Abstract
Within the study, the in vitro fertilization (ivF) in crucian carp, Carassius carassius (L.), with the use of three activating solutions (AS) [reverse osmosis water (RO), Woynarovich (WS) and Billard (BS) solutions] as well as the period of the capacity of eggs to be fertilized (up to 180 s post-egg-activation) in those solutions. Moreover, CASA analysis of sperm motility was conducted with each AS. In control groups (0 s), the highest (P < 0.05) fertilization rate (93.2 %) was observed with WS. The application of RO and BS affected lower (P < 0.05) embryo survival (63.5 and <5 %, respectively). High fertilization rate (over 90 %) was recorded up to 30 (RO) and 90 s (WS) post-egg-activation. Progressive sperm motility (pMOT) was high (over 48 %) in all treatment groups up to 30 s. In RO and WS groups, pMOT significantly decreased (P < 0.05) after 45 s post activation. BS affected pMOT over 10 % up to the 165 s. No differences (P > 0.05) between the groups were found considering the curvilinear velocity (VCL) 15 s post-sperm-activation. Between 30 and 135 s, the lowest (P < 0.05) VCL after application of RO was noted. The highest VCL (P < 0.05) up to 135 s was noted after application of BS and WS. All AS activated crucian carp sperm. The lowest fertilization rate was noted when BS was used, despite the high pMOT and VCL. It suggest that the AS which activates the sperm properly may not activate the eggs probably due to high osmolality of BS (262 mOsm kg−1). Because eggs retained the longest period of activity in WS, this AS is suggested for ivF in crucian carp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, sustainable aquaculture has become a very important tool in the conservation and restoration of endangered fish species and populations. Complete restitution or support of natural recruitment is usually based on the restocking operations, where high quality fry is needed (e.g., Cowx 1994). To this end, most frequently larvae and fry reared in controlled conditions are used (Shiri Harzevili et al. 2003; Sarkar et al. 2006; Hamácková et al. 2009). These larvae usually originate from wild spawners, which are typically caught during the spawning season (e.g., Targońska et al. 2010). However, effectiveness of the reproduction of wild fish is very often characterized by very low effectiveness and high mortality of the spawners (e.g., Krejszeff et al. 2008, 2010; Targońska et al. 2011a, 2012). Therefore, in many cases, growth in captivity away from cultured stocks was needed, since controlled reproduction of domesticated fish is usually much more effective than with wild spawners (e.g., Krejszeff et al. 2009). However, in that case, specific selective breeding programs, considering the monitoring of the genetic variation of the domesticated stock, should be considered (e.g., Pereira et al. 2010) since the genetic diversity of restocked fish is of obvious importance when wild populations are restored (e.g., González-Wangüemert et al. 2012). Thus, it is extremely important to optimize every step of culture, where in vitro fertilization (ivF) is one of the most important procedures directly affecting reproduction results (e.g., Linhart et al. 2006; Żarski et al. 2012a).
Crucian carp, Carassius carassius (L.), is a cyprinid fish species native to Europe and some parts of Asia (Lelek 1987). For many decades in Central Europe, this fish was one of the most important in commercially exploited freshwater reservoirs (Skrzypczak and Mamcarz 2005). However, in recent years, a serious decline of many populations of this species has been reported (e.g., Wheeler 2000; Skrzypczak and Mamcarz 2005; Tarkan et al. 2009; Sayer et al. 2011). One of the main factors responsible for this situation is the expansion of the goldfish, Carassius auratus (L.) and prussian carp, Carassius gibelio (Bloch 1782), which has degraded local populations of the crucian carp through excessive hybridization (Wheeler 2000; Hänfling et al. 2005; Wouters et al. 2012). However, climatic changes and anthropogenic transformation of natural habitats have also played a significant role in the degradation of the crucian carp (Sayer et al. 2011). This has resulted in an urgent need for development of procedures for the production of fry for restocking purposes under controlled conditions. In the aquaculture of this species, the first attempts have been made to reproduce wild spawners in captivity (Targońska et al. 2012) and to determine the basic protocols for larval rearing under controlled conditions (Żarski et al. 2011; Demény et al. 2012). There are few articles allowing to understand the mechanisms of spermatozoa motility (Morisawa et al. 1983) and the effect of different stimulants (pheromonal and hormonal) on the sperm quantity and motility (Olsen et al. 2006; Cejko et al. 2013) in crucian carp. However, according to our knowledge, there have been no published data on ivF and gamete management in this species.
After contact with water, eggs acquire the ability to be fertilized for a certain period of time (Coward et al. 2002; Minin and Ozerova 2008). This period is not known for many fish species, including crucian carp. Recently, it has been proven that the type of activating solution (AS) may affect the duration of egg fertilization ability in Eurasian perch, Perca fluviatilis L. (Żarski et al. 2012a). The different AS are commonly used for ivF in aquaculture since it has been proven that species-specific AS may positively affect spermatozoa motility and its movement duration (e.g., Billard 1983; Sarosiek et al. 2012). However, there are very limited data, which actually prove the usefulness of particular AS for ivF, and sometimes, the published data are mutually exclusive. For example, Billard solution (developed for salmonids) proved to be useful also in percid fish such as Eurasian perch (Żarski et al. 2012a). Also, Woynarovich solution (developed for cyprinids) affected high fertilization rate in the same percid species (Żarski et al. 2012a), whereas in cyprinid species such as common tench, Tinca tinca (L.), this solution decreased fertilization success (Geldhauser 1992). Therefore, development of species-specific AS or evaluation of an already existing one is very important in the practice of ivF. To date, there are data on the effectiveness of the application of different activating solutions on fertilization effectiveness in crucian carp.
The aim of the study was to investigate the effectiveness of ivF in crucian carp with the use of three different AS, as well as to investigate the period of time during which the eggs of this species are able to be fertilized in those solutions.
Materials and methods
Broodstock management and collection of gametes for fertilization trial
Wild crucian carp spawners (6 females and 14 males with an average body weight of 302 ± 122 g and 160 ± 65 g for females and males, respectively) were caught with electrofishing in Lake Sasek Wielki and, immediately after catching fish, were transported in plastic bags with oxygen to the laboratories of the Department of Lake and River Fisheries, University of Warmia and Mazury (Olsztyn, Poland). The fish were then placed in 1,000-L tanks with controllable photo-thermal conditions (17 °C; 16L:8D) (Kujawa et al. 1999). Hormonal stimulation was applied after 2 days of acclimation. For stimulation of females, Ovaprim (containing 20 μg of sGnRHa and 10 mg of domperidone in 1 mL of preparation) in two doses—0.1 and 0.5 mL kg−1—was used. After the first injection, the temperature was raised to 21 °C. Males were kept separately in the same photo-thermal conditions. Males were not stimulated hormonally (according to Targońska et al. 2012).
After ovulation, the certified eggs were stripped manually to dry plastic containers separately from each of the females. For further procedures, only portions of eggs without white or opaque eggs were used (n = 3). Sperm was stripped manually to dry plastic syringes and was kept at about 4 °C before fertilization, however, no longer than 1 h. For ivF, pooled sperm (from at least 5 males) was used.
Effectiveness of fertilization
For the ivF trial, eggs from three females were used (constituting three replicates). As an AS, three different media were used:
-
RO—water obtained after the filtration of tap water with the reverse osmosis filter (control AS)
-
BS—Billard solution (125 mM NaCl, 30 mM Glycine, 20 mM Tris–HCl), which was developed for salmonids (Billard 1983)
-
WS—Woynarovich solution (3 g of urea and 4 g of NaCl in 1 L of water), which was developed for cyprinids (Woynarovich and Woynarovich 1980)
Osmolality (measured with the Advanced Micro-Osmometer 3MO Plus, Advanced Instruments, USA) of the solutions used was 13, 262 and 191 mOsm kg−1 for RO, BS and WS, respectively.
The fertilization trial was performed on each batch of eggs with the use of the three tested activating solutions. To this end, 5 mL of AS was poured onto each Petri dish (50 mm in diameter). Next, one egg sample (about 100 eggs) was put on each of the dish and gently stirred to spread all eggs around the dish. Afterward, a sperm sample (5 μL) was added to the activated eggs at 0 (control group where sperm was added together with the eggs), 15, 30, 60, 90 and 120 s, after egg activation. After 5 min post-sperm-addition, Petri dishes with the egg samples were placed in an experimental recirculating aquaculture system for incubation at 21 °C. At the eyed-egg stage, survival rate was recorded under a stereoscopic microscope (MZ 12.5, Leica, Switzerland).
Sperm analysis
The results obtained after the fertilization trial necessitated the objective verification of sperm motility with the use of tested AS. To this end, for sperm analysis, five crucian carp males (135.3 ± 12.5 g) were obtained from Lake Rákospalota (Pest County, Hungary) and brought to the laboratory of the Department of Aquaculture of Szent Istvan University (Gödöllő, Hungary). Fish were hormonally stimulated with a single injection of carp pituitary homogenate (CPH) at a dose of 6 mg kg−1 (as described by Demény et al. 2012). This hormonal preparation was chosen since it affects only sperm volume and it does not affect sperm motility parameters (Cejko et al. 2012). Sperm was collected into dry syringes by stripping 16 h following hormonal stimulation. Next, the sperm samples were kept in Eppendorf tubes at about 4 °C before the analysis. Sperm motility was recorded with the use of a CASA system, which consisted of a microscope (Olympus BX41, Tokyo, Japan) and a PC with “Sperm Vision™” (Minitüb, Tiefenbach, Germany) software. The percentage of progressively motile spermatozoa (pMOT) and curvilinear velocity (VCL, μm s−1) was recorded 15 s post-sperm-activation and then every 15 s up to 180 s post-activation. The sperm analysis was performed for each of the 5 males with the use of each of the three tested AS. To each of the AS, a BSA (bovine serum albumin) was added at a concentration of 1 % (as described by Kowalski et al. 2013) in order to prevent spermatozoa adhesion to the glass.
Statistical analysis
All the data were presented as mean ± SD. All data expressed in percentages before the statistical analysis were subjected to arc-sine transformation. Data regarding the survival rate of embryos and spermatozoa motility (between the treatment groups at a respective time post-egg-activation as well as among the treatment groups at different time post-activation) were analyzed with a one-way analysis of variance (ANOVA) and subjected to a Duncan post hoc test at a significance level of 5 % (P = 0.05). The obtained data for particular parameter (survival rate of embryos, pMOT, VCL) were analyzed among respective experiment between different treatment groups at a particular time post-activation (for eggs and sperm motility parameters separately). The differences among particular treatment group (particularly activating solution used) and between different time post-activation (for eggs and sperm motility parameters separately) were analyzed separately. The statistical analysis was performed with STATISTICA 9.1 (StatSoft, Inc., USA).
Results
Effectiveness of fertilization
The lowest (P < 0.05) fertilization rate of embryos (below 5 % up to 30 s post-egg-activation) among all the tested post-egg-activation times was recorded in group where BS was used for gametes activation. The highest (P < 0.05) fertilization rate in the control groups (where sperm was added at the time 0 s post-egg-activation) was recorded in group where WS was used as an AS. Such high fertilization success was recorded up to 90 s post-egg-activation. Between 15 and 30 s post-egg-activation, no differences (P > 0.05) between the RO and WS were noted. When eggs were activated with RO, a significantly lower fertilization rate was noted at 60 s post-egg-activation. With the application of RO as an AS, eggs retained ability to be fertilized for no longer than 90 s (4.7 % of viable embryos), whereas after 120 s, over 46 % of eggs were able to be fertilized in a group where WS was applied. It was comparable (P > 0.05) to the RO group where sperm was added at the onset of egg activation (63.5 %) (Fig. 1).
The results of crucian carp eggs fertilization with different activating solutions and inseminated at different time after egg activation. Data marked with different uppercase letters (A, B, C, etc.) indicate statistical differences (P < 0.05) among the treatment groups (activating solution tested) and between different times post-activation. Data marked with different lowercase letters (a, b, c, etc.) indicate statistical differences (P < 0.05) among particular times post-activation and between the treatment groups (activating solution tested)
Sperm analysis
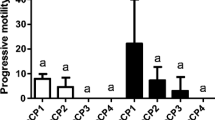
All tested AS activated spermatozoa. Up to 45 s following sperm activation, no differences (P > 0.05) in pMOT between the three AS were recorded. Between 60 and 180 s post-activation in the group where RO was used as an AS, less than 5 % of spermatozoa were found to be motile. In sperm activated with WS, up to 60 s post-activation, pMOT was similar (P > 0.05) to that recorded in the group activated with BS. However, between 75 and 180 s (except 120 s), pMOT was the highest (P < 0.05) in group activated with BS, whereas in group activated with WS, the pMOT did not exceed 6 % from 75 s onward (Fig. 2).
Results of crucian carp spermatozoa progressive motility at different time post-activation when activated with different activating solutions. Data marked with different uppercase letters (A, B, C, etc.) indicate statistical differences (P < 0.05) among the treatment groups (activating solution tested) and between different times post-activation. Data marked with different lowercase letters (a, b, c, etc.) indicate statistical differences (P < 0.05) among particular times post-activation and between the treatment groups (activating solution tested)
At 15 s following sperm activation, no differences (P > 0.05) between the groups were found considering the VCL of the spermatozoa. Between 30 and 135 (with the exception of 90 s post-activation), the lowest (P < 0.05) VCL was noted in a group where RO was used as an AS. The highest VCL (P < 0.05) was always noted where sperm was activated with the BS and WS (with the exception of 60 s following sperm activation). However, from 150 s onward, no differences in VCL between the groups could be observed (P > 0.05) (Fig. 3).
Results of crucian carp spermatozoa curvilinear velocity at different time post-activation when activated with different activating solutions. Data marked with different uppercase letters (A, B, C, etc.) indicate statistical differences (P < 0.05) among the treatment groups (activating solution tested) and between different times post-activation. Data marked with different lowercase letters (a, b, c, etc.) indicate statistical differences (P < 0.05) among particular times post-activation and between the treatment groups (activating solution tested)
Discussion
The results obtained in the present study indicate that the type of AS used has a significant effect on fertilization success in crucian carp. It has been clearly shown, for the first time, that AS (particularly BS) which activates sperm motility efficiently may not be an effective solution in in vitro fertilization.
Fertilization success is strictly related to the quality of the gametes (Bobe and Labbe 2010), the sperm-to-egg ratio (Linhart et al. 2006), the time of contact of active sperm with active eggs (Liley et al. 2002) and the activating solution used (e.g., Kucharczyk et al. 2010; Żarski et al. 2012a). Many studies have aimed at investigating different activating media on spermatozoa motility (e.g., Billard 1983, Ohta et al. 1997). However, there is very little information on the effect of different activating solution on fertilization effectiveness (e.g., Linhart et al. 2006; Żarski et al. 2012a). The results obtained in the present study indicate that the type of the AS affects fertilization rate directly. A similar effect was reported by Żarski et al. (2012a), when the same AS were used as in the present study in an ivF trial of Eurasian perch eggs. Those authors, similar to the present study, found the highest ivF success recorded after the application of WS, regardless of the time following egg activation. However, Żarski et al. (2012a) noted high fertilization results after the application of BS (over 70 % up to 150 s following activation), despite the lowest motility parameters of spermatozoa (pMOT below 10 %) when activated with this AS. This is in contrast with the data recorded for the crucian carp where, despite satisfying motility parameters (Fig. 2, 3), a very low survival rate was recorded (Fig. 1) when BS was used as an AS. On the basis of the presented data, it may be suggested that there are huge interspecific differences in the activation of gametes between crucian carp and Eurasian perch. However, it may be concluded that it is more related to egg activation rather than sperm.
Generally, spermatozoa motility in cyprinids and percids is triggered by hypo-osmotic shock (see e.g., Morisawa et al. 1983; Alavi et al. 2010). The seminal plasma of cyprinids, including crucian carp, is close to 300 mOsm kg−1 (Morisawa et al. 1983). The AS used in the present study had osmolality equal or lower than 262 mOsm kg−1, which resulted in high initial pMOT and VCL in all the tested AS. However, there was a significant effect in motility duration, which was the longest in the group where BS was used as an AS (Fig. 2). In addition, the VCL, when sperm was activated with BS, was as high as with other AS used. On the contrary, the sperm motility duration after application of RO (whose osmolality is very similar to the “hatchery water” commonly used in in vitro fertilization [personal, unpublished data]) was the shortest (about 45 s) (Fig. 1). It may be speculated that such difference may stem from the fact that the application of AS with lower osmolality caused shorter period of the spermatozoa movement. However, on the basis of the results obtained, it is impossible to make any conclusions, since the ionic composition of tested AS was also different and this plays an essential role in triggering spermatozoa movement, and affects velocity parameters and duration of movement (see, e.g., review by Alavi and Cosson 2006). Kucharczyk et al. (2010) suggested that the fertilization success is affected more by the duration of spermatozoa motility than the percentage of sperm motility. Moreover, Targońska et al. (2011b) reported that only 5 % of motile spermatozoa affected fertilization rate in more than 50 % of embryos when sperm was added in excess. On the other hand, Lahnsteiner et al. (1998) and Gage et al. (2004) reported that the fertilization success is strictly related to the sperm velocity. In the present study, all the parameters were comparable regardless the AS used for sperm activation. This confirms that the very low fertilization rate after application of BS observed in the present study was strictly related to the low effectiveness of egg activation by the BS.
Following egg activation, an intracellular Ca2+ wave occurs in an egg, which is then followed by a cortical reaction (Ohta et al. 1990; Lee et al. 1999; Coward et al. 2002; Żarski et al. 2012b). However, the mechanism which triggers this Ca2+ wave is still not clear in freshwater finfishes. To date, three different types of mechanisms of fish egg activation have been described where spermatozoa were pointed out as an important factor in this process (Coward et al. 2002; Coward and Parrington 2003; Kinsey et al. 2007). However, eggs of freshwater finfish species were seen to be activated in the aquatic environment (so called spontaneous egg activation) even without the presence of sperm, which resulted in formation of fertilization membrane (Minin and Ozerova 2008) or cortical reaction occurrence (Żarski et al. 2012b) within a few minutes following activation. This is in contrast, for example, with the marine finfish species, Gadus morhua L., where even 2-h exposition to marine water did not affect cortical reaction (Davenport et al. 1981). On the other hand, in eggs of freshwater weatherfish, Misgurnus fossilis (L.), it was proven that 10-min exposition affected egg activation and that some protease inhibitors (leupeptin and aprotinin) may abolish this process (Minin and Ozerova 2008). This suggests that mechanisms of eggs activation, as well as its inhibitory effect, may be different for marine and freshwater fish species. The AS used in the present study were the simple buffered aqueous solutions with different osmolality (without any proteinase inhibitors). Therefore, it may be suggested that the different osmolality of those solutions affected fertilization ability of crucian carp eggs, where AS with the highest osmolality (BS with 262 mOsm kg−1) negatively affected fertilization results (Fig. 1). It has been already reported that osmolality of the medium affects the effectiveness of short-term storage (up to 2 h) of the common carp, Cyprinus carpio L., eggs (Glenn and Tiersch 2002) which confirms the significant effect of this factor on eggs of freshwater fish. However, more studies are needed to determine the actual effect of osmolality of AS (as a single variable) on fertilization success in order to exclude the effect of different buffers used as well as glycine content (in the BS) in the tested AS.
The obtained results indicate that eggs of crucian carp in RO, which is similar to the normally used hatchery water, lost their fertilization ability within 60 s following activation. This was over twofold shorter than in Eurasian perch, where after 150 s following activation, 70 % of live embryos were recorded (Żarski et al. 2012a). However, the crucian carp eggs remained capable of fertilization 20 s longer than in the case of salmonids and close to the other cyprinid, which is ide, Leuciscus idus (L.), whose eggs remained active for 60 s (Targońska et al. unpublished). It has been suggested that such differences may stem from the reproductive strategies of those species where different water flow regimes in rivers or lakes on the spawning grounds occur (Mann 1996; Merz et al. 2004; Probst et al. 2009), which affect the possible time of contact of gametes with each other. However, crucian carp spawns in the lakes, and ide is a rheophilic cyprinid, which spawns in the rivers. This fact excludes possibility of such relationship. Additionally, both Eurasian perch and crucian carp are species which spawn in the littoral zone of the lakes where the water flow is limited. Thus, it may be suggested that differences in egg fertilization ability for those two species may stem from different thermal regimes during the spawning act (12 and 21 °C in Eurasian perch and crucian carp, respectively) or from different eggs characteristics, where Eurasian perch ovulates eggs situated within the cylindrical gelatinous ribbon (e.g., Probst et al. 2009), whereas crucian carp ovulates a batch of single eggs (typical for cyprinids). However, this topic requires further studies.
The application of different AS in the present study has shown that eggs remained active in different AS for a different period of time. This is consistent with the data published by Żarski et al. (2012a) for Eurasian perch, where WS also affected the longest period of egg activation as compared to the hatchery water (similar to RO in the present study). Interestingly, a similar effect of fertilization success in the control group (RO water at 0 s, Fig. 1) was noted in crucian carp and Eurasian perch (Żarski et al. 2012a), where a significantly lower fertilization rate was recorded in the control group as compared with 15 s post-egg-activation. Żarski et al. (2012a) have suggested that in Eurasian perch, the eggs could acquire the ability to be fertilized after a certain period of time, and the sperm in the hatchery water (or RO in the present study) was motile too short to fertilize some of the active eggs. In the case of crucian carp, this effect could stem from the same phenomenon. However, the effect of manipulation (when ivF procedure was applied) could not be excluded since eggs, after contact with the water, were seen to stick in a small clusters before they were distributed (with stirring) all over the Petri dish, and sperm motility in this AS decreased the earliest. Nevertheless, reduced fertilization success in this case was most probably affected by the short period of sperm motility in this AS.
The results presented in this study clearly indicate that the type of AS used for ivF in crucian carp affects not only fertilization success, but also the period of time when eggs are able to be successfully fertilized. It has been shown that AS which activates sperm motility successfully may be useless in ivF in particular species. It is very important from a practical point of view, where a species-specific fertilizing solution should not only include activation of spermatozoa motility but also egg activation effectiveness. On the basis of the results presented, it may be suggested that the time of contact of crucian carp eggs with sperm should be no shorter than 45 and 60 s (considering the duration of sperm motility) when RO and WS are used as an AS, respectively. However, when using RO water as an AS, addition of sperm 15 s following egg activation may be used to improve the fertilization rate. Nevertheless, as a result of this study, WS may be suggested for ivF in crucian carp since it may improve the general reproduction outcome.
Abbreviations
- AS:
-
Activating solution
- BS:
-
Billard solution (125 mM NaCl, 30 mM Glycine, 20 mM Tris–HCl)
- CASA:
-
Computer-assisted sperm analysis
- ivF:
-
In vitro fertilization
- pMOT:
-
Progressive spermatozoa motility
- RO:
-
Water obtained after the filtration of tap water with the reverse osmosis filter
- sGnRHa:
-
Salmon gonadoliberin analogue
- VCL:
-
Curvilinear spermatozoa motility
- WS:
-
Woynarovich solution (3 g of urea and 4 g of NaCl in 1 L of water)
References
Alavi SMH, Cosson J (2006) Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell Biol Int 30:1–14
Alavi SMH, Rodina M, Hatef A, Stejskal V, Policar T, Hamáčková J, Linhart O (2010) Sperm motility and monthly variations of semen characteristics in perca fluviatilis (teleostei: Percidae). Czech J Anim Sci 55:174–182
Billard R (1983) Effects of coelomic and seminal fluids and various saline diluents on the fertilizing ability of spermatozoa in the rainbow trout, Salmo gairdneri. J Reprod Fert 68:77–84
Bobe J, Labbe C (2010) Egg and sperm quality in fish. Gen Comp Endocrinol 165:535–548
Cejko BI, Targońska K, Kowalski RK, Żarski D, Sarosiek B, Kucharczyk D, Glogowski J (2012) The effectiveness of hormonal preparations (Ovopel, Ovaprim, LHRHa, hCG and CPE) in stimulating spermiation in dace Leuciscus leuciscus (L.). J Appl Ichthyol 28:873–877
Cejko BI, Żarski D, Krejszeff S, Kucharczyk D, Kowalski RK (2013) Effect of hormonal stimulation of the crucian carp Carassius carassius (L.) on milt volume, number of sperm and its motility. Isr J Aquacult-Bamidgeh (in press)
Coward K, Parrington J (2003) New insights into the mechanism of egg activation in fish. Aquat Living Resour 16:395–398
Coward K, Bromage NR, Hibbitt O, Parrington J (2002) Gamete physiology, fertilization and egg activation in teleost fish. Rev Fish Biol Fish 12:33–58
Cowx IG (1994) Stocking strategies. Fish Manage Ecol 1:15–30
Davenport J, Lønning S, Kjørsvik E (1981) Osmotic and structural changes during early development of eggs and larvae of the cod, Gadus morhua L. J Fish Biol 19:317–331
Demény F, Trenovszki MM, Sokoray-Varga S, Hegyi A, Urbányi B, Zarski D, Ács B, Miljanović B, Specziár A, Müller T (2012) Relative efficiencies of artemia nauplii, dry food and mixed food diets in intensive rearing of larval crucian carp (Carassius carassius L.) introduction crucian carp Carassius carassius (L.). Turk J Fish Aquat Sci 12:693–700
Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA (2004) Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr Biol 14:44–47
Geldhauser F (1992) The interplay of egg swelling and sperm activity in the fertilization process of tench (Tinca tinca L.). In: Adamek Z, Flajshans M (eds) Proceedings of the scientific conference fish reproduction, 2–4 March. Czech Republic, Vodnany, pp 89–91
Glenn DW III, Tiersch TR (2002) Effect of extenders and osmotic pressure on storage of eggs of ornamental common carp Cyprinus carpio at ambient and refrigerated temperatures. J World Aquacult Soc 33:254–267
González-Wangüemert M, Fernández TV, Pérez-Ruzafa A, Giacalone M, D’Anna G, Badalamenti F (2012) Genetic considerations on the introduction of farmed fish in marine protected areas: the case of study of white seabream restocking in the Gulf of Castellammare (southern Tyrrhenian Sea). J Sea Res 68:41–48
Hamáčková J, Prokeš M, Kozák P, Peňáz M, Stanny LA, Policar T, Baruš V (2009) Growth and development of vimba bream (Vimba vimba) larvae in relation to feeding duration with live and/or dry starter feed. Aquaculture 287:158–162
Hänfling B, Bolton P, Harley M, Carvalho GR (2005) A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshwat Biol 50:403–417
Kinsey WH, Sharma D, Kinsey SC (2007) Fertilization and egg activation in fishes. In: Babin PJ et al (eds) The fish oocyte: from basic studies to biotechnological applications. Springer, Berlin, pp 397–409
Kowalski RK, Cejko BI, Krejszeff S, Sarosiek B, Judycka S, Targońska K, Kucharczyk D, Glogowski J (2013) Effect of albumin and casein supplementation on the common carp Cyprinus carpio L. sperm motility parameters measured by CASA. Aquacult Int. doi:10.1007/s10499-013-9673-2
Krejszeff S, Kucharczyk D, Kupren K, Targońska K, Mamcarz A, Kujawa R, Kaczkowski Z, Ratajski S (2008) Reproduction of chub, Leuciscus cephalus L., under controlled conditions. Aquacult Res 39:907–912
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2009) Domestication affects spawning of the ide (Leuciscus idus)—preliminary study. Aquaculture 295:145–147
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2010) Artificial reproduction of two different spawn-forms of the chub. Reprod Biol 10:67–74
Kucharczyk D, Gomułka P, Krejszeff S, Żarski D, Targońska K (2010) The effect of prepared activating liquid on the survivability of ide Leuciscus idus (L.) embryos. Pol J Nat Sci 25:200–208
Kujawa R, Kucharczyk D, Mamcarz A (1999) A model system for keeping spawners of wild and domestic fish before artificial spawning. Aquacult Eng 20:85–89
Lahnsteiner F, Berger B, Weismann T, Patzner RA (1998) Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture 163:163–181
Lee KW, Webb SE, Miller AL (1999) A Wave of free cytosolic calcium traverses zebrafish eggs on activation. Dev Biol 214:168–180
Lelek A (1987) The freshwater fishes of Europe, vol 9., Threatened fishes of EuropeAULA-Verlag, Wiesbaden 343
Liley NR, Tamkee P, Tsai R, Hoysak DJ (2002) Fertilization dynamics in rainbow trout (Oncorhynchus mykiss): effect of male age, social experience, and sperm concentration and motility on in vitro fertilization. Can J Fish Aquat Sci 59:144–152
Linhart O, Rodina M, Kocour M, Gela D (2006) Insemination, fertilization and gamete management in tench, Tinca tinca (L.). Aquacult Int 14:61–73
Mann RHK (1996) Environmental requirements of European non-salmonid fish in rivers. Hydrobiologia 323:223–235
Merz JE, Setka JD, Pasternack GB, Weathon JM (2004) Predicting benefits of spawning-habitat rehabilitation to salmonid (Oncorhynchus spp.) fry production in a regulated California river. Can J Fish Aquat Sci 61:1433–1446
Minin AA, Ozerova SG (2008) Spontaneous activation of fish eggs is abolished by protease inhibitors. Rus J Dev Biol 38:293–296
Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K (1983) Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Biol 107:95–103
Ohta T, Iwamatsu T, Tanaka M, Yoshimoto Y (1990) Cortical alveolus breakdown in the eggs of the freshwater teleost Rhodeus ocellatus. Anat Rec 227:486–496
Ohta H, Tanaka H, Kagawa H, Okuzawa K, Iinuma N (1997) Artificial fertilization using testicular spermatozoa in the Japanese eel Anguilla japonica. Fish Sci 63:393–396
Olsen KH, Sawisky GR, Stacey NE (2006) Endocrine and milt response of male crucian carp (Carassius carassius L.) to periovulatory females under field conditions. Gen Comp Endocrinol 149:294–302
Pereira JC, Lino PG, Leitão A, Joaquim S, Chaves R, Pousão-Ferreira P, Guedes-Pinto H, Neves dos Santos M (2010) Genetic differences between wild and hatchery populations of Diplodus sargus and D. vulgaris inferred from RAPD markers: implications for production and restocking programs design. J Appl Genet 51:67–72
Probst WN, Stoll S, Hofmann H, Fisher P, Eckmann R (2009) Spawning site selection by Eurasian perch (Perca fluviatilis L.) in relation to temperature and wave exposure. Ecol Freshw Fish 18:1–7
Sarkar UK, Deepak PK, Negi RS, Singh S, Kapoor D (2006) Captive breeding of endangered fish Chitala chitala (Hamilton–Buchanan) for species conservation and sustainable utilization. Biodiv Cons 15:3579–3589
Sarosiek B, Cejko BI, Glogowski J, Targońska K, Zarski D, Kowalski RK, Kucharczyk D (2012) Spermatozoa motility and short-term sperm storage of colourful orfe (Leuciscus idus aberr orfus). Ital J Anim Sci 11:e270–e274
Sayer CD, Copp GH, Emson D, Godard MJ, Zięba G, Wesley KJ (2011) Towards the conservation of crucian carp Carassius carassius: understanding the extent and causes of decline within parts of its native English range. J Fish Biol 79:1608–1624
Shiri Harzevili A, De Charleroy D, Auwerx J, Vught I, Van Slycken J (2003) Larval rearing of chub, Leuciscus cephalus (L.), using decapsulated Artemia as direct food. J Appl Ichthyol 19:123–125
Skrzypczak A, Mamcarz A (2005) Crucian carp, Carassius carassius (L.), in the fishery exploited lakes of north-eastern Poland in 1951–1994. Acta Sci Pol Piscaria 4:89–100
Targońska K, Kucharczyk D, Kujawa R, Mamcarz A, Żarski D (2010) Controlled reproduction of asp, Aspius aspius (L.) using luteinizing hormone releasing hormone (LHRH) analogues with dopamine inhibitors. Aquaculture 306:407–410
Targońska K, Kucharczyk D, Żarski D, Cejko B, Krejszeff S, Kupren K, Król R, Dryl K, Kowalski R, Glogowski J (2011a) Artificial reproduction of wild and cultured barbel (Barbus barbus, cyprinidae) under controlled conditions. Acta Vet Hung 59:363–372
Targońska K, Krejszeff S, Żarski D, Kowalski R, Cejko BI, Sarosiek B, Palińska K, Glogowski J, Kucharczyk D (2011b) Influence of time of contact sperm with water to fertilization ability in ide Leuciscus idus L. Abstracts of the 3rd international workshop on the biology of fish gametes Budapest, Hungary, September 7–9 2011
Targońska K, Żarski D, Muller T, Krejszeff S, Kozłowski K, Demeny F, Urbanyi B, Kucharczyk D (2012) Controlled reproduction of the crucian carp Carassius carassius (L.) combining temperature and hormonal treatment in spawners. J Appl Ichthyol 28:894–899
Tarkan AS, Copp GH, Zięba G, Godart MJ, Cucherousset J (2009) Growth and reproduction of threatened native crucian carp Carassius carassius in small ponds of Epping Forest, south–east England. Aquat Cons Mar Fresh Ecosyst 19:797–805
Wheeler A (2000) Status of the crucian carp, Carassius carassius (L.), in the UK. Fish Manag Ecol 77:315–322
Wouters J, Janson S, Lusková V, Olsén KH (2012) Molecular identification of hybrids of the invasive gibel carp Carassius auratus gibelio and crucian carp Carassius carassius in Swedish waters. J Fish Biol 80:2595–2604
Woynarovich E, Woynarovich A (1980) Modified technology for elimination of stickiness of common carp Cyprinus carpio eggs. Aquacult Hung 2:19–21
Żarski D, Targońska K, Krejszeff S, Kwiatkowski M, Kupren K, Kucharczyk D (2011) Influence of stocking density and type of feed on the rearing of crucian carp, Carassius carassius (L.), larvae under controlled conditions. Aquacult Int 19:1105–1117
Żarski D, Horvath A, Kotrik L, Targońska K, Palińska K, Krejszeff S, Bokor Z, Urbanyi B, Kucharczyk D (2012a) Effect of different activating solutions on the fertilization ability of Eurasian perch, Perca fluviatilis L., eggs. J Appl Ichthyol 28:967–972
Żarski D, Krejszeff S, Palińska K, Targońska K, Kupren K, Fontaine P, Kestemont P, Kucharczyk D (2012b) Cortical reaction as an egg quality indicator in artificial reproduction of pikeperch, Sander lucioperca. Reprod Fertil Dev 24:843–850
Acknowledgments
This study was partially supported by the projects: “Innovations in finfish aquaculture with special references to reproduction” (InnovaFish), Operational Programme Sustainable Development of the Fisheries Sector and Coastal Fishing Areas 2007–2013 (OR14-61724-OR1400003/09/10/11); Bolyai János research Grant by the Hungarian Academy of Sciences (BO 54/12/4); Project TÁMOP-4.2.2.B-10/1-2010-0011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Żarski, D., Horváth, Á., Bernáth, G. et al. Application of different activating solutions to in vitro fertilization of crucian carp, Carassius carassius (L.), eggs. Aquacult Int 22, 173–184 (2014). https://doi.org/10.1007/s10499-013-9692-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9692-z