Abstract
Blood analysis is a very important and powerful diagnostic tool in animal health and welfare control. It is routinely performed in higher vertebrates, for which reference values are well established, but fish hematology still needs further research. Many intrinsic and environmental factors have profound impact on fish hematological values, making determination of reference values difficult. Additionally, fish blood usually requires the addition of an anticoagulant agent, because of short clotting times. The choice of anticoagulant is vital for obtaining reliable blood test values. In the present study, the impact of two common anticoagulants, K2EDTA (1.8 mg/ml) and lithium heparin (18 I.U./ml), on hematological values of farmed brown trout Salmo trutta spawners during the spawning season was investigated. Results of basic hematological analysis, such as packed cell volume (PCV), hemoglobin concentration (HGB), red blood cell count (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and white blood cell count (WBC), were compared between these two compounds. Statistically significant differences were observed in PCV, MCV, and MCHC, whereas HGB, RBC, MCH, and WBC showed no such differences. These results suggest that lithium heparin gives more reliable results, because red blood cells in K2EDTA-treated samples have a tendency to swell. It is worth noting that ethylenediaminetetraacetic acid salt did not induce sample hemolysis in the present study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown trout (Salmo trutta) belongs to the Salmonidae family, together with Atlantic salmon (Salmo salar), Arctic charr (Salvelinus alpinus), rainbow trout (Oncorhynchus mykiss), common whitefish (Coregonus lavaretus), and grayling (Thymallus thymallus) (Frank-Gopolos et al. 2015). There are several ecological forms of brown trout—few of which show facultative migratory character (Bagliniere and Maisse 1999). It can utilize various watercourses, lakes, estuaries, and sea coastal zones (Jonsson and Jonsson 2011). Variability of ecological forms and the ability to adapt to new environmental conditions are the main reasons for its wide geographical distribution (Klemetsen et al. 2003). Among introduced salmonid species, brown trout achieved the greatest success in establishing stable populations outside of its native range (Bagliniere and Maisse 1999).
Brown trout is native to Western Europe, from the coast of Portugal to the White Sea, including the Baltic Sea and Iceland. It was introduced to many European countries (mostly in rivers and estuaries along the northern coast of the Mediterranean, Black, and Caspian Sea), both North and South America, Southern and Eastern Africa and Asia, Australia, and New Zealand (Freyhof 2011). It is considered as a game fish throughout its entire range of distribution (Klemetsen et al. 2003; Frank-Gopolos et al. 2015). Brown trout is also caught commercially at sea, but market statistics are not available (Frank-Gopolos et al. 2015).
Fish in hatcheries are mainly produced for restocking of natural populations exploited by recreational anglers (Frank-Gopolos et al. 2015). Hatcheries have to maintain excellent welfare of the animals used in the hatching process. Thus, the management of the physiological condition of brood stock fish is of great concern (Wedemeyer 2002).
The easiest, most comprehensive way to assess animal condition is to perform blood tests. Hematological indices are of great diagnostic value in veterinary medicine due to several reasons. Blood transports nutrients, oxygen, and metabolites in the body. It is essential in maintaining water and electrolyte balance, body temperature, and proper function of the immune system. Thus, changes in blood constituents reflect any deviation from the normal physiological state of the animal. Blood is also relatively easy to obtain (via venipuncture) and to test (Voigt and Swist 2011). Fish diagnostic hematology is much more challenging than it mammalian counterpart is. Review of the available literature shows disparities, among others, in the taxonomy and in fish blood cell functions. Nucleated red blood cells and leukocytes have similar size, so cell count can only be determined by manual methods. The diversity of ecological forms, ecologic functions, and the number of fish species makes generalization about this group almost impossible (Weiss and Wardrop 2010). Moreover, blood cell count in fish shows seasonal variability and is influenced by many environmental factors, inter alia water temperature and oxygen concentration. Age, sex, reproductive, and nutritional status can also produce variation in results (Řehulka and Adamec 2004; Witeska 2013).
Sexual maturation in salmonids is known to cause lymphocytopenia, which suppresses immunological response to various pathogens (Pickering 1986; Pickering and Pottinger 1987). This feature increases fish susceptibility to fungal and bacterial infections which are a serious threat to animals raised in aquaculture (Pickering and Pottinger 1987). Thus, spawning season is one of the most critical phases in trout lifecycle. Maintenance of fish welfare during this time is the key to success in breeding.
Fish blood usually requires treatment with an anticoagulant due to the large number of thrombocytes, causing rapid formation of clot (compared to other vertebrates) (Maqbool et al. 2013). The addition of an anticoagulant to the blood sample is helpful so long as it does not alter results which can lead to misinterpretation of the data (Lippi et al. 2006). Many authors believe that the choice of anticoagulant for fish hematology is species specific (Weiss and Wardrop 2010), but reports describing appropriate anticlotting agents for particular species are scarce and often contradictory.
The salts of ethylenediaminetetraacetic acid (EDTA) are recommended as an anticoagulant of choice for hemocytometry by the International Council for Standardization in Hematology (ICSH) and the Clinical and Laboratory Standards Institute (CLSI) (Turgeon 2012). EDTA acts as a chelator of Ca2+ and Mg2+ ions. It binds calcium, essential for enzymatic reactions in the coagulation cascade, causing anticoagulant effect (Harr et al. 2005; Gilor and Gilor 2011; Witeska and Wargocka 2011). It causes complete anticoagulation with minimal effect on the morphology of the cells (Turgeon 2012; Greer et al. 2014). EDTA allows the most dependable preservation of cells on stained blood films (Thrall et al. 2012). It is commonly used in routine hematology tests in humans, mammals, and other vertebrates (Voigt and Swist 2011; Turgeon 2012; Greer et al. 2014; Campbell 2015). It has also found some success in fish (Blaxhall 1972; Blaxhall and Daisley 1973; Řehulka et al. 2004; Ishikawa et al. 2010; Maqbool et al. 2013; Campbell 2015). Standard test tubes with EDTA salts in concentrations appropriate for hematologic tests are commercially available and relatively cheap (Maqbool et al. 2013). However, EDTA salts have some drawbacks. In some cases, they can cause blood hemolysis, especially in specimens anesthetized with unbuffered tricaine methanesulfonate (MS 222) (Korcock et al. 1988; Campbell 2015).
Heparin salts are mainly used for clinical biochemistry (Gilor and Gilor 2011; Thrall et al. 2012). It prevents prothrombin conversion into thrombin (factor II), thus inhibiting conversion of fibrinogen into fibrin (Harr et al. 2005; Gilor and Gilor 2011; Witeska and Wargocka 2011). Heparin use has been recommended in hemocytometry of animals with nucleated erythrocytes, based on scarce reports of hemolysis in EDTA-preserved blood samples from some reptiles and birds (Gilor and Gilor 2011). For this reason, many authors have used heparin as the anticoagulant of choice in fish hematology (Hesser 1960; Hattingh 1975; Smit et al. 1977; Korcock et al. 1988; Maqbool et al. 2013). However, heparin does not completely stop clumping of white blood cells and thrombocytes, which can lead to sample clotting, and also produces blue background staining of Wright-stained blood film (Gilor and Gilor 2011; Turgeon 2012; Greer et al. 2014; Campbell 2015; Bain et al. 2016). It is also more expensive than other anticoagulants (Banfi et al. 2007).
We decided to evaluate the effects of two common anticoagulants, dipotassium EDTA and lithium heparin, on basic hematological parameters including packed cell volume (PCV), hemoglobin concentration (HGB), red blood cell count (RBC), white blood cell count (WBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) of captive mature brown trout (S. trutta) spawners during the spawning season.
Based on available literature, we assume that there are differences in hematologic parameters related to the choice of anticoagulant used.
Materials and methods
All samples were collected on 1 day in November 2017 from 35 mature spawners of freshwater brown trout S. trutta (mean mass 1.53 ± 0.57 kg, mean length 55.68 ± 5.70 cm). All specimens originated and were provided by Dąbie Fish Hatchery (54° 12′ 23.4″ N 17° 28′ 05.8″ E), Northern Poland. Fish were held in a concrete recirculating raceway (125 m3) and fed with commercial pellets (Aller Rep Ex, Aller Aqua). Water temperature at the time of sampling was 6 °C. Before artificial spawning, fish were anesthetized in a bath containing 2-phenoxyethanol (Sigma-Aldrich) at 0.5 ml/l. Immediately after artificial spawning fish were euthanized by manual application of blunt force trauma to the head followed by pithing. Blood and other tissues were collected for further examination afterwards.
The blood for analysis was drawn from the caudal vein by means of an 18G needle and 5-ml syringe. Blood was then transferred to standard, commercially available, test tubes containing K2EDTA (1.8 mg/ml K2EDTA for 2 ml of blood) and lithium heparin (18 I.U./ml heparin for 2.5 ml of blood) (Medlab Products). This allowed two blood samples to be collected from each specimen. The blood samples were analyzed immediately after collection.
For PCV, known also as hematocrit, blood was transferred to non-heparinized capillary tubes (75 μl, Medlab-Products) and centrifuged at 12,000 RPM for 3 min. The percentage of blood cells to blood plasma was determined (Turgeon 2012).
HGB was determined with Drabkin’s (Drabkin 1945) cyanmethemoglobin method. Twenty microliters of blood was added to the test tube containing 5 ml of Drabkin’s reagent (Stamar) and stirred thoroughly. The solution was stored for 20 min and was then centrifuged at 3000 RPM for 5 min to separate nuclei and cell membranes. Supernatant was transferred to a standard plastic spectrophotometer cell. Solution absorbance against the cyanmethemoglobin standard (Stamar) was measured using UV-VIS spectrophotometer (λ = 540 nm) (Prove 300, Merck). The formula below was used to calculate HGB.
RBC was determined with a Bürker hemocytometer using a Natt and Herrick (1952) stain. Twenty microliters of blood was added to the test tube containing 4 ml of stain (1:200 dilution). After 15 min of continuous stirring, 20 μl of solution was discharged to the hemocytometer counting chamber and left for 2 min to settle. Red blood cells were counted in 80 small squares under 400 × magnification (MT5300, Meiji). The formula below was used to calculate RBC.
Red blood cell indices were calculated in accordance with Greer et al. (2014).
MCV, an average erythrocyte volume, was calculated using the formula:
MCH, an average weight of hemoglobin in an average erythrocyte, was calculated with the formula:
MCHC, an average HGB per erythrocyte volume unit, was calculated with the formula:
WBC was determined using the same method as RBC. White blood cells were counted in the whole Bürker counting chamber field under 400 × magnification (MT5300, Meiji). The formula below was used to calculate WBC.
Statistical analysis was performed to identify significant differences in hematological parameters obtained from different anticoagulants. We used Student’s t test for dependent samples. The differences in two parameters (HGB, MCHC) were not distributed normally, so Wilcoxon signed-rank test was used for these. Statistica 12.5 application (StatSoft 2006) was used for statistical analysis.
Results
A comparison of hematological parameters of the blood samples treated with K2EDTA and lithium heparin is presented in Table 1.
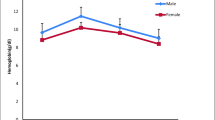
Statistically important differences (P < 0.01) between anticoagulants were observed in three hematological indices: PCV (P = 0.000001), MCV (P = 0.00002), and MCHC (P = 0.000001). PCV and MCV indices were higher in samples treated with K2EDTA, whereas greater MCHC values were observed in lithium heparin samples. There were no significant statistical differences (P > 0.05) between investigated anticoagulants in HGB, RBC, MCH, and WBC.
Discussion
In the present study of farmed brown trout spawners, significant differences between some hematological parameters values were obtained using two different anticoagulants.
Several authors observed that EDTA salts have a tendency to elevate PCV in various fish species (Blaxhall 1973; Korcock et al. 1988; Walencik and Witeska 2007; Witeska and Wargocka 2011; Maqbool et al. 2013). Elevation of this parameter has a profound impact on the indices calculated with mathematical formulas. MCV values rise proportionally to PCV; on the other hand, MCHC has an inverse relationship to the hematocrit, hence an increase in PCV produces a decrease in this parameter. Our results indicate that cell swelling in samples treated with K2EDTA occurs, and that the concentration of this anticoagulant has a hypotonic effect (Nemec et al. 2005). Concentrations beyond 2 mg/ml of blood are reported to produce significantly lower values of PVC and higher MCHC, which indicates cell shrinkage and morphological degeneration (Bain et al. 2016). An increase in red blood cell volume is thought to be a response to stress due to a variety of factors (Adeyemo et al. 2009). Calcium in the nucleated erythrocyte extracellular environment is vital for the maintenance of membrane integrity (Witeska and Wargocka 2011). EDTA salts produce their anticoagulant effect by chelation of Ca2+ and Mg2+ ions, thus disrupting the coagulation cascade (Harr et al. 2005; Gilor and Gilor 2011). Low level of Ca2+ ions in the cell surroundings increases membrane permeability, especially to Na+ ions, thus increasing water uptake leading to swelling (Lagunes et al. 1999). Erythrocyte swelling is also linked with high pCO2 and acidification caused by the acidic EDTA salt (Smit et al. 1977). An increase in cell size was observed by Walencik and Witeska (2007) in a study of the anticoagulant effect on blood morphology of Cyprinus carpio (L.). They noticed that a similar anticoagulant, Na2EDTA, in concentrations up to 1 mg/ml, induced anisocytosis, anisonucleosis, and hemolysis of blood cells in common carp. It also increased erythrocyte osmotic fragility. Na2EDTA is also reported to alter PCV, HGB, RBC, MCV, and MCHC in O. mykiss by causing hemolysis and cell membrane distortion affecting the parameters mentioned above (Maqbool et al. 2013).
There are no statistically significant differences between the anticoagulants we used, in HGB, RBC, MCH, and WBC, in the present study. Some authors observed significant differences in HGB and RBC obtained from EDTA and heparin salts (Korcock et al. 1988; Maqbool et al. 2013); however, simultaneously, they reported hemolysis of EDTA-treated samples. The hemolytic effect of EDTA on fish blood has been described by several authors (Smit et al. 1977; Van Vliet et al. 1985; Korcock et al. 1988; Walencik and Witeska 2007; Witeska and Wargocka 2011). However, this effect was not observed in the present study of brown trout. In our opinion, since hemolysis was not recorded, cell counts, HGB, and MCH should not show significant differences between anticoagulants. The insignificant differences in the means in these parameters in the current study are most likely due to inaccuracy of the manual methods.
In our opinion, lithium heparin is more suitable for hematologic assessment of farmed brown trout spawners, than dipotassium EDTA. Heparin does not influence the size of the red blood cells, thus reducing possibility of hemolysis to a minimum (Bain et al. 2016). Although results obtained in this study show statistically important PCV increase in K2EDTA-treated blood, authors do not consider this anticoagulant to be totally unsuitable for fish blood tests. There are reports suggesting that EDTA salts can be used for hematologic assessment if heparin is not available (Hesser 1960). Blood cell counts and HGB are not affected by salts of EDTA (Ahmed and Maqbool 2014). In addition, several authors reported that blood smears made with EDTA-treated blood have superior quality compared to those from heparin (Campbell 2015; Bain et al. 2016). Furthermore, the general availability and low cost of standard test tubes spray-coated with this compound make it easier for fish culturists to obtain them. Based on the present study, we suggest that K2EDTA can be used, with caution, for the determination of blood cell counts and HGB. Further research is required to better understand the effect of anticoagulants on hematological parameters. The analysis of blood collected without any anticoagulant could be of help in this process.
Our results can serve as reference values for mature brown trout during spawning season, but specific farming and environmental conditions should be taken into account. Temperature is especially important—seasonal variations in this parameter have a direct impact on some hematological indices, such as PCV and HGB (Campbell 2015). Blood parameters may also vary in relation to life cycle phase. Prolonged lymphocytopenia in brown trout during spawning season is documented (Pickering 1986; Pickering and Pottinger 1987).
Monitoring the condition of farmed fish is crucial for efficient fish production. Blood tests proved to be easy to obtain, and are a comprehensive tool for health assessment in higher vertebrate species bred in captivity (Weiss and Wardrop 2010; Thrall et al. 2012; Campbell 2015). In the light of the recent occurrence of potentially lethal ulcerative dermal necrosis (UDN)-like disease in Poland’s rivers (Kazuń et al. 2011; Grudniewska et al. 2012), monitoring and maintaining the welfare of cultured brown trout is vital for the future of salmonid aquaculture and the sustainability of hatcheries in Poland.
Abbreviations
- EDTA:
-
Ethylenediaminetetraacetic acid
- PCV:
-
Packed cell volume (hematocrit)
- HGB:
-
Hemoglobin concentration
- RBC:
-
Red blood cell count
- MCH:
-
Mean corpuscular hemoglobin
- MCV:
-
Mean corpuscular volume
- MCHC:
-
Mean corpuscular hemoglobin concentration
- WBC:
-
White blood cell count
References
Adeyemo OK, Okwilagwe OO, Ajani F (2009) Comparative assessment of sodium edta and heparin as anticoagulants for the evaluation of haematological parameters in cultured and feral african catfish (Clarias gariepinus). Braz J Aquat Sci Technol 13:19–24
Ahmed I, Maqbool A (2014) Effects of sodium-heparin and dipotassium EDTA on the haematological parameters and blood cell morphology of freshwater fish Schizothorax labiatus (Mcclelland, 1842). J Ecophysiol Occup Health 14:121. https://doi.org/10.15512/joeoh/2014/v14i3-4/59996
Bagliniere JL, Maisse G (1999) Biology and ecology of the brown and sea trout. Springer-Verlag, London
Bain BJ, Bates I, Laffan MA (2016) Dacie and Lewis practical haematology, 12th edn. Elsevier, London
Banfi G, Salvagno GL, Lippi G (2007) The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med 45:565–576. https://doi.org/10.1515/CCLM.2007.110
Blaxhall PC (1972) The haematological assessment of the health of freshwater fish. J Fish Biol 4:593–604. https://doi.org/10.1111/j.1095-8649.1972.tb05704.x
Blaxhall PC (1973) Error in haematocrit value produced by inadequate concentration of ethylenediamine tetra-acetate. J Fish Biol 5:767–769. https://doi.org/10.1111/j.1095-8649.1973.tb04509.x
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Campbell TW (2015) Exotic animal hematology and cytology, 4th edn. Wiley-Blackwell, Ames
Drabkin DL (1945) Crystallographic and optical properties of human hemoglobin - a proposal for the standardization of hemoglobin. Am J Med Sci 209:268–270
Frank-Gopolos T, Bekkevold D, Guyomard R, et al (2015) AquaTrace species leaflet - brown trout (Salmo trutta). https://aquatrace.eu/leaflets/trout. Accessed 19 Feb 2018
Freyhof J (2011) Salmo trutta. The IUCN Red List of Threatened Species 2011: e.T19861A9050312. In: Salmo trutta IUCN Red List Threat. Species 2011 ET19861A9050312. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T19861A9050312.en. Accessed 17 Mar 2017
Gilor S, Gilor C (2011) Common laboratory artifacts caused by inappropriate sample collection and transport: how to get the most out of a sample. Top Companion Anim Med 26:109–118. https://doi.org/10.1053/j.tcam.2011.02.003
Greer JP, Arber DA, Glader B et al (eds) (2014) Wintrobe’s clinical hematology, 13th edn. Wolters Kluwer Health, Philadelphia
Grudniewska J, Bartel R, Terech-Majewska E et al (2012) Evaluation of abundance, condition, and health of sea trout (Salmo trutta trutta) and salmon (Salmo salar) spawners threatened with UDN in some Polish rivers in 2010. Komun Rybackie 130:1–7
Harr KE, Raskin RE, Heard DJ (2005) Temporal effects of 3 commonly used anticoagulants on hematologic and biochemical variables in blood samples from macaws and Burmese pythons. Vet Clin Pathol 34:383–388. https://doi.org/10.1111/j.1939-165X.2005.tb00065.x
Hattingh J (1975) Heparin and ethylenediamine tetra-acetate as anticoagulants for fish blood. Pflugers Arch Eur J Physiol 355:347–352. https://doi.org/10.1007/BF00579855
Hesser EF (1960) Methods for routine fish hematology. Prog Fish Cult 22:164–171. https://doi.org/10.1577/1548-8659(1960)22[164:MFRFH]2.0.CO;2
Ishikawa MM, de Pádua SB, Satake F et al (2010) Heparin and Na2EDTA as anticoagulants for hybrid surubim catfish (Pseudoplatystoma reticulatum x P. corruscans): efficacy and hematological changes. Ciênc Rural 40:1557–1561. https://doi.org/10.1590/S0103-84782010005000113
Jonsson B, Jonsson N (2011) Ecology of Atlantic salmon and brown trout. Springer, Netherlands
Kazuń B, Grudniewska J, Terech-Majewska E et al (2011) Health assessments of sea trout, Salmo trutta trutta, spawners from Pomeranian rivers in 2010 based on immunological examinations. Komun Rybackie 124:1–4
Klemetsen A, Amundsen P-A, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12:1–59. https://doi.org/10.1034/j.1600-0633.2003.00010.x
Korcock DE, Houston AH, Gray JD (1988) Effects of sampling conditions on selected blood variables of rainbow trout, Salmo gairdneri Richardson. J Fish Biol 33:319–330. https://doi.org/10.1111/j.1095-8649.1988.tb05474.x
Lagunes R, Ruiz L, Frixione E (1999) Contraction of epithelial (MDCK) cells in response to low extracellular calcium is dependent on extracellular sodium. J Muscle Res Cell Motil 20:761–770. https://doi.org/10.1023/A:1005580425932
Lippi G, Bassi A, Brocco G, Montagnana M, Salvagno GL, Guidi GC (2006) Preanalytic error tracking in a laboratory medicine department: results of a 1-year experience. Clin Chem 52:1442–1443. https://doi.org/10.1373/clinchem.2006.069534
Maqbool A, Ahmed I, Sheikh ZA (2013) Effects of two commonly used anticoagulants on haematology and erythrocyte morphology of rainbow trout (Oncorhynchus mykiss). Int J Fish Aquat Stud 2:239–243
Natt MP, Herrick CA (1952) A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult Sci 31:735–738. https://doi.org/10.3382/ps.0310735
Nemec A, Drobnič–Košorok M, Butinar J (2005) The effect of high anticoagulant k3-edta concentration on complete blood count and white blood cell differential counts in healthy beagle dogs. Slov Vet Res 42:65–70
Pickering AD (1986) Changes in blood cell composition of the brown trout, Salmo trutta L., during the spawning season. J Fish Biol 29:335–347. https://doi.org/10.1111/j.1095-8649.1986.tb04950.x
Pickering AD, Pottinger TG (1987) Lymphocytopenia and interrenal activity during sexual maturation in the brown trout, Salmo trutta L. J Fish Biol 30:41–50. https://doi.org/10.1111/j.1095-8649.1987.tb05730.x
Řehulka J, Adamec V (2004) Red blood cell indices for rainbow trout (Oncorhynchus mykiss) reared in cage and raceway culture. Acta Vet Brno 73:105–114. https://doi.org/10.2754/avb200473010105
Řehulka J, Minařík B, Řehulková E (2004) Red blood cell indices of rainbow trout Oncorhynchus mykiss (Walbaum) in aquaculture. Aquac Res 35:529–546. https://doi.org/10.1111/j.1365-2109.2004.01035.x
Smit GL, Hattingh J, Schoonbee HJ (1977) Observations on some effects of disodium ethylenediamine tetra-acetate and heparin on fish blood. Comp Biochem Physiol C 57:35–38. https://doi.org/10.1016/0306-4492(77)90074-0
StatSoft (2006) Elektroniczny Podręcznik Statystyki PL. Krakow, Poland
Thrall MA, Weiser G, Allison R, Campbell T (eds) (2012) Veterinary hematology and clinical chemistry, 2nd edn. Wiley-Blackwell, Ames
Turgeon ML (2012) Clinical hematology: theory and procedures, 5th edn. Lippincott Williams & Wilkins, Baltimore
Van Vliet KJ, Smit GL, Pieterse JJ et al (1985) The effects of generally used anticoagulants on the haemolysis of fish erythrocytes. Water SA 2:87
Voigt GL, Swist SL (2011) Hematology techniques and concepts for veterinary technicians, 2nd edn. Wiley-Blackwell, Chichester
Walencik J, Witeska M (2007) The effects of anticoagulants on hematological indices and blood cell morphology of common carp (Cyprinus carpio L.). Comp Biochem Physiol C Toxicol Pharmacol 146:331–335. https://doi.org/10.1016/j.cbpc.2007.04.004
Wedemeyer GA (ed) (2002) Fish hatchery management, 2nd edn. CABI Publishing, New Jersey
Weiss DJ, Wardrop KJ (eds) (2010) Schalm’s veterinary hematology, 6th edn. Wiley-Blackwell, Ames
Witeska M (2013) Erythrocytes in teleost fishes: a review. Zool Ecol 23:275–281. https://doi.org/10.1080/21658005.2013.846963
Witeska M, Wargocka W (2011) Disodium EDTA used as anticoagulant causes hemolysis in common carp blood. Turk J Vet Anim Sci 35:99–104. https://doi.org/10.3906/vet-0908-51
Acknowledgements
The authors would like to thank the management and staff of “Dąbie Hatchery” for providing essential research material and for help and support during fieldwork.
Funding
The research was funded by the Faculty of Biological Sciences, University of Zielona Góra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ciepliński, M., Kasprzak, M., Grandtke, M. et al. The effect of dipotassium EDTA and lithium heparin on hematologic values of farmed brown trout Salmo trutta (L.) spawners. Aquacult Int 27, 79–87 (2019). https://doi.org/10.1007/s10499-018-0308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0308-5