, Brighton
FAO (2016) State of World Fisheries and Aquaculture 2016. Food and Agriculture Organization of the United Nations, Rome
Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K, Nichols PD (2009) Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci USA 106:15103–15110
Tocher DR (2015) Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449:94–107
Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77:198–208
Haslam RP, Usher S, Sayanova O, Napier JA, Betancor MB, Tocher DR (2015) The supply of fish oil to aquaculture: a role for transgenic oilseed crops? World Agric 5:15–23
Usher S, Haslam RP, Ruiz-Lopez N, Sayanova O, Napier JA (2015) Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: making fish oil substitutes in plants. Metab Eng Commun 2:93–98
Betancor MB, Sprague M, Usher S, Sayanova O, Campbell PJ, Napier JA, Tocher DR (2015) A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaced marine fish oil as a source of eicosapentaenoic acid for farmed Atlantic salmon (Salmo salar). Sci Rep 5:8104. doi:10.1038/srep08104
Betancor MB, Sprague M, Sayanova O, Usher S, Campbell PJ, Napier JA, Tocher DR (2015) Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): effects on tissue fatty acid composition, histology and gene expression. Aquaculture 444:1–12
Betancor MB, Sprague M, Sayanova O, Usher S, Metochis C, Campbell PJ, Napier JA, Tocher DR (2016) Nutritional evaluation of an EPA-DHA oil from transgenic Camelina sativa in feeds for post-smolt Atlantic salmon (Salmo salar L.). PLoS One. doi:10.1371/journal.pone.0159934
Bell JG, McEvoy J, Tocher DR, McGhee F, Campbell PJ, Sargent JR (2001) Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J Nutr 131:1535–1543
Caballero MJ, Obach A, Rosenlund G, Montero D, Gisvold M, Izquierdo MS (2002) Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214:253–271
Kamalam BS, Médale F, Larroquet L, Corraze G, Panserat S (2013) Metabolism and fatty acid profile in fat and lean rainbow trout lines fed with vegetable oil: effect of carbohydrates. PLoS One 8:e76570
Menoyo D, Izquierdo MS, Robaina L, Ginés R, Lopez-Bote CJ, Bautista JM (2004) Adaptation of lipid metabolism, tissue composition and flesh quality in gilthead sea bream (Sparus aurata) to the replacement of dietary fish oil by linseed and soyabean oils. Br J Nutr 92:41–52
Benedito-Palos L, Navarro JC, Kaushik S, Pérez-Sánchez J (2010) Tissue-specific robustness of fatty acid signatures in cultured sea bream (Sparus aurata) fed practical diets with a combined high replacement of fish meal and fish oil. J Anim Sci 88:1759–1770
Mourente G, Tocher DR (1993) Incorporation and metabolism of 14C-labelled polyunsaturated fatty acids in juvenile gilthead sea bream Sparus aurata L. in vivo. Fish Physiol Biochem 10:443–453
Izquierdo MS, Juárez-Carrillo E, Oliva V, Hernández-Cruz CM, Afonso JM (2008) Regulation of growth, fatty acid composition and delta 6 desaturase expression by dietary lipids in gilthead seabream larvae (Sparus aurata). Fish Physiol Biochem 34:117–127
National Research Council (NRC) (2011) Nutrient Requirements of Fish and Shrimp. The National Academies Press, Washington, DC
Fernández F, Miquel AG, Cumplido LR, Guinea J, Ros E (1996) Comparisons of faecal collection methods for digestibility determinations in gilthead sea bream. J Fish Biol 49:735–738
Folch J, Lees N, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
AOAC (2000) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC
Christie WW (2003) Lipid analysis, 3rd edn. Oily Press, Bridgwater
Tocher DR, Harvie DG (1988) Fatty acid compositions of the major phosphoglycerides from fish neural tissues; (n-3) and (n-6) polyunsaturated fatty acids in rainbow trout (Salmo gairdneri) and cod (Gadus morhua) brains and retinas. Fish Physiol Biochem 5:229–239
Martoja R, Martoja-Pearson M (1970) Técnicas de Histología Animal. Toray-Masson S.A., Barcelona
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:9
Benedito-Palos L, Navarro JC, Sitjà-Bobadilla A, Bell JG, Kaushik S, Pérez-Sánchez J (2008) High levels of vegetable oils in plant protein-rich diets fed to gilthead sea bream (Sparus aurata L.): growth performance, muscle fatty acid profiles and histological alterations of target tissues. Br J Nutr 100:992–1003
Montero D, Grasso V, Izquierdo MS, Ganga R, Real F, Tort L, Caballero MJ, Acosta F (2008) Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: effects of Mx expression and some immune parameters. Fish Shellfish Immunol 24:147–155
Fountoulaki E, Vasilaki A, Hurtado R, Grigorakis K, Karacostas I, Nengas I, Rigos G, Kotzamanis Y, Venou B, Alexis MN (2009) Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 289:317–326
Kalegeropoulos N, Alexis MN, Henderson RJ (1992) Effects of dietary soybean and cod-liver oil levels on growth and body composition of gilthead bream (Sparus aurata). Aquaculture 104:293–308
Ibeas C, Cejas JR, Fores R, Badía P, Gómez T, Lorenzo-Hernández A (1997) Influence of eicosapentaenoic to docosahexaenoic acid ratio (EPA/DHA) of dietary lipids on growth and fatty acid composition of gilthead seabream (Sparus aurata) juveniles. Aquaculture 150:91–102
Bessonart M, Izquierdo MS, Salhi M, Hernández-Cruz CM, González MM, Fernández-Palacios H (1999) Effect of dietary arachidonic acid levels on growth and survival of gilthead sea bream (Sparus aurata L.) larvae. Aquaculture 179:265–275
Koven W, Barr Y, Lutzky S, Ben-Atia I, Weiss R, Harel M, Behrens P, Tandler A (2001) The effect of dietary arachidonic acid (20:4n-6) on growth, survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae. Aquaculture 193:107–122
Koven W, van Anholt R, Lutzky S, Ben Atia I, Nixon O, Ron B, Tandler A (2003) The effect of dietary arachidonic acid on growth, survival, and cortisol levels in different-age gilthead seabream larvae (Sparus aurata) exposed to handling or daily salinity change. Aquaculture 228:307–320
Van Anholt RD, Spanings FAT, Koven WM, Nixon O, Wemdelaar Bonga SE (2004) Arachidonic acid reduces the stress response of gilthead seabream Sparus aurata L. J Exp Biol 207:3419–3430
Atalah E, Hernández-Cruz CM, Benítez-Santana T, Ganga R, Roo J, Izquierdo MS (2011) Importance of the relative levels of dietary arachidonic acid and eicosapentaenoic acid for culture performance of gilthead seabream (Sparus aurata). Aquacult Res 42:1279–1288
Montero D, Terova G, Rimoldi S, Betancor MB, Atalah E, Torrecillas S, Caballero MJ, Zamorano MJ, Izquierdo MS (2015) Modulation of the expression of components of the stress response by dietary arachidonic acid in European sea bass (Dicentrarchus labrax) larvae. Lipids 50:1029–1041
Lall SP (2000) Nutrition and health of fish. In: Cruz-Suárez LE, Ricque-Marie D, Tapia-Salazar M, Olvera-Novoa MA, Civera-Cerecedo R (eds). Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola. Mérida, Yucatán, Mexico, pp 19–22
Bell JG, Ashton I, Secombes CJ, Weitzel BR, Dick JR, Sargent JR (1996) Dietary lipid affects phospholipid fatty acid compositions, eicosanoid production and immune function in Atlantic salmon (Salmo salar). Prostag Leukot Essent Fatty Acids 54:173–182
Norambuena F, Rombenso A, Turchini GM (2016) Towards the optimization of Atlantic salmon reared at different water temperatures via the manipulation of dietary ARA/EPA ratio. Aquaculture 450:48–57
Rombenso AN, Trushenki JT, Jirsa D, Drawbridge M (2016) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential to meet LC-PUFA requirements of juvenile California Yellowatil (Seriola dorsalis). Aquaculture 463:123–134
Monroig Ó, Webb K, Ibarra-Castro L, Holt J, Tocher DR (2011) Biosynthesis of long-chain polyunsaturated fatty acids in marine fish: characterization of an Elovl4-like elongase from cobia Rachycentrum canadum and activation of the pathway during early life stages. Aquaculture 312:145–153
Betancor MB, Howarth FJE, Glencross BD, Tocher DR (2014) Influence of dietary docosahexaenoic acid in combination with other long-chain polyunsaturated fatty acids on expression of biosynthesis genes and phospholipid fatty acid compositions in tissues of post-smolt Atlantic salmon (Salmo salar). Comp Biochem Physiol 172–173B:74–89
Geay F, Wenon D, Mellery J, Tinti E, Mandiki SNM, Tocher DR, Debier C, Larondelle Y, Kestemont P (2015) Dietary linseed oil reduces growth while differentially impacting LC-PUFA synthesis and accretion into tissues in Eurasian perch (Perca fluviatilis). Lipids 50:1219–1232
Benítez-Dorta V, Caballero MJ, Izquierdo MS, Manchado M, Infante C, Zamorano MJ, Montero D (2013) Total substitution of fish oil by vegetable oils in Senegalese sole (Solea senegalensis) diets: effects on fish performance, biochemical composition and expression of some glucocorticoid receptor related genes. Fish Physiol Biochem 39:335–349
Peng M, Xu W, Mai K, Zhou H, Zhang Y, Liufu Z, Zhang K, Ai A (2014) Growth performance, lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophtalmus maximus L.) fed diets with various fish oil substitution levels by soybean oil. Aquaculture 433:442–449
Ballester-Lozano GF, Benedito-Palos L, Estensoro I, Sitjà-Bobadilla A, Kaushik S, Pérez-Sánchez J (2015) Comprehensive biometric, biochemical and histopathological assessment of nutrient deficiencies in gilthead sea bream fed semi-purified diets. Br J Nutr 114:713–726
Kim HK, Della-Fera M, Lin J, Baile CA (2006) Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J Nutr 136:2965–2969
Todorcević M, Vegusdal A, Gjøen T, Sundvold H, Torstensen BE, Kjær MA, Ruyter B (2008) Changes in fatty acids metabolism during differentiation of Atlantic salmon preadipocytes; effects of n-3 and n-9 fatty acids. Biochim Biophys Acta 1781:326–335
Horton JD, Goldstein JL, Brown MS (2003) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig 109:1125–1131
Moon YA, Hammer RE, Horton JD (2009) Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res 50:412–423
Geay F, Ferraresso S, Zambonino-Infante JL, Bargellioni L, Quentel C, Vandeputte M, Kaushik S, Cahu CL, Mazurais D (2011) Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genom 12:522
Morais S, Pratoomyot J, Taggart JB, Bron JE, Guy DR, Bell JG, Tocher DR (2011) Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: a liver transcriptomic analysis. BMC Genom 12:255
Limtipsuntorn U, Haga Y, Kondo H, Hirono I, Satoh S (2014) Microarray analysis of hepatic gene expression in juvenile Japanese flounder Paralichthys olivaceus fed diets supplemented with fish or vegetable oils. Mar Biotechnol 16:88–102
Stubhaug I, Lie Ø, Torstensen BE (2006) β-oxidation capacity in liver increases during parr-smolt transformation of Atlantic salmon (Salmo salar L.) fed vegetable and fish oil. J Fish Biol 69:504–517
Stubhaug I, Lie Ø, Torstensen BE (2007) Fatty acid productive value and β-oxidation capacity in Atlantic salmon tissues (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquacult Nutr 13:145–155
Evans R, Barish GD, Wang YX (2004) PPARs and the complex journey to obesity. Nat Med 20:355–361
Poudyal H, Panchal SK, Diwan V, Brown L (2011) Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res 50:371–387
Morais S, Silva T, Cordeiro T, Rodrigues P, Guy DR, Bron JE, Taggart JB, Bell JG, Tocher DR (2012) Effects of genotype and dietary fish oil replacement with vegetable oil on the intestinal transcriptome and proteome of Atlantic salmon (Salmo salar). BMC Genom 13:448
Morais S, Edvardsen RB, Tocher DR, Bell JG (2012) Transcriptomic analyses of intestinal gene expression of juvenile Atlantic cod (Gadus morhua) fed diets with Camelina oil as replacement for fish oil. Comp Biochem Physiol 161B:283–293
Li Y, Zhao Y, Zhang Y, Liang X, Zhang Y, Gao J (2015) Growth performance, fatty acid composition, peroxisome proliferator-activated receptors gene expressions, and antioxidant abilities of blunt snout bream, Megalobrama amblycephala, fingerlings fed different dietary oil sources. J World Aquac Soc 46:395–408
Roberts LD, Murray AJ, Menassa D, Ashmore T, Nicholls AW, Griffin JL (2011) The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol 12:R75
Tejera N, Vauzour D, Betancor MB, Sayanova O, Usher S, Cochard M, Rigby N, Ruiz-Lopez N, Menoyo D, Tocher DR, Napier JA, Minihane AM (2016) A transgenic Camelina sativa seed oil replaces fish oil as a dietary source of eicosapentaenoic acid in mice. J Nutr 146:227–235
Lands WE (1958) Metabolism of glycerolipids; a comparison of lecithin and triglyceride synthesis. J Biol Chem 231:883–888
Bonacic K, Campoverde C, Sastre M, Hachero-Cruzado I, Ponce M, Manchado M, Estevez A, Gisbert E, Morais S (2016) Mechanisms of lipid metabolism and transport underlying superior performance of Senegalese sole (Solea senegalensis, Kaup 1858) larvae fed diets containing n-3 polyunsaturated fatty acids. Aquaculture 450:383–396
Tocher DR, Bendiksen EÅ, Campbell PJ, Bell JG (2008) The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 280:21–34
Benedito-Palos L, Calduch-Giner JA, Ballester-Lozano GF, Pérez-Sánchez J (2013) Effect of ration size on fillet fatty acid compositions, phospholipid allostasis and mRNA expression patterns of lipid regulatory genes in gilthead sea bream (Sparus aurata). Br J Nutr 109:1175–1187
Rimoldi S, Benedito-Palos L, Terova G, Pérez-Sánchez J (2015) Wide-targeted gene expression infers tissue-specific molecular signatures of lipid metabolism in fed and fasted fish. Rev Fish Biol Fish 26:93–108
Lilleeng E, Frøystad MK, Vekterud K, Valen EC, Krogdahl Å (2007) Comparison of intestinal gene expression in Atlantic cod (Gadus morhua) fed standard fish meal or soybean meal by means of suppression subtractive hybridization and real-time PCR. Aquaculture 267:269–283
Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A (2002) Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol 161:575–585
Olsvik PA, Torstensen BE, Nermtssen MHG (2007) Effects of complete replacement of fish oil with plant oil on gastrointestinal cell death, proliferation and transcription of eight genes’ encoding proteins responding to cellular stress in Atlantic salmon Salmo salar L. J Fish Biol 71:550–568
Lajtha A, Latzkovits L, Toth J (1976) Comparison of turnover rates of proteins of the brain, liver and kidney in mouse in vivo following long term labelling. Biochim Byophys Acta 425(511):520
Sweet LI, Passino-Reader DR, Meier PG, Omann GM (1999) Xenobiotic-induced apoptosis: significance and potential application as a general biomarker of response. Biomarkers 4:237–253
Kadir NHA, David R, Rossiter JT, Gooderham NJ (2015) The selective cytotoxicity of the alkenyl glucosinolate hydrolysis products and their presence in Brassica vegetables. Toxicology 334:59–71
Akbar M, Calderon F, Wen Z, Kim HY (2005) Docosahexaenoic acid: a positive modulator of Akt signalling in neuronal survival. Proc Natl Acad Sci USA 102:10858–10863
Torrecillas S, Montero D, Caballero MJ, Pittman KA, Custódio M, Campo A, Sweetman J, Izquierdo M (2015) Dietary mannan oligosaccharides: counteracting the side effects of soybean meal oil inclusion in European sea bass (Dicentrachus labrax) gut health and skin mucosa mucus production? Front Immunol 6:397
Montero D, Izquierdo MS (2010) Welfare and health of fish fed vegetable oils as alternative lipid sources to fish oil. In: Turchini G, Ng W, Tocher D (eds) Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press, Cambridge, pp 439–485
Montero D, Benitez-Dorta V, Caballero MJ, Ponce M, Torrecillas S, Izquierdo M, Zamorano MJ, Manchado M (2015) Dietary vegetable oils: effects on the expression of immune-related genes in Senegalese sole (Solea senegalensis) intestine. Fish Shellfish Immunol 44:100–108
Acknowledgments
The authors are grateful to R. Cruz, D. Domínguez and N. Oubramy for their excellent technical assistance in fish rearing and fish sampling. MBB and this project were partly funded by a UK Biotechnology and Biological Sciences Research Council (BBSRC) Industrial Partnership Award (/BB/J001252/1). The trial was performed thanks to an Aquaexcel transnational action (0119//08/12/28) and trip costs were funded thanks to a Santander Staff Mobility Fund both awarded to MBB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interest to declare.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11745-017-4248-z.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Betancor, M.B., Sprague, M., Montero, D. et al. Replacement of Marine Fish Oil with de novo Omega-3 Oils from Transgenic Camelina sativa in Feeds for Gilthead Sea Bream (Sparus aurata L.). Lipids 51, 1171–1191 (2016). https://doi.org/10.1007/s11745-016-4191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4191-4