Abstract
Clarias gariepinus growth hormone (CgGH) transgenesis was previously used to develop a population of second-generation (G2) transgenic mutiara catfish (C. gariepinus). The third generation of these fish (1-month old fingerlings) had 2–3 times improvement in growth compared to non-transgenic fish in the commercial feed test for 6 weeks of rearing. We assessed the impact of CgGH transgene expression on growth and protein and amino acid content of the G3 generation of these transgenic mutiara catfish relative to non-transgenic catfish. Since variation in composition of feed mixes can affect protein and amino acid content of fish, we tested three mixtures of commercial feed and boiled tuna (Euthynnus affinis): feed A (50:50 feed to tuna), B (65:35), and C (80:20) to transgenic catfish. Feed A* (50:50) was fed as a control to non-transgenic catfish. Feed efficiency, including feed conversion ratio and protein use efficiency (i.e., protein retention and protein productive value), was assessed. Feed efficiency, protein content, and essential amino acid content in G3 transgenic catfish (feed A and B) were higher than in non-transgenic fish (feed A*). The latter were deficient in lysine and methionine. Transgenic catfish fed with feed C (80:20) showed lysine deficiency and lower growth than fish fed feeds A and B. Feed B (65:35) was the optimal feed mixture utilized; it increased growth, protein levels, and feed conversion efficiency in G3 catfish. The growth of transgenic fish was higher than non-transgenic fish when supported by feeding with balanced nutrients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the demand for aquaculture products increases, there is a concurrent need to improve the growth rate of fish in order to increase the efficiency of fish production. Increased production in commercial fish farming can be achieved by genetic improvement of fish growth. For example, insertion of exogenous growth hormone (GH) genes using transgenic technology can produce a 2–3-fold increase in fish growth relative to non-transgenic fish over a few months (Devlin et al. 2004). Studies of salmonids demonstrate that transgenic fish expressing exogenous GH constructs (e.g., OnMTGH1 and CMVOnGH1) display dramatic growth enhancement (Higgs et al. 2009; Pitkanen et al. 1999). Furthermore, Clarias gariepinus growth hormone (CgGH) transgenic technology, successfully developed for mutiara catfish (C. gariepinus), induces significantly greater growth in transgenic fish; second-generation (G2) animals showed a 150–200% increase in growth relative to non-transgenic fish (Buwono et al. 2019a). This technology also increased growth in transgenic offspring of other fish species, including G2 transgenic tilapia (125% increase, Rahman et al. 2001) and G5 transgenic Atlantic salmon (Salmo salar) (100% increase, Levesque et al. 2008). The aim of this study, therefore, was to evaluate mass production of G3 transgenic mutiara catfish, which requires that levels of expression and transmission of exogenous GH remain high; thus, transgene inheritance must be stable among generations of transgenic fish (Wang et al. 2001). In our previous study, CgGH transmission in the G2 strain reached 50%, indicating the occurrence of Mendelian transmission and suggesting that these fish can be used to produce G3 catfish (Buwono et al. 2019b).
Induction of increased growth in transgenic-CgGH catfish is mainly due to increased expression of exogenous GH, which stimulates IGF-1 (insulin-like growth factor 1) production in the liver. Previous studies show that plasma GH and IGF-1 levels in transgenic fish are higher than in non-transgenic fish (Eppler et al. 2007; Raven et al. 2008). Hence, transgenic fish are more responsive to feed (Cook et al. 2000; Raven et al. 2008), and growth phenotype is improved along with feed efficiency (Devlin et al. 2004; Oakes et al. 2007). Furthermore, overexpression of exogenous GH stimulates de novo protein synthesis from precursors available from feed protein (Fauconneau et al. 1996; Mauras and Haymond 2005; Rosa et al. 2008). Enhanced GH secretion plays an important role in stimulating fish growth, improving feed conversion, and increasing protein use efficiency. Thus, GH-transgenic salmon regulate energy metabolism by converting feed carbohydrates into metabolic energy, avoiding the use of protein (protein sparing) to increase protein synthesis (increased protein retention). This increased efficiency in protein use supports more rapid growth (Levesque et al. 2008). Growth hormones, including transgenic GH, also function to promote absorption of amino acids, especially essential amino acids that cannot be synthesized intrinsically in the fish (Farmanfarmaian and Sun 1999; Li et al. 2009). For example, the content of essential amino acids in fish feed determined protein use efficiency, represented by protein productive value (PPV) to support optimum fish growth in stinging catfish (Heteropneustes fossilis) fingerlings (Farhat and Khan 2012).
Therefore, maximum growth potential of transgenic fish depends on the quality of feed protein based on both protein content and amino acid composition (Wilson and Poe 1985; Cowey 1994). Feed composition indirectly influences protein retention, amino acid content, and growth of transgenic fish. Available commercial fish feed might provide transgenic fish with the content protein they need to achieve their growth potential, but alternative fish feeds should also be considered, especially since comparisons of the effects of feeds in transgenic fish growth have rarely been reported.
In the present study, we investigated an alternative feed protein source for transgenic catfish, namely boiled tuna (Euthynnus affinis), commonly known as pindang tongkol in Indonesia. The fish is prepared by a combination of boiling and salting. Pindang tongkol might replace commercial feed since it contains all nutritional elements required by commercial fish species (Uju et al. 2017; Kim et al. 2018). Specifically, we investigated whether overexpression of CgGH, increased levels of protein, and increased amino acid content are reflected in the growth rate of G3 transgenic mutiara catfish fed mixtures of commercial feed and pindang tongkol at different ratios. Finding an optimum balance of feeds would provide important information to help improve feed conversion efficiency and growth.
Materials and methods
Transgene construct and the production of G2 transgenic broodstock
The transgene constructs designed to produce G0 transgenic mutiara catfish were pCMV-CgGH (6285 bp) containing CgGH (Buwono et al. 2019b). The pCMV-CgGH plasmid was transferred to mutiara catfish sperm using the Xcell gene pulser eletroporator (BioRad Laboratories, Inc., Hercules, CA, USA) by the same procedure as in previous studies (Subyakto et al. 2011). Post electroporation, plasmid-carrying sperm (containing CgGH) was mixed with mutiara catfish eggs to produce G0 transgenic mutiara catfish.
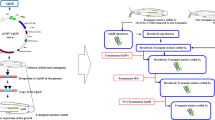
The production of G1 transgenic fish was carried out by crossing G0 transgenic and non-transgenic broodstock, and crossing G1 transgenic and non-transgenic broodstock was used to produce G2 transgenic fish. Furthermore, these G2 transgenic broodstock were used for the production of G3 transgenic fish.
Validation of the G2 transgenic mutiara catfish broodstock to produce G3 transgenic mutiara catfish
G2 catfish broodstock were derived from a cross from previous studies and showed a CgGH transmission rate of 50% (Buwono et al. 2019b); thus, both male and female catfish required validation for expression of the CgGH gene (GenBank accession number MN 249238.1). Transgene detection used RT-PCR and DNA isolated from a small sample of the catfish tail fin. Once positive G2 transgenic male and female broodstock were confirmed to carry the CgGH gene (a product of approximately 600 bp using primers GH-F and GH-R; see below), they were used for G3 transgenic mutiara catfish production. For controls, G2 non-transgenic broodstock pairs were used to produce G3 non-transgenic catfish.
G3 transgenic mutiara catfish were produced by first maintaining G2 transgenic broodstock until their gonads matured. Maturation of broodstock gonads (5 pairs of broodfish) took place over 1.5 months in a separate rearing tank (2 × 1.5 × 1-m3 round fiberglass). Broodstock were fed a mixture of commercial feed (39% protein content) and pindang tongkol (ratio 1:1) twice daily at 3% of their biomass by weight to promote gonad maturity. During this period, water temperature was maintained at 27 °C ± 1 °C using a water heater, aeration was provided to maintain dissolved oxygen, and photoperiod was set to 10:14 h light to dark. Maturity of broodstock gonads was determined by examining the female and male genital papillae; pink genitals are an indicator of sexual maturity (Das Neves et al. 2019). Subsequently, the broodstock was examined to validate G2 transgenic females and males as follows.
Production of G3 transgenic mutiara catfish
One pair of transgenic broodstock (Fig. 1) was spawned at around 10-month old (female body weight = 440 g, total length = 40 cm, male body weight = 600 g, total length = 44 cm); the breeding scheme is explained in Fig. 2. Semi-artificial spawning was achieved by an injection of Ovaprim hormone (Syndel Laboratories, Ltd., Vancouver, British Columbia, Canada) (0.5 mL/kg body weight of female catfish; and 0.4 mL/kg body weight of male catfish) combined with the use of kakaban, an egg-attachment substrate placed in the bottom of a round fiberglass tank (Buwono 2019b; Kasi et al. 2015). Immediately following injection, the pair was transferred to their spawning tank (1200 L), which was maintained at controlled water temperature (28 ± 1 °C), oxygen level (5 ± 1 ppm), and water volume (400 L). Twelve hours after injection, eggs attached to the kakaban substrate were examined. Fertilized eggs were then transferred into a glass aquarium (50 × 30 × 30 cm) filled with approximately 40 L of water maintained at 27 °C ± 1 °C, with homogenous aeration.
Eighteen hours after spawning, samples (n = 300) of fertilized eggs were taken and transferred to another aquarium for hatching. Two days after larvae hatched, they were fed Artemia nauplii ad libitum until the age of 14 days, and survival of larvae was calculated (Okomoda et al. 2018). G3 catfish fingerlings were then fed live Tubifex sp. twice a day and maintained until they were 1-month old, when they were used in the growth experiment.
CgGH gene transmission and expression
Inheritance and expression of the CgGH transgene in G3 catfish fingerlings was measured as an indication of stable Mendelian CgGH gene inheritance. Catfish that carried the CgGH transgene were determined using electrophoresis seeking observation of a 600-bp amplicon following PCR amplification of the introduced transgene construct. CgGH transgene transmission was calculated using 80 catfish fingerlings, with each sample being pooled from small pieces of fin (from four different fins of each fish) of 1-month old fingerlings. CgGH transmission was calculated using the following formula:
The level of CgGH mRNA expression in the G3 catfish fingerlings was measured with semi-quantitative RT-PCR (sqRT-PCR) with β-actin as an internal expression control (Alimuddin et al. 2008). Total RNA was extracted from larvae and fingerlings using a High Pure RNA Tissue Kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. Synthesis of cDNA and RT-PCR for CgGH was performed using a My Taq OneStep RT-PCR Mix (Bioline, London, UK) with the following program: 48 °C for 20 min; 40 cycles of 95 °C for 1 min, denaturation at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s; and final extension at 72 °C for 5 min. GH-F and GH-R primers were used for CgGH gene amplification, while βAct-F and βAct-R primers were used for amplification of the C. gariepinus β-actin gene (Table 1). The CgGH/β-actin expression ratio was calculated of the 30th and 25th cycles for CgGH and β-actin, respectively. Expression levels were analyzed using Image J software version 1.33 (NIH, USA).
Fish and test feed
Results from molecular analysis identified transgenic catfish carrying the CgGH gene and non-transgenic catfish, at 1-month old (initial weight 6 g ± 0.06 g). In total, 45 transgenic catfish and 15 non-transgenic catfish were used for growth evaluation with various test feed mixtures (Table 2). Also, 11 transgenic (total weight, 66 g) and nine non-transgenic (total weight, 54 g) fish were used for proximate analysis of fingerling bodies at the beginning of the experiment. Growth of G3 transgenic and non-transgenic fingerlings were assessed using mixtures of commercial feed (Prima Feed PF 500) and boiled tuna (pindang tongkol) as test feeds. Moist pindang tongkol was first mixed with commercial feed pellets, and then, 200 mL of warm water was added for every 1 kg of mixture. The final mixture was converted into pellets (i.e., re-pelleted).
G3 catfish were gradually adapted to feed mixtures by initially providing a mixed feed (75% commercial feed and 25% pindang tongkol) for 1 week while monitoring fish appetites and for 3 days before progressing to a mixture of 50% commercial feed and 50% pindang tongkol. Subsequently, the test feed experiment began using the respective mixtures described in Table 2.
Growth performance and amino acid and protein content of G3 catfish
Approximately 170 test fish (G3 fingerlings) were obtained from egg samples following assessment of larval survival. Test feeds (A, B, C, and A*, Table 2) were used as treatments, and growth of transgenic and non-transgenic mutiara catfish was evaluated in triplicate. Each replicate included 15 glass aquariums (60 × 50 × 40 cm) with a water height of 30 cm (90 L) and a fish density of five per aquarium. Catfish were fed twice a day (at 9 am and 5 pm) at a feeding rate of 5% of total biomass. The catfish were reared for 42 days (6 weeks), water was exchanged (50%) every week, water temperature was maintained at 26 °C ± 1 °C using a heater, and photoperiod was 12:12 h light to dark. Oxygen was supplied by an aeration system and maintained at a level of 5.0 mg L−1. Growth measurements from each treatment were taken weekly in order to adjust the amount of feed based on biomass, while the amount of feed residue in each treatment was also observed. At the end of the experiment, the final weight of fish were measured, and the total amount of feed consumed were determined to calculate feed conversion ratio (FCR). Finally, one catfish was taken from each replicate of each treatment for further analysis (50 g used for proximate analysis of whole body and 0.5 g for analysis of amino acids) (Table 3).
Proximate levels of water, ash, protein, lipids, and carbohydrates in fish followed the methods of AOAC (1990). Water content of a test sample (1 g) was calculated gravimetrically using an analytical balance (Mettler Toledo, Colombus, OH, USA). The sample was weighed, then dried in an oven (Heraeus Instruments Athens, Greece) at 105 °C for 4 h, stored in a desiccator, and then re-weighed to determine water content. Ash content (3 g samples) was calculated using dry ashing with a furnace (Thermolyne 1500) at 550–600 °C. The protein content (1 g samples) was measured using the Kjeldahl method. Lipid content (5 g samples) was determined using the Soxhlet method. Carbohydrate content (2.5 g samples) was determined using the titration method (Hall 2008).
Feed and protein conversion efficiency during fish growth was evaluated using the weight-gain parameters FCR, protein retention (PR), and protein productive value (PPV). The formulae used were:
where the final amount of stored protein = % final dry weight × average final weight × final body protein (g), the initial amount of stored protein = % initial dry weight × average initial weight × initial body protein (g), and the amount of feed protein = amount of feed consumed by fish/% mixed protein
The amino acid content of G3 transgenic and non-transgenic catfish was analyzed with 0.5 g of fish muscle tissue at the end of the experiment using ultra-performance liquid chromatography (UPLC; ACQUITY UPLC-H Class, Waters, Milford, MA, USA). Samples were homogenized and hydrolyzed in 5 mL of 6 N HCl and then heated to110 °C for 22 h. A total of 500 μL of solution were extracted using 0.45-μm filter paper, and 40 μL of alpha aminobutyric acid and 460 μL of AquaBidest were added. Approximately 10 μL of this solution was added to 70 μL of AccQ-Fluor Borate and 20 μL of Fluor A reagent, and total mixtures were vortexed for 1 min. Finally, solutions were incubated at 55 °C for 10 min before being injected into the UPLC system (Rosmawati et al. 2018; Ignatz et al. 2020).
Statistical analysis
Data on weight gain, FCR, protein content, and amino acid content were quantitatively analyzed using one-way ANOVA, with a significance level of p ˂ 0.05 used to test differences between the transgenic and non-transgenic fish, and a post hoc Duncan’s multiple range test used to assess significance of differences among treatments. The frequency of CgGH of inheritance and CgGH/β-actin expression ratio of G3 fish were analyzed using comparative descriptions.
Results
Detection of CgGH gene in G2 transgenic broodstock
RT-PCR analysis positively identified male and female G2 mutiara catfish broodstock with the CgGH transgene (i.e., exhibiting a PCR amplification products of 600 bp), indicating that both were transgenic and appropriate for G3 catfish production (Fig. 3).
Electropherogram for detection of female and male G2 transgenic mutiara catfish (Clarias gariepinus) broodstock. CgGH represents C. gariepinus growth hormone (600 bp) and β-actin (200 bp) represents the internal expression control. Tested broodstock: one female and two males. P, pCMV-CgGH plasmid; M1, 1-kb DNA ladder; M2, 100-bp DNA ladder
Transmission and expression of CgGH gene
Transmission of genes in transgenic catfish was expected to be inherited following the Mendelian law based on our previous study (Fig. 2). Observation based on the RT-PCR analysis of G3 catfish fingerlings showed that inheritance of the CgGH transgene was 70%, an increase over 50% inheritance reported in G2 transgenic mutiara catfish (Buwono et al. 2019a), therefore crossing between G2 transgenic broodstock tends to increase the transmission of CgGH in G3. The level of exogenous GH gene expression also tended to be higher in G3 than in G2 catfish (Table 4 and Fig. 4).
Electropherogram of CgGH expression in transgenic mutiara catfish (Clarias gariepinus) fingerlings with numbers 1–10 (a) and numbers 11–20 (b). CgGH represents the C. gariepinus growth hormone gene (600 bp), and β-actin (200 bp) represents the internal expression control. The numbers 1–20 represent the fish sample number. M1, 1-kb DNA ladder; M2, 100-bp DNA ladder; P, pCMV-CgGH plasmid
Growth performance
After 42 days, body weight measurements and proximate analysis of G3 catfish showed a tendency for body protein content of transgenic catfish in each treatment to be higher than in non-transgenic catfish (Table 5). A concurrent trend of increased body weight at the end of the experiment was also observed (Table 5 and Fig. 5a). Furthermore, feed conversion rate was higher in transgenic catfish than in non-transgenic catfish (Fig. 5b).
Average weight gain (a) and feed conversion ratio (b) for G3 transgenic and non-transgenic mutiara catfish (Clarias gariepinus). Data are represented as means ± SEM. Different lowercase letters indicate that the means are significantly different (p ˂ 0.05). Feeds contained commercial feed and boiled tuna (Euthynnus affinis) at the following ratios: A (50:50), B (65:35), C (80:20), and A* (50:50). Feeds A–C were fed to transgenic catfish, while feed A* was fed to non-transgenic catfish
Protein content of G3 catfish
The protein content of G3 transgenic mutiara catfish varied depending on the ratio between commercial feed and pindang tongkol. The feed composition was predicted to increase the responsiveness of fish to feed, thereby stimulating higher consumption and greater growth and promoted protein accumulation in the fish body (Table 5). Increased protein storage was reflected in PR values, which were typically greater for transgenic catfish than for non-transgenic catfish. The exception was fish fed feed C that showed no increase in PR (Fig. 6a). PPV of transgenic catfish (given feeds A, B, or C) was higher than PPV of non-transgenic catfish (given feed A*, Fig. 6b).
Protein retention (a) and protein productive value (b) of G3 transgenic and non-transgenic mutiara catfish (Clarias gariepinus). Different lowercase letters indicate that means are significantly different (p ˂ 0.05). Feeds contained commercial feed and boiled tuna (Euthynnus affinis) at the following ratios: A (50:50), B (65:35), C (80:20), and A* (50:50). Feeds A–C were fed to transgenic catfish, while feed A* was fed to non-transgenic catfish
Amino acid content of G3 fish
Analysis of essential and non-essential amino acid content in G3 transgenic and non-transgenic mutiara catfish indicated that both groups of amino acids tended to show higher levels in transgenic catfish (Table 6; Fig. 7a and b).
Essential (a) and non-essential (b) amino acid content of G3 transgenic and non-transgenic mutiara catfish. Data are represented as means ± SD. Different lowercase letters indicate that the means are significantly different (p ˂ 0.05). Feeds contained commercial feed and boiled tuna (Euthynnus affinis) at the following ratios: A (50:50), B (65:35), C (80:20), and A* (50:50). Feeds A–C were fed to transgenic catfish, while feed A* was fed to non-transgenic catfish
Discussion
Stability of CgGH expression and inheritance in G3 transgenic mutiara catfish
The transmission of exogenous transgene from one generation to the next depends on stable integration into host genomes. Expression of the exogenous gene may produce a phenotypic improvement of transgenic fish (Chen et al. 2015). A high percentage of transgene transmission is vital for ensuring inheritance of exogenous genes in future generations. CgGH gene inheritance and expression levels in the present study were higher in G3 transgenic mutiara catfish fingerlings (70%) than in G2 catfish (50%) in a previous study (Buwono 2019a), as was CgGH/β-actin expression ratio (1.80–3.21 vs. 0.96–1.37, respectively). We suspected that the increased transmission of CgGH transgene in the third generation was related to the use of G2 transgenic female broodstock crossed between transgenics in the homozygous state making it possible for more individuals to carry the transgene (Wang et al. 2001; Wu et al. 2003). Based on that theory, our G2 transgenic catfish broodstock was potentially possible for G3 catfish production. It was known that transmission of genes in transgenic fish suggest inheritance in a Mendelian fashion as reported in studies of G3 transgenic mud loach (Misgurnus mizolepis) where 50–53.7% of inheritance was found, suggesting Mendelian inheritance in transgenic mud loach (Nam et al. 1999), while studies in transgenic tilapia also supports this theory (Martinez et al. 1999). Stable inheritance of exogenous GH genes will assure accompanying efficient expression in offspring (Philips and Devlin 2010).
The growth of G3 mutiara catfish
On average, G3 transgenic mutiara catfish weighed approximately 2 to 3 times more than non-transgenic fish at the end of the experiment. Significant weight difference was observed in G3 transgenic catfish compared to non-transgenic (Table 5 and Fig. 8). Higher growth performance in transgenic fish was suggested to be caused by increase in food intake in transgenic fish (Raven et al. 2006; Higgs et al. 2009). Leggatt et al. (2009) suggested that transgenic coho salmon fish had an increased ability to use carbohydrates for energy to spare protein and lipid, hence supporting higher growth. This may explain results in our study where transgenic fish given the mixture of commercial feed with pindang tongkol had higher growth and protein content compared to the non-transgenic counterparts. In previous studies, increased growth was reported in transgenic common carp (containing transgene pCAgcGH) at G1 (1.6-fold) (Wang et al. 2001), G2 (1.8–2.5-fold), and G3 (1.4–1.9-fold) (Li et al. 2007) relative to non-transgenic fish. Thus, overexpression of exogenous GH gene may result in increased growth of transgenic fish, with increases > 100% possible (Nam et al. 2001).
We found that G3 transgenic mutiara catfish gained the most weight when fed feed B (65% commercial feed and 35% pindang tongkol). We suggest that the balanced protein mixture in feed B promoted increased expression of the CgGH gene that stimulates IGF-I production saying that this should be examined in future studies of GH gene expression in this line. IGF-1 increases responsiveness to the feed and, in turn, accelerates the conversion of feed into body protein (Cook et al. 2000; Raven et al. 2008). This hypothesis is supported by the increase in catfish body protein levels observed at the end of the present study.
G3 transgenic mutiara catfish fed with feed B showed both increased body weight and increased conversion of feed protein into body tissue protein. Consequently, these fish had 17.99% body protein content at the end of the experiment, which was > 2% higher than transgenic catfish fed on any other test feeds. Increased protein levels were shown previously in transgenic common carp, expressing rainbow trout growth hormone gene, with protein levels of 19.48% and 18.02% observed in G1 and G2, respectively (Chatakondi et al. 1995; Dunham et al. 2002). GH overexpression was suggested to be general importance for increasing fish growth, improving the efficiency of feed use, and increasing body protein (Oakes et al. 2007). In our study, higher performance was found for feed B. We infer that the nutrient composition in feed B increased the responsiveness of fish to feed compared to other test feeds.
Feed conversion ratio
G3 transgenic catfish showed improved FCR (means 0.78–1.00) relative to non-transgenic catfish (mean 1.36), consistent with increased fish growth. A previous study on transgenic Nile tilapia (Oreochromis niloticus) showed a similar FCR value of 0.76 (Rahman et al. 1998). In our study, feed B—with 37.88% protein—led to the lowest FCR, resulting in the greatest growth. The nutritional value of feed is determined by both the amount of protein and the composition of feed protein mixture. The latter is critical for providing essential amino acids (Fu et al. 2000; Wilson 1986; Raven et al. 2006; Rosa et al. 2007).
GH-transgenic fish efficiently convert lipids and carbohydrates in feed into energy for use in routine metabolic activities; therefore, feed protein is spared from the maintenance of basal metabolism. Excess protein is instead used for more rapid growth, as previously observed in GH-transgenic coho salmon (Oakes et al. 2007) and GH-transgenic Nile tilapia (Rahman and Maclean 1999). Protein sparing that promotes growth also was shown in the present study with transgenic catfish. Specifically, catfish were fed feeds A and B that had lipid contents of 1.86% and 1.92%, respectively, and carbohydrate contents of 6.29% and 6.53%, respectively, at the end of the experiment. These were the lowest levels among treatments. Final measurements in transgenic fish showed significantly lower carbohydrate content in transgenic fish samples compared to non-transgenic fish. This suggests the ability of transgenic fish to utilize carbohydrate as an energy source, hence lowering the carbohydrate content in fish. In addition, a protein-sparing action in transgenic fish may also occur causing an increase in protein storage.
Protein retention in G3 mutiara catfish
We found that PR differed among G3 transgenic and non-transgenic mutiara catfish according to feed. Feed B, with a commercial feed to pindang tongkol ratio of 65:35, increased PR in transgenic catfish relative to that in catfish fed feeds A (50:50 ratio) or C (80:20 ratio). Non-transgenic catfish fed a 50:50 ratio showed the lowest PR. These observations are supported by concurrent increases in catfish body protein content measured at the end of the experiment. The difference between PRs resulting from feeds B and C is perhaps due to different levels of pindang tongkol in these feeds. The lower amount of pindang tongkol in feed C likely reduced available amino acids that, in turn, reduced body protein content and therefore PR.
Protein stored in the body that includes sufficient essential amino acids to meet metabolic requirements has more influence on growth than high levels of feed protein (Wilson and Poe 1985; Cowey 1994; Fu et al. 2000). In the present study, reduced PR resulted in less average weight gain in G3 transgenic mutiara catfish fed feed C, and non-transgenic fish fed feed A*. The utilization limit for pindang tongkol that resulted in optimum PR was 35%. Using 50% pindang tongkol did not result in higher PR in non-transgenic catfish (feed A*); however, PR in transgenic catfish fed feed A, also a 50:50 ratio, was higher than that of non-transgenic catfish. Including 50% pindang tongkol in feed did improve PR in transgenic catfish, but using 35% (feed B) provided better results. Overexpression of the CgGH gene thus effectively promoted the conversion of feeds A and B protein into body protein, and we conclude that the balance of essential to non-essential protein in these two feeds was likely sufficient for maximum catfish growth. Overexpression of exogenous GH increased protein levels and PR in transgenic coho salmon (Oakes et al. 2007; Higgs et al. 2009), and increased growth and PR in transgenic Arctic charr (Salvelinus alpinus) (Pitkanen et al. 1999) and transgenic Nile tilapia (Kobayashi et al. 2007).
Protein productive value of G3 catfish
PPV, the ratio of a fish’s body protein at the end of an experiment to feed protein consumed (Hepher 1988), can be used to evaluate protein use efficiency. G3 transgenic mutiara catfish were more efficient at using feed protein than non-transgenic catfish: PPVs for G3 transgenic mutiara catfish were 6.94–12.3%, but only 4.39% for non-transgenic catfish. Previous studies of GH-transgenic Atlantic salmon (Salmo salar) reported a similar PPV, 10% (Cook et al. 2000), whereas PPVs in GH-transgenic Nile tilapia were somewhat higher, 15–20% (Rahman et al. 2001). Our observed increased in PPV is likely due to CgGH gene expression, which increases protein synthesis and, in turn, the protein content of transgenic catfish. Exogenous GH induced glycolytic enzymes in transgenic coho salmon, which caused the catabolism of carbohydrates and lipids to produce metabolic energy and led to increased body protein deposits (Leggatt et al. 2009). Such an increase in protein correlates with the levels of exogenous GH expression observed in our study. Further, higher PPVs of G3 transgenic mutiara catfish relative to those of non-transgenic catfish also supports these conclusions. Expression of exogenous GH in transgenic fish resulted in GH levels higher than found in non-transgenic individuals in previous studies. These levels led to increased body protein content (Devlin et al. 2000; Dunham et al. 2002; Raven et al. 2008; Higgs et al. 2009). Transgenesis increases the activity of enzymes involved in the Krebs cycle (TCA cycle); these enzymes break down carbohydrates and lipids and allow proteins to feed essential amino acid pools (Melzer 2011; Leggatt et al. 2009).
In our study, PPVs were closely correlated with fish PR values as well as ratios of mixed proteins in feeds. The PPVs of catfish fed feeds B and C were proportional to the increases and decreases, respectively, in the PR of fish that consumed these feeds. In addition, protein-sparing was observed in G3 transgenic catfish fed feeds A and B, as indicated by the decreased end-of-experiment lipid and carbohydrate levels of catfish that consumed these feeds; this decrease likely caused PR and PPV to be higher in transgenic catfish.
Amino acid content of G3 transgenic catfish
The protein content in feed can affect the ability of the feed to provide essential amino acids; the content of the amino acids in feed protein determines the balance between essential and non-essential amino acids. An imbalance in essential and non-essential can lead to amino acid deficiency, which will limit weight gain (Wilson and Poe 1985; Cowey 1994; Ahmed 2012; Cao et al. 2012; Zhang et al. 2017).
G3 transgenic mutiara catfish had higher essential amino acid content than non-transgenic catfish. The suggested effect of GH was its importance in affecting the breakdown of carbohydrates and lipids through a complex enzymatic pathways into pyruvic acid and then converted to amino acids; hence, both contribute to the increase of amino acid content (Melzer 2011). Feed B led to the highest essential amino acid content among transgenic fish; the content in these fish was greater than the essential amino acid requirement for C. gariepinus (Rosa et al. 2007). While feeds A, B, and C increased the weight gain of transgenic fish by 2.8-, 3.5-, and 2.3-fold, respectively, non-transgenic catfish fed feed A* (equivalent to feed A) were deficient in histidine, lysine, and methionine (Rosa et al. 2007). Thus, increasing the amount of essential amino acids in feed above standard requirements can substantially boost weight gain. Other transgenic fish species also showed an increase in essential amino acid content, e.g., GH-transgenic olive flounder (Paralichthys olivaceus) when fed commercial feed (Liu et al. 2008) and GH-transgenic Cyprinus carpio (Chatakondi et al. 1995; Fu et al. 2000). Essential amino acid content increased in the presence of exogenous GH, which may reflect the role of GH in influence intestinal absorption and renal reabsorption of protein and amino acid metabolism in fish (Farmanfarmaian and Sun 1999).
Deficiency in any one of the three limiting essential amino acids that must be available in fish feed, namely arginine, lysine, and methionine, leads to reduced fish growth (Green and Hardy 2002; Ahmed 2012). These amino acids are thus limiting factors for growth (Wilson and Poe 1985) and must be available in feed at levels sufficient to meet requirements for protein synthesis (Small and Soares 1998; Green and Hardy 2002). Our results showed that arginine, lysine, and methionine were present at increased levels, above the standard requirement for C. gariepinus (Rosa et al. 2007), in G3 catfish fed feeds A and B; however, consuming feed C led to a lysine deficiency and decreased weight gain, PR, and PPV. Non-transgenic catfish having been fed only feed A* were deficient in both lysine and methionine. Feed B increased the content of limiting essential amino acids in transgenic catfish to levels that were sufficient for optimum growth.
Feed B also increased the levels of non-essential amino acids (glutamic acid, aspartic acid, glycine, serine, proline, tyrosine, and alanine) in G3 transgenic mutiara catfish above those required for C. gariepinus (Rosa et al. 2007). In contrast, consuming feed A led to proline deficiency; feed C led to aspartic acid and proline deficiency; and feed A* led to deficiencies in aspartic acid, proline, and tyrosine. Given that consuming feed B led to optimal growth in G3 transgenic mutiara catfish, these results indicate that enough non-essential amino acids are required in fish feed to support the optimal growth of transgenic catfish.
Conclusions
The effect of CgGH gene transgenesis on growth and protein and amino acid content of G3 transgenic mutiara catfish was affected by the ratio of commercial feed and pindang tongkol in the feed mix. We identified a feed mix that improved body protein content and increased levels of essential and non-essential amino acids of G3 transgenic mutiara catfish. Using feeds deficient in essential or non-essential or amino acids reduced fish growth, PR, and efficiency of feed protein utilization. By finding an optimum balance of one feed component mixture, we provided important information that can be used to improve growth and feeding efficiency in transgenic catfish.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CgGH:
-
Clarias gariepinus growth hormone
- CMVOnGH1:
-
cytomegalovirus Oncorhynchus growth hormone 1
- FCR:
-
feed conversion ratio
- OnMTGH1:
-
Oncorhynchus metallothionein growth hormone 1
- pCAgcGH:
-
plasmid Carp actin grass carp growth hormone
- pCMV-CgGH:
-
plasmid Cytomegalovirus Clarias gariepinus growth hormone
- PPV:
-
protein productive value
- PR:
-
protein retention
- UPLC:
-
ultra-performance liquid chromatography
References
Ahmed I (2012) Dietary amino acid L-tryptophan requirement of fingerling Indian catfish, Heteropneustes fossilis (Bloch), estimated by growth and haemato-biochemical parameters. Fish Physiol Biochem 38:1195–1209. https://doi.org/10.1007/s10695-012-9609-1
Alimuddin A, Kiron V, Satoh S, Takeuchi T, Yoshizaki G (2008) Cloning and over-expression of a masu salmon (Oncorhynchus masou) fatty acid elongase-like gene in zebrafish. Aquaculture 282:13–18. https://doi.org/10.1016/j.aquaculture.2008.06.033
AOAC (1990) The official methods of analyses 15th edn. Association of Official Analytical Chemists International. Arlington, Virginia, USA
Buwono ID, Junianto J, Iskandar I, Alimuddin A (2019a) Growth and expression level of growth hormone in transgenic mutiara catfish second generation. J Biotech Res 10:102–109
Buwono ID, Junianto J, Iskandar I, Alimuddin A (2019b) Reproduction performance of transgenic mutiara catfish (G1) comprising the growth hormone gene. J Biotech Res 10:199–212
Cao JM, ChenY ZX, Huang YH, Zhao HX, Li GL, Lan HB, Chen B, Pan Q (2012) A study on dietary L-lysine requirement of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac Nutr 18:35–45. https://doi.org/10.1111/j.1365-2095.2011.00874.x
Chatakondi N, Lovell RT, Duncan PL, Hayat M, Chen TT, Powers DA, Weete JD, Cummins K, Dunham RA (1995) Body composition of transgenic common carp, Cyprinus carpio, containing rainbow trout growth hormone gene. Aquaculture 138:99–109. https://doi.org/10.1016/0044-8486(95)01078-5
Chen J, Luo Q, Bao H, Liao L, LiY ZZ, Wang Y, Hu W (2015) The integration characteristics of the exogenous growth hormone gene in a transgenic common carp (Cyprinus carpio L.) with fast-growth performance. Sci Bull 60:1654–1660. https://doi.org/10.1007/s11434-015-0893-x
Cook JT, McNiven MA, Richardson GF, Sutterlin AM (2000) Growth rate, body composition and feed digestibility/conversion of growth-enhanced transgenic Atlantic salmon (Salmo salar). Aquaculture 188:15–32. https://doi.org/10.1016/S0044-8486(00)00331-8
Cowey CB (1994) Amino acid requirements of fish: a critical appraisal of present values. Aquaculture 124:1–11. https://doi.org/10.1016/0044-8486(94)90349-2
Das Neves JL, Coetzee H, Barnhoorn I, Wagenaar I (2019) The urogenital papillae as an indicator of sexual maturity in male African catfish, Clarias gariepinus (Burchell, 1822). Aquac Res 50:3519–3527. https://doi.org/10.1111/are.14283
Devlin RH, Swanson P, Clarke WC, Plisetskaya E, Dickhoff W, Moriyama S, Yesaki TY, Hew CL (2000) Seawater adaptability and hormone levels in growth-enhanced transgenic coho salmon, Oncorhynchus kisutch. Aquaculture 191:367–385. https://doi.org/10.1016/S0044-8486(00)00484-1
Devlin RH, Biagi CA, Yesaki TY (2004) Growth, viability and genetic characteristics of GH transgenic coho salmon strains. Aquaculture 236:607–632. https://doi.org/10.1016/j.aquaculture.2004.02.026
Dunham RA, Chatakondi N, Nichols AJ, Kucuktas H, Chen TT, Powers DA, Weete JD, Cummins K, Lovell RT (2002) Effect of rainbow trout growth hormone complementary DNA on body shape, carcass yield, and carcass composition of F1 and F2 transgenic common carp (Cyprinus carpio). Mar Biotechnol 4:604–611. https://doi.org/10.1007/s10126-002-0034-9
Eppler E, Caelers A, Shved N, Hwang G, Rahman AM, Maclean N, Zapf J, Reinecke M (2007) Insulin-like growth factor I (IGF-I) in a growth-enhanced transgenic (GH-overexpressing) bony fish, the tilapia (Oreochromis niloticus): indication for a higher impact of autocrine/paracrine than of endocrine IGF-I. Transgenic Res 16(4):479–489. https://doi.org/10.1007/s11248-007-9093-z
Farhat MA, Khan MA (2012) Effects of dietary arginine levels on growth, feed conversion, protein productive value and carcass composition of stinging catfish fingerling Heteropneustes fossilis (Bloch). Aquac Int 20:935–950. https://doi.org/10.1007/s10499-012-9519-3
Farmanfarmaian A, Sun LZ (1999) Growth hormone effects on essential amino acid absorption, muscle amino acid profile, N-retention and nutritional requirements of striped bass hybrids. Genet Anal 15:107–113. https://doi.org/10.1016/S1050-3862(99)00012-1
Fauconneau B, Mady MP, Le Bail PY (1996) Effect of growth hormone on muscle protein synthesis in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Fish Physiol Biochem 15:49–56. https://doi.org/10.1007/BF01874837
Fu C, Cui Y, Hung SSO, Zhu Z (2000) Whole-body amino acid pattern of F4 human growth hormone gene-transgenic red common carp (Cyprinus carpio) fed diets with different protein levels. Aquaculture 189:287–292. https://doi.org/10.1016/S0044-8486(00)00381-1
Green JA, Hardy RW (2002) The optimum dietary essential amino acid pattern for rainbow trout (Oncorhynchus mykiss), to maximize nitrogen retention and minimize nitrogen excretion. Fish Physiol Biochem 27:97–108. https://doi.org/10.1023/B:FISH.0000021878.81647.6e
Hall MB (2008) Determination of starch, including maltooligosaccharides, in animal feeds: comparison of methods and a method recommended for AOAC collaborative study. J AOAC Int 92:42–49
Hepher B (1988) Nutrition of pond fishes. Cambridge University Press, Cambridge, UK
Higgs DA, Sutton JN, Kim H, Oakes JD, Smith J, Biagi C, Rowshandeli M, Devlin RH (2009) Influence of dietary concentrations of protein, lipid and carbohydrate on growth, protein and energy utilization, body composition, and plasma titres of growth hormone and insulin-like growth factor-1 in non-transgenic and growth hormone transgenic coho salmon, Oncorhynchus kisutch (Walbaum). Aquaculture 286:127–137. https://doi.org/10.1016/j.aquaculture.2008.08.036
Ignatz EH, Dumas A, Benfey TJ, Hori TS, Braden LM, Runighan CD, Rise ML, Westcott JD (2020) Growth performance and nutrient utilization of growth hormone transgenic female triploid Atlantic salmon (Salmo salar) reared at three temperatures in a land-based freshwater recirculating aquaculture system (RAS). Aquaculture 519:1–11. https://doi.org/10.1016/j.aquaculture.2019.734896
Kasi M, Nirmell S, Aminur Rahman M, Arshad A, Gokul RM, Arockiaraj J (2015) Induced ovulation and spawning of African catfish Clarias gariepinus (Bloch) using ovaprim. Environ Biotechnol Res 1:2–9
Kim KD, Jang JW, Kim KW, Lee BJ, Hur SW, Han HS (2018) Tuna by-product meal as a dietary protein source replacing fish meal in juvenile Korean rockfish Sebastes schlegeli. Fish Aquat Sci 21:1–8. https://doi.org/10.1186/s41240-018-0107-y
Kobayashi S, Alimuddin MT, Miwa M, Lu J, Endo M, Takeuchi T, Yoshizaki G (2007) Transgenic Nile tilapia (Oreochromis niloticus) over-expressing growth hormone show reduced ammonia excretion. Aquaculture 270:427–435. https://doi.org/10.1016/j.aquaculture.2007.05.016
Leggatt RA, Raven PA, Mommsen TP, Sakhrani D, Higgs D, Devlin RH (2009) Growth hormone transgenesis influences carbohydrate, lipid and protein metabolism capacity for energy production in coho salmon (Oncorhynchus kisutch). Comp Biochem Physiol Part B 154:121–133. https://doi.org/10.1016/j.cbpb.2009.05.010
Levesque HM, Shears MA, Fletcher GL, Moon TW (2008) Myogenesis and muscle metabolism in juvenile Atlantic salmon (Salmo salar) made transgenic for growth hormone. J Exp Biol 211:128–137. https://doi.org/10.1242/jeb.006890
Li D, Fu C, HuW ZS, Wang Y, Zhu Z (2007) Rapid growth cost in “all-fish” growth hormone gene transgenic carp: reduced critical swimming speed. Chin Sci Bull 52:1501–1506. https://doi.org/10.1007/s11434-007-0217-x
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53. https://doi.org/10.1007/s00726-008-0171-1
Liu S, Zhang X, Zang X, Liu B, Arunakumara K, Xu D, Zhang X (2008) Growth, feed efficiency, body muscle composition, and histology of flounder (Paralichthys olivaceus) fed GH transgenic Synechocystis. Aquaculture 277:78–82. https://doi.org/10.1016/j.aquaculture.2008.02.011
Martınez R, Arenal A, Estrada MP, Herrera F, Huerta V, Vazquez J, Sanchez T, de la Fuente J (1999) Mendelian transmission, transgene dosage and growth phenotype in transgenic tilapia (Oreochromis hornorum) showing ectopic expression of homologous growth hormone. Aquaculture 173:271–283
Mauras N, Haymond MW (2005) Are the metabolic effects of GH and IGF-I separable? Growth Hormon IGF Res 15:19–27. https://doi.org/10.1016/j.ghir.2004.12.003
Melzer K (2011) Carbohydrate and fat utilization during rest and physical activity. J Clin Nutr Metab 6:e45–e52. https://doi.org/10.1016/j.eclnm.2011.01.005
Nam YK, Noh CH, Kim DS (1999) Transmission and expression of an integrated reporter construct in three generations of transgenic mud loach (Misgurnus mizolepis). Aquaculture 172:229–245. https://doi.org/10.1016/S0044-8486(98)00433-5
Nam YK, Noh JK, Cho YS, Cho HJ, Cho KN, Kim CG, Kim DS (2001) Dramatically accelerated growth and extraordinary gigantism of transgenic mud loach Misgurnus mizolepis. Transgenic Res 10:353–362. https://doi.org/10.1023/A:101669610418
Oakes JD, Higgs DA, Eales JG, Devlin RH (2007) Influence of ration level on the growth performance and body composition of non-transgenic and growth-hormone-transgenic coho salmon (Oncorhynchus kisutch). Aquaculture 265:309–324. https://doi.org/10.1016/j.aquaculture.2007.01.015
Okomoda VT, Koh ICC, Shahreza SM (2018) A simple technique for accurate estimation of fertilization rate with specific application to Clarias gariepinus (Burchell, 1822). Aquac Res 49:1116–1121. https://doi.org/10.1111/are.13528
Phillips RB, Devlin RH (2010) Integration of growth hormone gene constructs in transgenic strains of coho salmon (Oncorhynchus kisutch) at centromeric or telomeric sites. Genome 53:79–82. https://doi.org/10.1139/G09-074
Pitkänen TI, Krasnov A, Teerijoki H, Mölsä H (1999) Transfer of growth hormone (GH) transgenes into Arctic charr (Salvelinus alpinus L.): I. Growth response to various GH constructs. Genetic Analysis: Biomol Eng 15:91–98
Raghuveer K, Senthilkumaran B (2010) Cloning and differential expression pattern of vasa in the developing and recrudescing gonads of catfish, Clarias gariepinus. Comp Biochem Physiol Part A: Mol Integr Physiol 157:79–85. https://doi.org/10.1016/j.cbpa.2010.04.017
Rahman MA, Maclean N (1999) Growth performance of transgenic tilapia containing an exogenous piscine growth hormone gene. Aquaculture 173:333–346. https://doi.org/10.1016/S0044-8486(98)00456-6
Rahman MA, Mak R, Ayad H, Smith A, Maclean N (1998) Expression of a novel piscine growth hormone gene results in growth enhancement in transgenic tilapia (Oreochromis niloticus). Transgenic Res 7:357–369. https://doi.org/10.1023/A:1008837105299
Rahman MA, Ronyai A, Engidaw BZ, Jauncey K, Hwang GL, Smith A, Roderick E, Penman D, Varadi L, Maclean N (2001) Growth and nutritional trials on transgenic Nile tilapia containing an exogenous fish growth hormone gene. J Fish Biol 59:62–78. https://doi.org/10.1111/j.1095-8649.2001.tb02338.x
Raven PA, Devlin RH, Higgs DA (2006) Influence of dietary digestible energy content on growth, protein and energy utilization and body composition of growth hormone transgenic and non-transgenic coho salmon (Oncorhynchus kisutch). Aquaculture 254:730–747. https://doi.org/10.1016/j.aquaculture.2005.11.009
Raven PA, Uh M, Sakhrani D, Beckman BR, Cooper K, Pinter J, Leder EH, Silverstein J, Devlin RH (2008) Endocrine effects of growth hormone overexpression in transgenic coho salmon. Gen Comp Endocrinol 159:26–37. https://doi.org/10.1016/j.ygcen.2008.07.011
Rosa R, Bandarra NM, Nunes ML (2007) Nutritional quality of African catfish Clarias gariepinus (Burchell 1822): a positive criterion for the future development of the European production of Siluroidei. Int J Food Sci Technol 42:342–351. https://doi.org/10.1111/j.1365-2621.2006.01256.x
Rosa CE, Figueiredo MA, Lanes CFC, Almeida DV, Monserrat JM, Marins LF (2008) Metabolic rate and reactive oxygen species production in different genotypes of GH-transgenic zebrafish. Comp Biochem Physiol Part B 149:209–214. https://doi.org/10.1016/j.cbpb.2007.09.010
Rosmawati AE, Tawali AB, Said MI, Sari DK (2018) Effect of body weight on the chemical composition and collagen content of snakehead fish Channa striata skin. Fish Sci 84:1081–1089. https://doi.org/10.1007/s12562-018-1248-8
Small BC, Soares JH (1998) Estimating the quantitative essential amino acid requirements of striped bass Morone saxatilis, using fillet A/E ratios. Aquac Nutr 4:225–232. https://doi.org/10.1046/j.1365-2095.1998.00075.x
Subyakto S, Alimuddin R, Sasmito JM, Faizal I, Aliah RS, Triastutik G, Sumantadinata K (2011) Comparison of three different techniques of gene transfer in Humpback Grouper (Cromileptes altivelis). Biotropia-The Southeast Asian Journal of Tropical Biology 18:13–23
Terlouw EMC, Arnould C, Auperin B, Berri C, Le Bihan-Duval E, Deiss V, Lefevre F, Lensink BJ, Mounier L (2008) Pre-slaughter conditions, animal stress and welfare: current status and possible future research. Animal: an International Journal of Animal Bioscience 2:1501–1517. https://doi.org/10.1017/S1751731108002723
Uju BR, Ibrahim B, Ramadhan W, Tanjung IS (2017) Recovery process and concentration of drinking water protein “pindang tongkol” (Euthynnus affinis) through ultrafiltration. J Agroind Technol 27:281–290. https://doi.org/10.24961/j.tek.ind.pert.2017.27.3.281
Wang Y, Hu W, Wu G, Sun Y, Chen S, Zhang F, Zhu Z, Feng J, Zhang X (2001) Genetic analysis of “all-fish” growth hormone gene transferred carp (Cyprinus carpio L.) and its F1 generation. Chin Sci Bull 46:a1–a4. https://doi.org/10.1007/BF02900596
Wilson RP (1986) Protein and amino acid requirements of fishes. Annu Rev Nutr 6:225–244. https://doi.org/10.1146/annurev.nu.06.070186.001301
Wilson RP, Poe WE (1985) Relationship of whole body and egg essential amino acid patterns to amino acid requirement patterns in channel catfish, Ictalurus punctatus. Comp Biochem Physiol B 80:385–388. https://doi.org/10.1016/0305-0491(85)90224-X
Wu G, Sun Y, Zhu Z (2003) Growth hormone gene transfer in common carp. Aquat Living Resour 16:416–420. https://doi.org/10.1016/S0990-7440(03)00087-1
Zhang MQ, Chen CX, Guo YJ, Guo J, Wang XM (2009) Cloning and sequence analysis of full-length growth hormone cDNA from Clarias gariepinus. Acta Agric Boreali-Sinica 24:27–32
Zhang S, Zeng X, Ren M, Mao X, Qiao S (2017) Novel metabolic and physiological functions of branched chain amino acids: a review. J Ani Sci Biotech 8:1–12. https://doi.org/10.1186/s40104-016-0139-z
Acknowledgments
The authors are thankful to the Directorate of Research and Community Service of the Universitas Padjadjaran for support of research. The authors also thank the research team for technical support during the research work. Thanks to Mr. Rosadi, Mr. Udin, Maulana Iqbal, Rahayu, and Pratiwi for help with the rearing of fish and the data collection.
Funding
This work was supported by the Directorate of Research and Community Service of Padjadjaran University through the financial support of Daftar Isian Pelaksana Anggaran Badan Layanan Umum (Contract Number: 1427/UN6.3.1/LT/2020).
Author information
Authors and Affiliations
Contributions
IDB and II conducted the fish trial, reared fish, and collected data; IDB and RG analyzed and interpreted data and wrote the manuscript. The design of the study and data analysis and manuscript formatting involved all authors. All authors also critically reviewed the manuscript for intellectual content and gave final approval for the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
This article does not require ethic approval since fish was not listed by the Ethic Commission of Education Ministry and Universitas Padjadjaran Ethic Commission. However, guidelines on fish handling during and after research were based on literature on fish welfare (Terlouw et al. 2008).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buwono, I.D., Iskandar, I. & Grandiosa, R. Growth hormone transgenesis and feed composition influence growth and protein and amino acid content in transgenic G3 mutiara catfish (Clarias gariepinus). Aquacult Int 29, 431–451 (2021). https://doi.org/10.1007/s10499-020-00628-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00628-8