Abstract
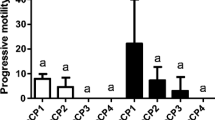
Sperm handling procedures and its usage for in vitro fertilization are crucial for standardized experimental operations on evaluation of reproductive performance, gamete quality, and optimization of fertilization protocols. In our study, the changes in perch sperm motility parameters within 6-h chilled storage and using 4 different activating solutions were compared. Eight different sperm-to-egg ratio was also compared during fertilization. Sperm activated with modified activating solution for cyprinids (78±11%), common perch activating solution (68±16%), modified Lahnsteiner activating solution (75±16%), and Woynárovich solution (76±13%) showed similar progressive motility at 10 s after activation. At 30 s after activation, progressive motility decreased below 5%, regardless the activating solution used. Progressive motility decreased significantly already after 2 h of storage (51±19%) in comparison with 0 h (78±5%). The highest average fertilization rate (using common perch activating solution) was observed with a sperm-to-egg ratio 2.5×105:1 (80±9%), where the smallest variability in the values was also recorded (coefficient of variation: 11%). However, no significant difference was detected among the 8 sperm-to-egg ratio groups. According to our findings, undiluted fresh perch sperm is recommended to use in 1 h post-stripping. Modified Lahnsteiner’s activating solution can be applied efficiently for quality assessment where common perch activating solution is applicable for fertilization in Eurasian perch. A sperm-to-egg ratio 2.5×105:1 already allows to achieve a high fertilization rate; however, the finding is needed to be tested also at hatchery level (higher number of eggs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Control of gamete quality (both sperm and egg) is a major issue for the aquaculture industry both for the production of species with already high commercial interest and of new potential candidates as well (Cabrita et al. 2014; Samarin et al. 2017). The quality of sperm is related to their capacity to produce viable embryos during fertilization of high-quality eggs in an appropriate environment (Cabrita et al. 2009). Efficient management of fish reproduction requires the use of best males as breeders in order to promote aquaculture or conservation of wild stocks (Cabrita et al. 2011).
Short-term gamete preservation can support different breeding programs, artificial fertilization, and hybridization (Riley 2002). Fertilizing capacity decreases during the post-stripping storage depending on several factors (fish age, spawning season, storage conditions: temperature, storage time, oxygen availability, diluent composition etc., Babiak et al. 2006; DeGraaf and Berlinsky 2004; Mylonas et al. 2003; Risopatrón et al. 2018; Trigo et al. 2015; Vuthiphandchai et al. 2009). Chilled storage methods have been already developed for numerous known fish species of commercial interest. During in vitro storage of fish sperm, its quality can reduce significantly (motility, viability, integrity of plasma membrane, mitochondrial membrane potential, Contreras et al. 2017; Risopatrón et al. 2018; Trigo et al. 2015). Sperm storage time (hours to days) highly depend on the appropriate method and on the species (Beirão et al. 2019; Contreras et al. 2020).
Among several other extrinsic parameters, an optimal fertilizing medium or activating solution is also required for the successful fertilization mechanism (Beirão et al. 2019). Hypotonic solutions in freshwater and hypertonic media in marine species can trigger sperm movement. The ionic composition as well as the final osmolality plays an important role in the activation process (Alavi et al. 2007; Beirão et al. 2019). There is no universal solution which can enhance sperm movement in all fish species; however, various highly conserved mechanisms can help to design species-specific media (Beirão et al. 2019). Longevity of sperm in most freshwater and marine species extends for periods from seconds to minutes. The osmolality and the temperature of the fertilization environment can affect longevity of sperm of freshwater fish (Browne et al. 2015; Cosson et al. 2008; Dreanno et al. 1999).
One of the most important parameters affecting fertilization success is the sperm-to-egg-ratio (Żarski et al. 2017b). A direct relationship between sperm density and fertilizing capacity of sperm was revealed by former studies (Horváth et al. 2015). Too frequent stripping intensity, over-exploitation of males, and deficit of the sperm (leading to low fertilization rate) may reduce the effectiveness of the controlled reproduction. Nonetheless, in case of hatchery practice, this aspect is often ignored (Żarski et al. 2017b). Sperm quality is a limiting factor in the appropriate sperm-to-egg ratio. Correspondingly, sperm motility was defined as one of the most important quality parameters during fertilization. In the case of a low-quality sperm (<50%), the effective number of spermatozoa per egg is needed to be adjusted (enhanced) to maximize the fertilization rate (Beirão et al. 2019; Gallego et al. 2013).
Eurasian perch (Perca fluviatilis) is one of the most promising candidate freshwater species for intensive aquaculture. It has already a notable commercial production in several European countries (Judycka et al. 2019). Its reproduction, although feasible under controlled conditions, is constantly being improved (Żarski et al. 2019). Especially, that its effectiveness, by affecting number of juveniles produced, significantly influences effectiveness of further production process. Several studies were investigating the physiological features of sperm quality (Alavi et al. 2007; Boryshpolets et al. 2009). Furthermore, Lahnsteiner (2011) presented an effective activating medium for sperm motility assessment as well. Sperm was able to move (motility: 41±10%) more than 2 h at 4 °C (Lahnsteiner 2011). Sarosiek et al. (2014) published the positive effect of an extender on the possible short-term storage time (prolonged for 17 days) comparison with the control undiluted sperm (7 days) where motility was measured in every 3–5 days. In shorter period (4, 8, and 24 h), Król et al. (2018) proved that the quality of undiluted perch sperm is negatively affected by urine contamination. However, the possible quality changes during chilled storage of undiluted perch sperm in laboratory conditions (1 to a few hours) were (according to our knowledge) not tested, so far. Fertilization procedure was already optimized for Eurasian perch by Żarski et al. (2012a); however, according to our knowledge, the optimal sperm-to-egg ratio was not investigated whenever fresh sperm was used for fertilization in Eurasian perch. A controlled ratio was used in stripped perch sperm (9.2 or 12×105 spermatozoa per oocyte, hatching rate: 80.3±14%, 49.3±21.0%) and testicular sperm of perch neomales (12×105 spermatozoa per oocyte, hatching rate: 42.5±22.7%) for fertilization by Rodina et al. (2008). In former study, the high fertilizing capacity (73–77%) of cryopreserved perch sperm was proven using a ratio ranged from 5×104 to 5×105 spermatozoa per oocyte (Judycka et al. 2019).
The aim of our study was to determine optimal sperm handling protocol by focusing on changes in sperm motility during the short-term storage (for 6 h) of the freshly collected sperm as well as finding the best activating solution for evaluation of spermatozoa motility with CASA system. Additionally, the study aimed at evaluation of sperm-to-egg ratio during the in vitro fertilization procedure.
Materials and methods
In our study, 3 different experiments were carried out. The experiments 1 and 2 were carried out in Poland, whereas experiment 3 was performed in Hungary.
Broodstock management
In experiments 1 and 2, Wild Eurasian perch broodstock (collected at the beginning of the reproductive season from Saska river, North-East Poland) was kept in 1 m3 fiberglass tanks at 12 °C in recirculating aquaculture system (RAS; equipped with automatic photo-thermal control, biological filtration, UV sterilization, and aeration) at the laboratories of Aquaculture and Ecological Engineering Center of University of Warmia and Mazury in Olsztyn, Poland (N= 30 males, average bodyweight: 113±49 g). In experiment 3, a broodstock (provided by Szabolcsi Halászati Ltd.) of Eurasian perch males (N=13, 88±69 g) and females (N=24, 167±188 g) was kept (according to the hatchery temperature average on 9 °C) at the Department of Aquaculture, Szent István University in Hungary. Perch individuals were placed into 1 m3 at a temperature varied according to the hatchery temperature. In both cases, the daily fresh water supply was provided.
Gamete collection
The male broodstock kept in experiments 1 and 2 at 14 °C where individuals were hormonally not stimulated prior to the sampling (individuals were already spermiating because of the spawning season). In experiment 3, both males and females were hormonally induced using 500 IU per kg−1 of hCG (human chorionic gonadotropin Ferring, Switzerland). In this experiment, injected females were kept at 12 °C for 3 days whereas males were kept for 6 days at various temperatures (according to the hatchery temperature) before sampling (Żarski et al. 2017a). In the case of females, genital pores were closed with surgical suture after injection to prevent spontaneous release of eggs. Synchronization of ovulation was achieved using the oocyte categorization described by Żarski et al. (2011 and 2012b) for Eurasian perch. Genital apertures of anesthetized (in experiments 1 and 2: 150 mg L−1 MS-222, tricaine-methanesulfonate; in experiment 3: 0.4 mL L−1, 2-phenoxyethanol) males and females were wiped dry immediately before stripping. Sperm was collected in all experiments using catheter (connected to the genital pore) into 1.5-mL Eppendorf tubes and were stored at 4 °C. Eggs were stripped into plastic dishes and were stored at 4 °C prior to fertilization experiment.
Sperm motility assessments
In experiments 1 and 2, progressive motility (pMOT, %), curvilinear velocity (VCL, μm s−1), and straightness (STR, %) was recorded using a CASA (Sperm Class Analyzer v. 4.0.0. by Microptic S.L., Barcelona, Spain) system whereas in experiment 3, another system was used (computer-assisted sperm analysis, Sperm VisionTM v. 3.7.4., Minitube of America, Venture Court Verona, USA). Sperm was diluted in experiments 1 and 2 in an ionic immobilizing solution designed for perch (150 mM NaCl, 5 mM KCl,1 mM MgSO47H2O,1 mM CaCl2 2H2O, 20 mM Tris, pH 8, Lahnsteiner 2011) because of the high cell concentration at a ratio 1:50 and 1:100 (according to the visually observed sperm density using a brightfield microscope) prior to the motility assessment. Four different activating solutions (which were already tested in perch or other species) were used with the addition of 0.01 g mL−1 BSA according to the experimental design:
-
1.
Modified activating solution for cyprinids (As, 45 mM NaCl, 5 mM KCl, 30 mM Tris, pH 8 (Saad et al. 1988)) in experiment 1.
-
2.
Common perch activating solution (Co, 50 mM NaCl, pH 8.0 (Lahnsteiner 2011)) in experiments 1 and 3.
-
3.
Modified Lahnsteiner’s activating solution (La, 75 mM NaCl, 2 mM KCl, 1 mM MgSO4×7H2O, 1 mM CaCl2× 2H2O, 20 mM Tris, pH 8 (Lahnsteiner 2011)) in experiments 1 and 2.
-
4.
Woynárovich solution (Wo, 50 mM urea, 68 mM NaCl, pH 8 (Woynarovich and Woynarovich 1980)) in experiment 1.
Experimental design
Experiment 1.1: Selection of the best activating solution (first trial)
Sperm was collected from 10 males. Samples were diluted in modified Lahnsteiner’s immobilizing solution only prior to motility assessments (just immediately prior to the measurement, because of the too high sperm density). Sperm was activated immediately after dilution with common perch activating solution, modified activating solution for cyprinids, modified Lahnsteiner’s activating, and Woynarovich solution. Movement was recorded at 10, 30, 60, 90, and 120 s following activation.
Experiment 1.2: Selection of the best activating solution (second trial)
Based on the results of experiment 1.1, the abovementioned investigation was repeated with minor changes. In this case, sperm from 10 males was diluted in Lahnsteiner’s immobilizing solution (just immediately prior to the measurement, because of the too high sperm density) and was activated using the 4 activating solutions. However, motility was recorded at 10 and 20 s after activation.
Experiment 2: Short-term storage of undiluted sperm
Ten samples following stripping were stored for up for 6 h at 4 °C. Sperm was diluted only immediately prior to motility assessments using modified Lahnsteiner’s immobilizing solution (just immediately prior to the measurement, because of the too high sperm density). Movement was recorded in 1-h interval using modified Lahnsteiner’s activating solution.
Experiment 3: The investigation of the effective sperm-to-egg ratio
Prior to fertilization, motility of 8 stripped males was evaluated. According to the results, 3 samples (showed the highest pMOT) were chosen. The cell concentration of the mentioned 3 samples was measured as well. Individual samples were diluted in modified Tanaka’s extender (137 mM NaCl and 76.2 mM NaHCO3, Bernáth et al. 2016; Szabó et al. 2005) at a ratio of 1:99 and were loaded into a Bürker-type hemocytometer (Marienfield Superior, Paul Marienfield GmBH & CO. KG, Lauda-Königshofen, Germany). Concentration was calculated visually using a digital camera (QImaging Micro Publisher 3.3, QImaging, Surrey, Canada) connected to a brightfield microscope (Nikon Eclipse 600, Auroscience Kft., Budapest, Hungary) equipped with a ×20 objective. For fertilization, eggs (ribbon, perch eggs are held together in a long ribbon-like structure, Rougeot et al. 2008) from 1 female (to reduce individual variation obtained from different females) were used. To estimate the number of eggs used for fertilization, the total number of eggs in a 1 g of ribbon was calculated by direct counting of number of eggs under the microscope (Carl Ceiss Jena Technival, Carl Zeiss Microscopy GmbH, Germany). Next, eight different sperm-to-egg ratios were tested experimentally: 2.5×104:1, 5×104:1, 105:1, 1.5×105:1, 2×105:1, 2.5×105:1, 3×105:1, 5×105:1 (spermatozoa:egg) for each sperm sample separately. The amount of sperm was adjusted according to the sperm-to-egg ratio. Egg batches were placed into petri dishes onto which common perch activating solution was added. Eggs were incubated for 15 s in the common perch activating solution thereafter sperm samples were pipetted on them (according to the method of Żarski et al. (2012a)). Petri dishes were roughly mixed for 20 s. Embryos were incubated in plastic cups (400 mL) at a temperature between 10 and 12 °C. Water was changed daily. Eggs were identified as “fertilized” if they reached the neurula stage and viable embryos were counted directly under the microscope (Carl Ceiss Jena Technival, Carl Zeiss Microscopy GmbH, Germany). Fertilization was performed not later than 2 h following collection of the gametes, which included all the necessary procedures allowing estimation of sperm concentration and number of eggs in 1 g of ribbon (Samarin et al. 2017).
Statistical analyses
The precise calculations were performed using the MS Excel (Microsoft Excel 2007, Microsoft Corporation) spreadsheet considering number of eggs in 1 g and sperm concentration calculated. The statistical software packages SPSS 14.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 5.0 for Windows (GraphPad Software, La Jolla, CA, USA) were used to analyze results of motility and fertilization experiments. Normal distribution of data was verified using a Kolmogorov-Smirnov test at a significance level of p<0.05. Data were log transformed (VCL) or transformed using arcsine square root (pMOT, STR, fertilization rate) if distribution was not normal. The differences among experimental groups were investigated using one- and two-way ANOVA (analysis of variance) and Kruskal-Wallis test combined with Tukey’s, Dunnett’s, Bonferroni’s, and Dunn’s multiple comparison post hoc tests (at a significance level of p<0.05).
Results
Experiment 1.1: Selection of the best activating solution (first trial)
No significant difference was recorded pMOT at 10 and 30 s following activation using the 4 solutions. At 30 s after activation, progressive motility decreased with every activating solutions around or below 5%. A significantly higher VCL was observed with La than in Co at 10 s. At 30 s, a significantly higher VCL was also measured using La and AS in comparison with Co and Wo. No significant difference was found in STR during the comparison of the 4 different solutions at 10 s. Similarly to VCL, significantly higher STR values were recorded using La and AS than in Co and Wo at 30 s post-activation (Table 1).
Experiment 1.2: Selection of the best activating solution (second trial)
The lowest reduction in pMOT was measured during 20 s with La (80±26–69±22%), compare to AS (73±26–53±21%), Wo (80±25–60±20%), and Co (79±25–43±17%). Using AS, VCL showed a significant reduction at 20 s following activation compare to La (65±19 and 80±19 μm s−1). A similar significant decrease was observed in VCL using Co already at 20 s in comparison with La (54±14 and 80±19 μm s−1) and Wo (54±14 and 66±15 μm s−1). In contrast, no significant difference in STR was recorded among the 4 different activating solutions at 10 and 20 s following thawing (Table 2).
Experiment 2: Short-term storage of undiluted sperm
Motility decreased significantly already after 2 h of storage (51±19%) in comparison with 0 h (78±5%). VCL decreased significantly also after 2 (105±39 μm s−1), where STR reduced only after 5 (73±5%) h of storage time in comparison with 0 h (155±14 μm s−1 and 81±4%) (Fig. 1 a–c).
Experiment 3: Evaluation of the effective sperm-to-egg ratio
Prior to fertilization, 566 eggs were counted in 1g of egg. The egg batches were calculated according to the sperm concentration measured in the 3 sperm samples. Used fresh sperm samples showed just after stripping an average motility 88±4% and a high concentration (1: 2.34×107, 2: 4.52×107, 3: 3.575×107 spermatozoa μL−1). The highest average fertilization rate was observed with a ratio 2.5×105:1 (80±9%); however, no significant difference was detected among the 8 sperm-to-egg ratio group. The lowest coefficient of variation values were recorded by the ratios 2×105:1 (17%) and 2.5×105:1 (11%) where the highest variability was observed using 5×104:1 (80%) and 1.5×105:1 (80%) (Table 3).
Discussion
The decrease of pMOT after 30 s following activation observed in our study, regardless the activating solution used, was comparable to the observations of Bernáth et al. (2016), who also recorded 30 s as a threshold for motility in cryopreserved-thawed sperm. Similarly to our results, frozen-thawed sperm showed identical tendency in progressive motility at 10 s after activation (Bernáth et al. 2016). The lowest reduction in pMOT after 20 s following activation was recorded in La, perch sperm movement decreased drastic 30 s post-activation (5% or less) in comparison with the findings of Lahnsteiner (2011) where sperm showed 41±10% motility more than 2 h post-activation at 4 °C using the similar activating solution (La). Dilution ratio is a key factor in the longevity of sperm movement. High ratio and two-step dilution (first: immobilizing solution, second: activating solution) is recommended for observation of sperm motility (Alavi et al. 2007). In our study, the too high sperm density hypothetically could have a negative effect on sperm longevity in Eurasian perch. Co achieved a high fertilization rate using fresh perch sperm. In our former study, cryopreserved and thawed perch sperm showed a similar high (72 ± 14%) fertilization rate using Co (Bernáth et al. 2016). Because of the simple chemical composition, Co can be a recommended solution for the regular hatchery practice during the standard propagation process (Bernáth et al. 2016). According to our results and the study by Lahnsteiner (2011), La for semen analysis and Co for fertilization tests can be efficiently used in Eurasian perch.
Short-term storage can support the successful fertilization through hatchery practice in the case of a possible asynchronous spemiation and ovulation with the elongated applicability of fresh sperm (Cabrita et al. 2010). After 2 h, a significant negative effect of storage time was recorded on the progressive motility of perch sperm. In contrast with our result, Sarosiek et al. (2014) found that drastic reduction in motility parameters in undiluted semen was recorded only after 3 days of chilled storage (4 °C). Furthermore, Król et al. (2018) presented a dose-dependent negative effect of urine contamination on the perch sperm motility. In both studies, a similar flexible catheter was used during stripping allowing collection of urine-free sperm. Despite to the precise sperm stripping method, a relatively low-quality sperm (low VCL and high STR values) was collected in our study. Żarski et al. 2017a) presented the clear positive effects of hCG and sGnRHa on sperm motility at 6 days following injection in Eurasian perch. Contrary, in our study, already spermiating males were used without hormonal induction during the spawning season. According to our hypothesis, the lacks of hormonal stimulation lead to a low initial sperm quality and a reduced tolerance to short-term storage. Furthermore, former studies proved that gaseous exchange (O2-Co2) is generally important in the stored samples (Bobe and Labbe 2009). In our experiment, the Eppendorf tubes used for storage could not allow the efficient oxygen supply for the samples. The notable reduction measured in motility could cause by the sedimentation of the samples. In a future method, a periodic gentle mixing, stirring, or rocking can avoid the mentioned phenomenon (Babiak et al. 2006; Beirão et al. 2019; Dorsey et al. 2011; McNiven et al. 1993; Park and Chapman 2005; Santos et al. 2018).
The effective sperm-to-egg ratio was investigated by several authors and its importance during artificial propagation was also presented. However, different species specific ratios were suggested in different fish species. The highest fertilization rate was achieved in Eurasian perch using 2.5×105 spermatozoa for one oocyte. Furthermore, an increasing tendency was observed between the sperm-to egg-ratios 2.5×104:1–2.5×105:1. Comparable result (hatching rate: 80.3±14%) was found in perch males by Rodina et al. (2008) where 9.2 ×105 spermatozoa per oocyte were used. Furthermore, Judycka et al. (2019) achieved also high fertilization rates (73%) with frozen-thawed perch sperm at as low ratio as 5×104. The authors also suggested that a relatively small sperm-to-egg ratio can be successfully applied for fertilization of an elevated amount (25 g) of perch egg using cryopreserved sperm (Judycka et al. 2019). Contrary, the results showed in our experiment that in controlled (laboratory) conditions, higher amount of spermatozoa was more efficient and showed smaller variation in the fertilization success. According to the average cell concentration, lower and higher optimal sperm-to-egg ratios were reported in different fish species (Beirão et al. 2019). In comparison with our results, a higher effective sperm-to-egg-ratio was suggested in Caspian brown trout (Salmo trutta caspius, 3 or 6×105, Golshahi et al. 2015) and brook trout (Salvelinus fontinalis, 3×105, Nynca et al. 2015). Contrary, lower sperm-to-egg ratio was observed in Atlantic cod (Gadus morhua, L., 105, Butts et al. 2009), Adriatic grayling (Thymallus thymallus, 5×104 Horváth et al. 2015), and in turbot (Scophthalmus maximus, 6×103, Suquet et al. 1995). According to our and the results of Judycka et al. (2019), a medium high (5×104–2.5×105:1) sperm-to-egg ratio is recommended to maximize the fertilization success both using fresh and cryopreserved perch sperm as well. This information can support the intensive culture of the species via optimization of gamete usage during fertilization.
Conclusion
According to our results, modified Lahnsteiner’s activating solution should be applied during CASA measurements. The possible storage time without significant decrease in pMOT and VCL for undiluted perch sperm was defined in maximum 1 h (refrigerated storage at 4 °C, without additional O2 supply). The common perch activating solution is suitable for fertilization during standard propagation process. An effective sperm-to-egg ratio was experimentally defined for fresh Eurasian perch sperm to 2.5×105:1, for laboratory-scale fertilization. Our findings can contribute to the improvement of the artificial propagation of perch at hatchery conditions.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- Co:
-
Common perch activating solution
- CASA:
-
Computer-assisted sperm analysis
- VCL:
-
Curvilinear velocity
- As:
-
Modified activating solution for cyprinids
- La:
-
Modified Lahnsteiner’s activating solution
- pMOT:
-
Progressive motility
- STR:
-
Straightness
- Wo:
-
Woynárovich solution
References
Alavi SMH, Rodina M, Policar T, Kozak P, Psenicka M, Linhart O (2007) Semen of Perca fluviatilis L.: sperm volume and density, seminal plasma indices and effects of dilution ratio, ions and osmolality on sperm motility. Theriogenology 68:276–283. https://doi.org/10.1016/j.theriogenology.2007.05.045
Babiak I, Ottesen O, Rudolfsen G, Johnsen S (2006) Chilled storage of semen from Atlantic halibut, Hippoglossus hippoglossus L. I: Optimizing the protocol. Theriogenology 66:2025–2035. https://doi.org/10.1016/j.theriogenology.2006.06.003
Beirão J, Boulais M, Gallego V, O’Brien JK, Peixoto S, Robeck TR, Cabrita E (2019) Sperm handling in aquatic animals for artificial reproduction. Theriogenology 133:161–178. https://doi.org/10.1016/j.theriogenology.2019.05.004
Bernáth G, Bokor Z, Żarski D, Várkonyi L, Hegyi Á, Staszny Á, Urbányi B, Radóczi IJ, Horváth Á (2016) Commercial-scale out-of-season cryopreservation of Eurasian perch (Perca fluviatilis) sperm and its application for fertilization. Anim Reprod Sci 170:170–177. https://doi.org/10.1016/j.anireprosci.2016.05.005
Bobe J, Labbe C (2009) Chilled storage of sperm and eggs. In: Cabrita E, Robles V, Herráez P (eds) Methods in reproductive aquaculture: marine and freshwater species. Taylor & Francis Group, Boca Raton, pp 219–231
Boryshpolets S, Dzyuba B, Stejskal V, Linhart O (2009) Dynamics of ATP and movement in Eurasian perch (Perca fluviatilis L.) sperm in conditions of decreasing osmolality. Theriogenology 72:851–859. https://doi.org/10.1016/j.theriogenology.2009.06.005
Browne RK, Kaurova SA, Uteshev V, Shishova NV, McGinnity D, Figiel CR, Mansour N, Agney D, Wu M, Gakhova EN et al (2015) Sperm motility of externally fertilizing fish and amphibians. Theriogenology 83:1–13. https://doi.org/10.1016/j.theriogenology.2014.09.018
Butts IAE, Trippel EA, Litvak MK (2009) The effect of sperm to egg ratio and gamete contact time on fertilization success in Atlantic cod Gadus morhua L. Aquaculture 286:89–94. https://doi.org/10.1016/j.aquaculture.2008.09.005
Cabrita E, Robles V, Herráez MP (2009) Sperm quality assessment. In: Cabrita E, Robles V, Herráez MP (eds) Methods in reproductive aquaculture: marine and freshwater species. Biology Series. CRC Press (Taylor and Francis Group), Boca Raton, pp 93–148
Cabrita E, Sarasquete C, Martínez-Páramo S, Robles V, Beirão J, Pérez-Cerezales S, Herráez MP (2010) Cryopreservation of fish sperm: applications and perspectives. J Appl Ichthyol 26:623–635. https://doi.org/10.1111/j.1439-0426.2010.01556.x
Cabrita E, Robles V, Sarasquete C, Herráez MP (2011) New insights on sperm quality analysis for the improvement of broodstock. In: Tiersch TR, Green CC (eds) Cryopreservation in aquatic species, 2nd edn. World Aquaculture Society, Baton Rouge, pp 146–161
Cabrita E, Martínez-Páramo S, Gavaia PJ, Riesco MF, Valcarce DG, Sarasquete C, Herráez MP, Robles V (2014) Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 432:389–401. https://doi.org/10.1016/j.aquaculture.2014.04.034
Contreras P, Ulloa P, Merino O, Valdebenito I, Figueroa E, Farías J, Risopatrón J (2017) Effect of short-term storage on sperm function in Patagonian blenny (Eleginops maclovinus) sperm. Aquaculture 481:58–63. https://doi.org/10.1016/j.aquaculture.2017.08.022
Contreras P, Dumorné K, Ulloa-Rodríguez P, Merino O, Figueroa E, Farías JG, Valdebenito I, Risopatrón J (2020) Effects of short-term storage on sperm function in fish semen: a review. Rev Aquac 12:1373–1389. https://doi.org/10.1111/raq.12387
Cosson J, Groison AL, Suquet M, Fauvel C, Dreanno C, Billard R (2008) Studying sperm motility in marine fish: an overview on the state of the art. J Appl Ichthyol 24:460–486. https://doi.org/10.1111/j.1439-0426.2008.01151.x
DeGraaf JD, Berlinsky DL (2004) Cryogenic and refrigerated storage of Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) spermatozoa. Aquaculture 234:527–540. https://doi.org/10.1016/j.aquaculture.2003.11.037
Dorsey KM, Guthrie HD, Welch GR, Mohler J, Theisen DD, Siewerdt F et al (2011) Quality assessment of wild atlantic sturgeon semen under conditions of short-term storage. N Am J Aquac 73:418–425. https://doi.org/10.1080/15222055.2011.629945
Dreanno C, Cosson J, Suquet M, Cibert C, Fauvel C, Dorange G, Billard R (1999) Effects of osmolality, morphology and intracellular nucleotid content during the movement of sea bass (Dicentrarchus labrax) spermatozoa. J Reprod Fertil 116:113–125. https://doi.org/10.1530/jrf.0.1160113
Gallego V, Pérez L, Asturiano JF, Yoshida M (2013) Relationship between spermatozoa motility parameters, sperm/egg ratio, and fertilization and hatching rates in pufferfish (Takifugu niphobles). Aquaculture 416-417:238–243. https://doi.org/10.1016/j.aquaculture.2013.08.035
Golshahi K, Shabani N, Aramli MS, Noori E (2015) Motility and fertilizing ability of cryopreserved Caspian brown trout (Salmo trutta caspius) sperm: effect of post-thaw storage time and different sperm-to-egg ratios. Cryobiology 71:360–363. https://doi.org/10.1016/j.cryobiol.2015.08.005
Horváth Á, Bokor Z, Bernáth G, Csenki Z, Gorjan A, Herráez MP, Urbányi B, Jesenšek D (2015) Very low sperm–egg ratios result in successful fertilization using cryopreserved sperm in the Adriatic grayling (Thymallus thymallus). Aquaculture 435:75–77. https://doi.org/10.1016/j.aquaculture.2014.09.032
Judycka S, Żarski D, Dietrich MA, Palińska-Żarska K, Karol H, Ciereszko A (2019) Standardized cryopreservation protocol of European perch (Perca fluviatilis) semen allows to obtain high fertilization rates with the use of frozen/thawed semen. Aquaculture 498:208–216. https://doi.org/10.1016/j.aquaculture.2018.08.059
Król J, Żarski D, Bernáth G, Palińska-Żarska K, Krejszeff S, Długoński A, Horváth Á (2018) Effect of urine contamination on semen quality variables in Eurasian perch Perca fluviatilis L. Anim Reprod Sci 197:240–246. https://doi.org/10.1016/j.anireprosci.2018.08.034
Lahnsteiner F (2011) Spermatozoa of the teleost fish Perca fluviatilis (perch) have the ability to swim for more than two hours in saline solutions. Aquaculture 314:221–224. https://doi.org/10.1016/j.aquaculture.2011.02.024
McNiven MA, Gallant RK, Richardson GF (1993) Fresh storage of rainbow trout (Oncorhynchus mykiss) semen using a non-aqueous medium. Aquaculture 109:71–82. https://doi.org/10.1016/0044-8486(93)90487-J
Mylonas C, Papadaki M, Divanach P (2003) Seasonal changes in sperm production and quality in the red porgy Pagrus pagrus (L.). Aquac Res 34:1161–1170. https://doi.org/10.1046/j.1365-2109.2003.00922.x
Nynca J, Dietrich GJ, Dobosz S, Zalewski T, Ciereszko A (2015) Effect of postthaw storage time and sperm-to-egg ratio on fertility of cryopreserved brook trout sperm. Theriogenology 83:253–256. https://doi.org/10.1016/j.theriogenology.2014.09.009
Park C, Chapman FA (2005) An extender solution for the short-term storage of sturgeon semen. N Am J Aquac 67:52–57. https://doi.org/10.1577/FA03-068.1
Riley K (2002) Refrigerated storage and cryopreservation of sperm for the production of red snapper and snapper hybrids. Master in Sciences Thesis, Louisiana State University, pp 204.
Risopatrón J, Merino O, Cheuquemán C, Figueroa E, Sánchez R, Farías JG, Valdebenito I (2018) Effect of the age of broodstock males on sperm function during cold storage in the trout (Oncorhynchus mykiss). Andrologia 50:e12857. https://doi.org/10.1111/and.12857
Rodina M, Policar T, Linhart O, Rougeot C (2008) Sperm motility and fertilizing ability of frozen spermatozoa of males (XY) and neomales (XX) of perch (Perca fluviatilis). J Appl Ichthyol 24:438–442. https://doi.org/10.1111/j.1439-0426.2008.01137.x
Rougeot C, Fontaine P, Mandiki SMN (2008) Perch description and biology. In: Toner D, Rougeot C (eds) Farming of Eurasian Perch, vol. 1—juvenile production. Aquaculture explained n°24. Aquaculture Development Division, Dublin, pp 12–15
Saad A, Billard R, Theron MC, Hollebecq MG (1988) Short-term preservation of carp (Cyprinus carpio) semen. Aquaculture 71:133–150. https://doi.org/10.1016/0044-8486(88)90280-3
Samarin AM, Żarski D, Palińska-Żarska K, Krejszeff S, Blecha M, Kucharczyk D, Policar T (2017) In vitro storage of unfertilized eggs of the Eurasian perch and its effect on egg viability rates and the occurrence of larval malformations. Animal 11:78–83. https://doi.org/10.1017/S1751731116001361
Santos M, Soares F, Moreira M, Beirao J (2018) Evaluation of different extenders for the cold storage of meagre (Argyrosomus regius) semen. Aquac Res 49:2723–2731. https://doi.org/10.1111/are.13733
Sarosiek B, Dryl K, Kucharczyk D, Żarski D, Kowalski RK (2014) Motility parameters of perch spermatozoa (Perca fluviatilis L.) during short-term storage with antioxidants addition. Aquac Int 22:159–165. https://doi.org/10.1007/s10499-013-9679-9
Suquet M, Billard R, Cosson J, Normant Y, Fauvel C (1995) Artificial insemination in turbot (Scophthalmus maximus): determination of the optimal sperm to egg ratio and time of gamete contact. Aquaculture 133:83–90. https://doi.org/10.1016/0044-8486(94)00395-5
Szabó G, Müller T, Bercsényi M, Urbányi B, Kucska IB, Horváth A (2005) Cryopreservation of European eel (Anguilla anguilla) spermusing different extenders and cryoprotectants. Short communication. Acta Biol Hung 56:173–175. https://doi.org/10.1556/ABiol.56.2005.1-2.18
Trigo P, Merino O, Figueroa E, Valdebenito I, Sánchez R, Risopatrón J (2015) Effect of short-term semen storage in salmon (Oncorhynchus mykiss) on sperm functional parameters evaluated by flow cytometry. Andrologia 47:407–411. https://doi.org/10.1111/and.12276
Vuthiphandchai V, Thadsri I, Nimrat S (2009) Chilled storage of walking catfish (Clarias macrocephalus) semen. Aquaculture 296:58–64. https://doi.org/10.1016/j.aquaculture.2009.07.018
Woynarovich E, Woynarovich A (1980) Modified technology for elimination of stickiness of common carp Cyprinus carpio eggs. Aquac Hung 2:19–21
Żarski D, Bokor Z, Kotrik L, Urbanyi B, Horváth A, Targońska K, Krejszeff S, Palińska K, Kucharczyk D (2011) A new classification of a preovulatory oocyte maturation stage suitable for the synchronization of ovulation in controlled reproduction of Eurasian perch, Perca fluviatilis L. Reprod Biol 11:194–209. https://doi.org/10.1016/S1642-431X(12)60066-7
Żarski D, Horváth Á, Kotrik L, Targońska K, Palińska K, Krejszeff S, Bokor Z, Urbányi B, Kucharczyk D (2012a) Effect of different activating solutions on the fertilization ability of Eurasian perch, Perca fluviatilis L., eggs. J Appl Ichthyol 28:967–972. https://doi.org/10.1111/jai.12098
Żarski D, Krejszeff S, Horváth Á, Bokor Z, Palińska K, Szentes K, Łuczyńska J, Targońska K, Kupren K, Urbányi B et al (2012b) Dynamics of composition and morphology in oocytes of Eurasian perch, Perca fluviatilis L., during induced spawning. Aquaculture 364–365:103–110. https://doi.org/10.1016/j.aquaculture.2012.07.030
Żarski D, Bernáth G, Król J, Cejko BI, Bokor Z, Palińska-Żarska K, Milla S, Fontaine P, Krejszeff S (2017a) Effects of hCG and salmon gonadoliberine analogue on spermiation in the Eurasian perch (Perca fluviatilis). Theriogenology 104:179–185. https://doi.org/10.1016/j.theriogenology.2017.08.022
Żarski D, Horváth Á, Bernáth G, Krejszeff S, Radóczi J, Palińska-Żarska K, Bokor Z, Kupren K, Urbányi B (2017b) Sperm-to-egg ratio. In: controlled reproduction of wild Eurasian perch: a hatchery manual. Springer Briefs in Environmental Science, Cham, p 76
Żarski D, Palińska-Żarska K, Krejszeff S, Król J, Milla S, Fontaine P, Bokor Z, Urbányi B (2019) A novel approach for induced out-of-season spawning of Eurasian perch, Perca fluviatilis. Aquaculture 512:734300. https://doi.org/10.1016/j.aquaculture.2019.734300
Funding
Open access funding provided by Hungarian University of Agriculture and Life Sciences. Research was supported by the EUREKA_HU_12-1-2012-0056 and by the EFOP-3.6.3-VEKOP-16-2017-00008 projects. The project is co-financed by the European Union and the European Social Fund. The study was also supported two scholarships for Gergely Bernáth: the Bolyai János Postdoctoral (BO/00508/18/4, Hungarian Academy of Sciences) and the ÚNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. This research was supported by the Ministry of Innovation and Technology within the framework of the Thematic Excellence Programme 2020, National Challenges Subprogramme (TKP2020-NKA-16). Our experiments were also supported by the fish farm Szabolcsi Halászati Ltd.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethical approval
All experiments were conducted in accordance with the Government Decree 40/2013 (II. 14.) on Animal Experiments and the Act XXVIII of 1998 of the Hungarian Parliament on the protection and humane treatment of animals. Following consultation with the Scientific Ethical Committee on Animal Experiments of the Ministry of Agriculture, it was concluded that no specific permit was required for the experiments.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bokor, Z., Żarski, D., Palińska-Żarska, K. et al. Standardization of sperm management for laboratory assessment of sperm quality and in vitro fertilization in Eurasian perch (Perca fluviatilis). Aquacult Int 29, 2021–2033 (2021). https://doi.org/10.1007/s10499-021-00731-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00731-4