Abstract
The potential for predation by the sea stars Asterias rubens and Marthasterias glacialis on seed-size (41 ± 3 mm shell height) juvenile scallops (Pecten maximus), ready for seeding in sea ranching areas, was investigated in a 30-day laboratory predation experiment. There was no significant difference (P > 0.05) in predation rate of large A. rubens (95–115 mm radium) and large M. glacialis (120–164 mm radius), which averaged 0.88 and 0.71 scallops individual−1 day−1, respectively. Maximum rates of predation were 2.44 scallops individual−1 day−1 for large A. rubens and 3.00 scallops individual−1 day−1 for large M. glacialis. Small M. glacialis (76–87 mm radius) had a significantly lower predation rate than large individuals of either species (average 0.13 scallops individual−1 day−1, P < 0.05). Small A. rubens (50–80 mm radius) only began to prey on scallops when average scallop size was reduced to 35 mm. Based on estimated density of sea stars at a Norwegian sea ranching site and average predation rates, a population of scallops seeded at 10 m−2 would be reduced by between 0.5 and 11% in 1 month. Furthermore, using the highest observed predation rate, the degree of loss of scallops indicated that scallop culture via sea ranching would not be economically viable and thus methods for reducing scallop predation by sea stars are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of scallop species are harvested and cultured around the world, accounting for approximately 11% of global mollusc production in 2008 (Fisheries and Agriculture Organisation [FAO] 2010). As a result of increasing demand, there has been increased development of scallop farming. Sea ranching of scallops utilises the sea bottom to grow seeded scallops to commercial size. During sea ranching, a population of seeded scallops may be reduced by dispersion and predation within the sea ranching area (Cliche et al. 1994; Hatcher et al. 1996; Minchin 2002). Thus, the rate of survival of seeded scallops, termed the survival rate, is determined by the level of losses from dispersion and predation during the sea ranching phase. Sea stars and crabs are generally thought to have the largest predatory impact on seeded scallops of a variety of species (Barbeau and Scheibling 1994a; Barbeau and Scheibling 1994b; Cliche et al. 1994; Barbeau et al. 1996; Hatcher et al. 1996; Barbeau et al. 1998; Strand et al. 2002; Grefsrud et al. 2003; Wong and Barbeau 2003, 2005; Wong et al. 2005), although fish also may reduce numbers of smaller scallops (Strohmeier et al. 2006). Trials have shown that fencing areas of the seabed can limit the access of crabs to seeded scallops (Strand et al. 2004); however, methodologies commonly used to exclude crabs do not exclude sea stars. Sea stars are often removed using different devices or are caught in traps (Hoshikawa et al. 1997; Martin and Proctor 2000). Chemical control or biological control methods using other sea star species (for example, the predacious sea star Crossaster papposus) have also been proposed (Spencer 2002).
The king scallop, Pecten maximus, is a candidate for sea ranching on the west coast of Norway. During sea ranching, fences have been shown to reduce predation by brown crabs resulting in increased P. maximus survival from <5 to 89% (Strand et al. 2004). Although both crabs (Grefsrud et al. 2003; Strand et al. 2004) and fish (Strohmeier et al. 2006) have been identified as potential predators on P. maximus in Norway, there is no known study of the impact of sea stars. The sea stars Asterias rubens and Marthasterias glacialis are important bivalve predators in shallow water ecosystems in western Norway (Wiborg 1946). Scallops may escape from predatory sea stars following contact using a swimming escape response (Barbeau and Scheibling 1994a; Wong and Barbeau 2003, 2005; Himmelman et al. 2005; Nadeau et al. 2009). Hatchery reared scallops, however, may have a less well-developed escape response than wild scallops (Minchin et al. 2000; Lafrance et al. 2003; Brokordt et al. 2006) and, therefore, may be more susceptible to sea star predation. Predation by A. rubens on the mussel, Mytilus edulis, has been the focus of most prior A. rubens research (O’Neill et al. 1983; Norberg and Tedengren 1995; Saier 2001; Gallagher et al. 2008), although there have been some studies of Asterias vulgaris, considered synonymous with A. rubens (Clark and Downey 1992), on several scallop species (Barbeau and Scheibling 1994a, b; Wong and Barbeau 2003, 2005). Only a few studies have considered the predatory activity of M. glacialis (Penney and Griffiths 1984; Frid 1992; Verling et al. 2003).
It has been suggested that recovery rates of >25% are necessary for scallop sea ranching production to be economically viable (Hébert et al. 2006). In Norway, however, scallops are seeded after intermediate culture (>30 mm), so that >60% of the seeded individuals must be harvested for the culture to be economically viable (Skjæggestad and Magnesen 2006).
Better understanding of the predation rates of sea stars will help to improve culture practices and increase recovery rates. The present study report results of laboratory experiments which evaluated the rate at which A. rubens and M. glacialis prey upon juvenile scallops ready for seeding and the potential impact of predation.
Methods
The experiment was carried out at the Department of Biology, University of Bergen, between 8 September and 7 October 2009 (maximum experimental period 30 days). A total of 15 tanks were prepared for the experiment by introducing a coarse shell-sand substrate bottom. Water flowed out from near the bottom of the tank. Tanks were provided with water from 105 m depth, filtered to 50 μm with partial UV-treatment. Water temperature and salinity measurements were made regularly using a WTW Cond 315i handheld meter. Temperature was kept at 14.9 ± 0.5°C (ambient natural temperature) throughout the experiment, and salinity varied between 34.5 and 34.6 ppt. The light regime was 13:11 light/dark at the start of the experimental period, decreasing to 11:13 (natural light cycle in Bergen, Norway; N60.22.881 E5.19.924).
Scallops were from spawning local broodstock in 2008 and were grown in intermediate culture from the autumn of 2008 until the time of the experiment. At this time, the scallops were ready for seeding at sea ranching localities at Norskjell AS (Huglo, Norway; N59.500 E5.5056). Prior to introduction to the tanks, any fouling on the scallop shell was removed using a sharp knife to standardise the outer appearance of each shell. Twelve tanks (1 × 1 × 0.6 m, 600 l water) were stocked (10 scallops m−2) with hatchery produced scallops (Pecten maximus; 41 ± 3 mm) from Scalpro AS. Three control tanks without predators (dimensions 0.6 × 0.6 × 0.6 m, filled to 180 l), each containing 10 scallops from the same size distribution as the experiment, were monitored daily for survival during the course of the experiment. The scallops were introduced to the experimental tanks and allowed to recess in the substrate. Sea stars (Asterias rubens and Marthasterias glacialis) were collected from the actual sea ranching locality, randomly allocated to groups (N = 3) according to size (measured from the mouth to the end of a normal arm) as shown in Table 1. Each size class was replicated three times. Sea star size reflected the actual size distribution at the sea ranching site.
After 1 week of the experiment, one large A. rubens was found to be missing an arm. The same was seen in another of the large A. rubens replicates after 17 days. These sea stars were removed from their respective tanks as previous studies have indicated that autotomised sea stars exert less predation pressure (Ramsay et al. 2001; Barrios et al. 2008), and the average predation rate was adjusted.
All experimental tanks were examined each day (approximately 11am) to count the number of dead scallops. All dead scallops (empty shells) were removed from the tank and were replaced to keep prey density constant. All scallops removed from tanks were assumed to have been eaten by sea stars. New scallops for the experiment were received in two batches and held in a small tank (max volume 200 l) with flowing seawater.
Due to variable predation between tanks, the groups of sea stars were removed from their original tanks after 13 days and the entire group was re-distributed to a randomly selected tank (see Table 1). Predation rate for each tank was calculated as the number of scallops eaten per day per individual sea star, and the average predation rate was taken over the duration of the experimental period. Where relevant, predation rate was calculated for pre- and post-move experimental periods. The end of the experimental period (Table 1) varied with tank dependent on the availability of scallops to maintain scallop density in the tanks.
On 27 September 2009, after 3 weeks of observing no predation by small A. rubens, the scallops were removed from the tanks and replaced with significantly smaller scallops (34.8 ± 0.3 mm shell height). Scallop mortality was monitored daily for 1 week.
Sea star density was estimated by two divers picking all sea stars in an area of 62,500 m2 (N = 1,008, L. Nordveit, pers. comm). Estimates of sea star population species composition were made based on 5 transects of 20 m length and 1.725 m width (T. Strohmeier, pers. comm.). This information was used in combination with the experimental predation rates to estimate the potential predation rate on newly seeded scallops. Potential predation was calculated by the formula:
where N scallop was the estimated number of scallops within the area occupied by one sea star, and PredPot was the upper predation rate or average predation rate (where applicable) for 1 month from experimental work.
Statistical analysis was carried out in SPSS (version 15.0, SPSS Inc., U.S.A) and R for Mac OS (version 2.12, R: A language and environment for statistical computing R Foundation for Statistical Computing, Austria). The difference between average predation rates per individual for M. glacialis and A. rubens was tested using a paired t test assuming equal variance following a F test for sample variance. The difference in slope of the regression lines was tested using a one-way ANOVA. All tests were assumed significant when P < 0.05.
Results
The average daily predation rate of large Marthasterias glacialis (144 ± 14 mm radius) and large A. rubens (103 ± 8 mm radius) was 0.71 ± 0.4 and 0.88 ± 0.5 scallops individual−1 day−1 (mean ± SD), respectively (Fig. 1). There was no significant difference (P > 0.05) between the two species in the large size class. Large M. glacialis, held in the fourth tank, did not prey upon scallops until they were moved to a different tank. The predation rate of small M. glacialis (81 ± 4 mm radius) was 0.13 ± 0.1 scallops individual−1 day−1, significantly lower than that of large M. glacialis and large A. rubens.
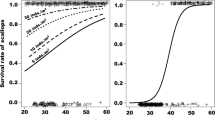
Predation by small A. rubens (64 ± 11 mm radius) on scallops 41.3 ± 0.8 mm was not observed during the main experiment. Predators were thereby only 1.6 times the shell height of the scallop. When provided with significantly smaller scallops (34.8 ± 0.3 mm), the small A. rubens began to prey on scallops (Fig. 2) in 2 of 3 tanks at an average rate of 0.33 (±0.47) and 0.29 (±0.36) scallops individual−1 day−1 (mean ± SD). The radius of small A. rubens was 1.8 times the shell height of the smaller scallops.
Size–frequency distribution of scallops eaten or not eaten by small (64 ± 11 mm) Asterias rubens during a feeding experiment. Scallops not eaten were randomly distributed amongst 12 tanks including 3 tanks containing 3 small A. rubens. Scallops were provided at a density of 10 m−2, and thus those not eaten could be any 30 from the above size distribution. Size of scallops eaten were measured after A. rubens had consumed the scallop
Both large M. glacialis and A. rubens showed increased predation rates over the duration of the experiment (Fig. 3). The highest average daily predation rate, in a single tank, during the experiment was recorded at 2.44, 3.00, and 0.44 scallops individual−1 day−1 for large A. rubens, large M. glacialis, and small M. glacialis, respectively. The predation rate of all three groups continued to increase following transfer to a different tank (post-move, Fig. 3). The increase in predation rate was significantly greater (P < 0.05) after transfer (post-move). The average daily predation rate prior to tank change (Fig. 4) was significantly different from the post-move predation rate for large M. glacialis and large A. rubens (P < 0.05), but not significant for small M. glacialis (P = 0.06).
Predation rate of sea stars Asterias rubens (AR) and Marthasterias glacialis (MG) of differing sizes, on scallops (Pecten maximus) over the course of the experiment, divided into two groups; pre-move (08 September–20 September 2009) and post-move (21 September–08 October 2009). Vertical bars indicate standard error of the mean
Divers estimated sea star density at the Huglo sea ranching site (Norskjell AS) as 1 sea star per 50 m2, with M. glacialis and A. rubens representing 78 and 22% of the sea stars collected, respectively (T. Strohmeier and L. Nordtveit, pers. comm.). In a month, large M. glacialis could on average consume 2.7%, or at the highest rate 11%, of a seeded population. Whilst A. rubens had a similar predation rates, the predation potential based on local densities was much lower; 0.9% as average and highest rate 3% of the population per month.
There was no mortality in the holding tank containing additional prey scallops, and only 2 of 30 scallops died over the duration of the 26-day experiment in the three control tanks.
Discussion
Results showed predation rates ranged between no predation to a daily average predation rate of 3 scallops individual−1 day−1. The higher estimates of daily predation rate were greater than the predation rates (0.2–2.5 scallops individual−1 day−1) found by Wong and Barbeau (2003) and Himmelman et al. (2005) for Placopecten magellanicus, but the average was consistent with the average P. magellanicus predation rate (0.9 scallops individual−1 day−1) reported by Nadeau et al. (2009). In the current experiment, sea stars had a higher chance of encountering scallops; the ability of scallops to escape was restricted by tank walls and this may lead to overestimation of predation rate (Barbeau and Scheibling 1994a; Wong and Barbeau 2003). We maintained prey density at 10 scallops m−2 on a daily basis. In the field, prey density is likely to be reduced as scallops either disperse or as they are consumed by predators. The predation rate of large individuals of both species increased over the course of the experiment. The gradual change in predation rate after moving large A. rubens and large M. glacialis is directly attributable to one tank of each species beginning to prey upon scallops following transfer. A similar pattern was seen by Barrios et al. (2008), when observing sea star predation on mussels in a 4 week period. In comparison, small M. glacialis individuals appeared to prey upon scallops at a relatively constant rate which may have reflected their smaller size.
The experimental period (21–30 days) was long in comparison with previous studies of sea stars feeding on scallops. The extension of an experiment by Barbeau and Scheibling (1994a) did not result in a reduction in variance, suggesting that sea star feeding behaviour is naturally erratic. Our 21–30 day experiment provided evidence that the feeding rate of A. rubens and M. glacialis naturally varies, despite constant conditions. The slope of the daily predation rates following transfer to new tanks is exaggerated by a slight depression of predation rate following movement between tanks. Both sea star species are highly plastic consumers and are thereby able to change their diet according to prey availability (Thrush 1988 and references therein). Animals with a flexible diet should, in theory, have a flexible digestive system which alters activity to optimise digestion in response to different food types (Penry and Jumars 1986). The diet of sea stars prior to transfer to the laboratory was unknown, and some of the sea stars may not have been acclimated to scallops as prey items prior to initiation of the experiment. Thus, increased predation over the course of the experiment could reflect an increased digestibility of the scallop prey as the digestive system became acclimated. In addition, predation rates may have increased as sea stars became familiar with the capture methodology required for scallop prey (Landenberger 1968; Murdoch 1969).
There is an array of factors that may be involved in prey choice decisions, including the ease of capture, size relationships between predator and prey and the energetic value of prey items (Menge 1972). During our experiments, no scallops larger than 37 mm shell height were eaten by small Asterias rubens (<60 mm radius). Sea star prey may find a refuge in size, whereby they can not physically be consumed by sea stars (Menge 1972) or because the energetic and time investments needed for capturing and consuming that prey item exceed the energetic gain (Gaymer et al. 2001). A number of studies have reported an apparent preference for prey of a certain size (Frid 1992; Barbeau and Scheibling 1994a; Norberg and Tedengren 1995; Sommer et al. 1999) and larger sea stars may be more efficient at opening scallops, as seen for the oyster (Needler 1941) and mussel (Penney and Griffiths 1984). Scallop size may affect predation rates by sea stars because larger scallops have a more intense escape response reaction than smaller scallops (Barbeau and Scheibling 1994a; Barbeau et al. 1994). If the size distribution within a local sea star population is known, then it may be possible to seed scallops once they have reached a size, which minimises the risk of predation (Barbeau et al. 1996).
Temperature was controlled at about 15°C during the current experiment, which was the same as ambient temperature at the sea ranching location at the beginning of September and similar to the average water temperature for Norwegian fjords on the south west coast around September (Strand and Brynjeldsen 2003). Temperature has been shown to affect predation rates of sea stars (Sloan 1980; Gaymer et al. 2002; Gil and Zaixso 2007) by altering search time, movement velocity, and encounter rate (Watts and Lawrence 1990; Barbeau and Scheibling 1994b; Barbeau et al. 1994). Sea star feeding rates at different temperatures can have a strong impact on the predation pressure placed upon newly seeded scallops. Barbeau et al. (1994) suggested that the success of bottom culture may be improved by seeding scallops at low temperatures thus inducing a protective refuge for newly seeded scallops by seeding in water temperatures which reduce predatory activity, as documented for scallop density and alternative prey by Wong et al. (2005). However, care must be taken to ensure that the seeding temperature is also appropriate for scallop juvenile survival. Results have shown that P. maximus suffered high mortalities below 2°C (2–4°C leads to 52–100% mortality), and mortality rates less than 30% only occurred when the minimum temperature was 4–5°C (Strand and Brynjeldsen 2003). Given that water temperatures in south west Norway are generally too low to allow for scallop handling during the winter, the best time to seed scallops for maximal survival would be after cleaning and recovery in July or August, when temperatures are above 10°C. However, this corresponds to increased potential predation pressure from sea stars; an alternative solution could be to hold the scallops in intermediate culture longer, increasing the size of the prey. Wong et al. (2005) found that the presence of both other prey (mussels) and reduced densities slowed the predation rate of sea stars. Despite the final density of scallops being the same independent of treatment, reduced density could have resulted in lower losses.
In our experiment, tanks originally contained sea stars at densities of 3 sea stars m−2. Natural densities of adult (50 to 130 mm radius) A. rubens in sub tidal areas have been reported at around 0.2 ± 0.01 m−2 (Saier 2001). M. glacialis may attain densities of up to 2 m−2 down to 6 m depth (Verling et al. 2003). Divers found a density of approximately 0.02 sea stars m−2 (or 1 sea star 50 m−2) at the Huglo sea ranching site (T. Strohmeier and L. Nordtveit, pers. comm.). High density aggregations of A. rubens have been found to occur at dredging sites (Dare 1982; Veale et al. 2000; Saier 2001). Increased predation rates at higher densities may reflect improved capture probability (Barbeau et al. 1998; Wong and Barbeau 2005), and the presence of conspecifics is thought to stimulate feeding through chemosensory signals and contact (Gaymer et al. 2002). Sea star populations have been shown to increase following introduction of scallops for sea ranching (Cliche et al. 1994; Barbeau et al. 1996; Hatcher et al. 1996; Barbeau et al. 1998; Silina 2008), and furthermore, aggregations of sea stars have been shown to move between years, and these changes have overlapped with areas of high scallop densities (Marino et al. 2009). The current study indicated that a population of seeded scallops could be reduced by 0.5–11% per month. Our estimates of potential predation were likely to be overestimates due to dispersion and possible reduced predation at reduced density. However, at the estimated predation rates, this would represent a significant mortality of 20–30% of seeded scallops in a 3 month summer period during high temperatures. Large M. glacialis had the highest estimated potential predation rate per month.
Results of this study emphasise the importance of sea star size when controlling predation on newly seeded scallops. Increased knowledge about the density and size structure of the local sea star population, and methods for preventing build up of sea star biomass, will be needed to reduce predation on seeded Pecten maximus.
References
Barbeau MA, Scheibling RE (1994a) Behavioral mechanisms of prey size selection by sea stars (Asterias vulgaris Verrill) and crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)). J Exp Mar Biol Ecol 180:103–136
Barbeau MA, Scheibling RE (1994b) Temperature effects on predation of juvenile sea scallops [Placopecten magellanicus (Gmelin)] by sea stars (Asterias vulgaris Verrill) and crabs (Cancer irroratus Say). J Exp Mar Biol Ecol 182:27–47
Barbeau MA, Scheibling RE, Hatcher BG et al (1994) Survival analysis of tethered juvenile sea scallops Placopecten magellanicus in field experiments: effects of predators, scallop size and density, site and season. Mar Ecol Prog Ser 115:243–256
Barbeau MA, Hatcher BG, Scheibling RE et al (1996) Dynamics of juvenile sea scallop (Placopecten magellanicus) and their predators in bottom seeding trials in Lunenburg Bay, Nova Scotia. Can J Fish Aquat Sci 53:2494–2512
Barbeau MA, Scheibling RE, Hatcher BG (1998) Behavioural responses of predatory crabs and sea stars to varying density of juvenile sea scallops. Aquaculture 169:87–98
Barrios JV, Gaymer CF, Vásquez JA et al (2008) Effect of the degree of autotomy on feeding, growth, and reproductive capacity in the multi-armed sea star Heliaster helianthus. J Exp Mar Biol Ecol 361:21–27
Brokordt KB, Fernández M, Gaymer CF (2006) Domestication reduces the capacity to escape from predators. J Exp Mar Biol Ecol 329:11–19
Clark AM, Downey ME (1992) Starfishes of the Atlantic. Chapman and Hall, London
Cliche G, Giguère M, Vigneau S (1994) Dispersal and mortality of sea scallops, Placopecten magellanicus (Gmelin 1791), seeded on the sea bottom off Îles-de-la-Madeleine. J Shellfish Res 13:565–570
Dare PJ (1982) Notes on the swarming behaviour and population density of Asterias rubens L. (Echinodermata: Asteroidea) feeding on the mussel, Mytilus edulis L. J Cons Int Explor Mer 40:112–118
FAO (2010) Yearbook
Frid CLJ (1992) Foraging behaviour of the spiny starfish Marthasterias glacialis in Lough Ine, Co. Cork. Mar Behav Physiol 19:227–239
Gallagher T, Richardson CA, Seed R et al (2008) The seasonal movement and abundance of the starfish, Asterias rubens in relation to mussel farming practice: a case study from the Menai Strait, UK. J Shellfish Res 27:1209–1215
Gaymer CF, Himmelman JH, Johnson LE (2001) Use of prey resources by the seastars Leptasterias polaris and Asterias vulgaris: a comparison between field observatons and laboratory experiments. J Exp Mar Biol Ecol 262:13–30
Gaymer CF, Himmelman JH, Johnson LE (2002) Effect of intra- and interspecific interactions on the feeding behavior of two subtidal sea stars. Mar Ecol Prog Ser 232:149–162
Gil DG, Zaixso HE (2007) The relation between feeding and reproduction in Anasterias minuta (Asteroidea: Forcipulata). Mar Biol Res 3:256–264
Grefsrud ES, Strand Ø, Haugum GA (2003) Handling time and predation behaviour by the crab, Cancer pagurus, preying on cultured scallop, Pecten maximus. Aquac Res 34:1191–1200
Hatcher BG, Scheibling RE, Barbeau MA et al (1996) Dispersion and mortality of a population of sea scallop (Placopecten magellanicus) seeded in a tidal channel. Can J Fish Aquat Sci 53:38–54
Hébert D, Nadeau M, Presse N et al (2006) Contrôle des prédateurs avec les dragues à pétoncles et le faubert. In: Nadeau M, Tita G (eds) Atelier de travail “Prédation du pétoncle et gestion des ensemencements”. Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec. Îles-de-la-Madeleine, Quebec. 29 February–1 March 2004
Himmelman JH, Dutil C, Gaymer CF (2005) Foraging behavior and activity budgets of sea stars on a subtidal sediment bottom community. J Exp Mar Biol Ecol 322:153–165
Hoshikawa H, Tajima K-I, Fujisawa C (1997) Field experiment on traps for starfish on a rocky shore to protect released abalone spats from predation. Sci Rep Hokkaido Fish Exp Stat 50:19–26
Lafrance M, Cliche G, Haugum GA et al (2003) Comparison of cultured and wild sea scallops Placopecten magellanicus, using behavioral responses and morphometric and biochemical indicies. Mar Ecol Prog Ser 250:183–195
Landenberger DE (1968) Studies on selective feeding in the Pacific starfish Pisaster in southern California. Ecology 49:1062–1075
Marino MCI, Juanes F, Stokesbury KDE (2009) Spatio-temporal variations of sea star Asterias spp. distributions between sea scallop Placopecten magellanicus beds on Georges Bank. Mar Ecol Prog Ser 382:56–68
Martin R, Proctor C (2000) Tasmanian marine farm pest monitoring project. Final report. Centre for Research on Introduced Marine Pests, Tasmania, Australia, p 25
Menge BA (1972) Foraging strategy of a starfish in relation to actual prey availability and environmental predictability. Ecol Monogr 42:25–50
Minchin D (2002) The potential for ranching the scallop, Pecten maximus - past, present and future: problems and opportunities. ICES Mar Sci 215:416–423
Minchin D, Skjaeggestad H, Haugum GA et al (2000) Righting and recessing ability of wild and naive cultivated scallops. Aquac Res 31:473–474
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335–354
Nadeau M, Barbeau MA, Brêthes J-C (2009) Behavioural mechanisms of sea stars (Asterias vulgaris Verrill and Leptasterias polaris Müller) and crabs (Cancer irroratus Say and Hyas araneus Linnaeus) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)), and procedural effects of scallop tethering. J Exp Mar Biol Ecol 374:134–143
Needler AWH (1941) Oyster farming in eastern Canada. B Fish Res Board Can 60:1–83
Norberg J, Tedengren M (1995) Attack behaviour and predatory success of Asterias rubens L. related to differences in size and morphology of the prey mussel Mytilus edulis L. J Exp Mar Biol Ecol 186:207–220
O’Neill SM, Sutterlin AM, Aggett D (1983) The effects of size-selective feeding by starfish (Asterias vulgaris) on the production of mussels (Mytilus edulis) cultured on nets. Aquaculture 35:211–220
Penney AJ, Griffiths CL (1984) Prey selection and the impact of the starfish Marthasterias glacialis (L.) and other predators on the mussel Choromytilus meridionalis (Krauss). J Exp Mar Biol Ecol 75:19–36
Penry DL, Jumars PA (1986) Chemical reactor analysis and optimal digestion. Bioscience 36:310–315
Ramsay K, Kaiser MJ, Richardson CA (2001) Invest in arms: behavioural and energetic implications of multiple autotomy in starfish (Asterias rubens). Behav Ecol Sociobiol 50:360–365
Saier B (2001) Direct and indirect effects of seastars Asterias rubens on mussel beds (Mytilus edulis) in the Wadden Sea. J Sea Res 46:29–42
Silina AV (2008) Long-term changes in intra- and inter-specific relationships in a community of scallops and sea stars under bottom scallop mariculture. J Shellfish Res 27:1189–1194
Skjæggestad H, Magnesen T (2006) Strategiplan for havbeite med kamskjell i Norge. Department of Biology, University of Bergen, p 35
Sloan NA (1980) Aspects of the feeding biology of asteroids. Oceanogr Mar Biol Ann Rev 18:57–124
Sommer U, Meusel B, Stielau C (1999) An experimental analysis of the importance of body-size in the seastar-mussel predator-prey relationship. Acta Oecol 20:81–86
Spencer BE (2002) Molluscan shellfish farming. Blackwell Science, Oxford
Strand Ø, Brynjeldsen E (2003) On the relationship between low winter temperatures and mortality in juvenile scallops, Pecten maximus L., cultured in western Norway. Aquac Res 34:1417–1422
Strand Ø, Grefsrud ES, Haugum GA et al (2002) Release strategies in scallop (Pecten maximus) sea ranching vulnerable to crab predation. In: Leber KM, Kitada S, Blankenship HL, Svasand T (eds) Stock enhancement and sea ranching: developments, pitfalls, and opportunities, 2nd edn. Blackwell Publishing, Oxford
Strand Ø, Grefsrud ES, Haugum GA et al (2004) Release strategies in scallop (Pecten maximus) sea ranching vulnerable to crab predation. In: Leber KM, Kitada S, Blankenship HL, Svåsand T (eds) Stock enhancement and sea ranching. Blackwell Science Ltd, Oxford
Strohmeier T, Oppegård GG, Strand Ø (2006) Predation of hatchery-reared scallop spat (Pecten maximus L.) by the Ballan wrasse (Labrus bergylta)—consequences for sea ranching. Aquaculture 254:341–346
Thrush SF (1988) Behavioural observations on sublittoral populations of starfish in Lough Hyne. Irish Nat J 22:430–436
Veale LO, Hill AS, Brand AR (2000) An in situ study of predator aggregations on scallop (Pecten maximus (L.)) dredge discards using a static time-lapse camera system. J Exp Mar Biol Ecol 255:111–129
Verling E, Crook AC, Barnes DKA et al (2003) Structural dynamics of a sea-star (Marthasterias glacialis) population. J Mar Biol Ass UK 83:583–592
Watts SA, Lawrence JM (1990) The effect of temperature and salinity interactions on righting, feeding and growth in the sea star Luidia clathrata (Say). Mar Behav Physiol 17:159–165
Wiborg KF (1946) Undersøkelser over oskjellet (Modiola modiolus (L.)). Rep Norw Fishery Mar Invest 8:1–85
Wong MC, Barbeau MA (2003) Effects of substrate on interactions between juvenile sea scallops (Placopecten magellanicus Gmelin) and predatory sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say). J Exp Mar Biol Ecol 287:155–178
Wong MC, Barbeau MA (2005) Prey selection and the functional response of sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)) and blue mussels (Mytilus edulis Linnaeus). J Exp Mar Biol Ecol 327:1–21
Wong MC, Barbeau MA, Hennigar AW et al (2005) Protective refuges for seeded juvenile scallops (Placopecten magellanicus) from sea star (Asterias spp.) and crab (Cancer irroratus and Carcinus maenas) predation. Can J Fish Aquat Sci 62:1766–1781
Acknowledgments
The authors would like to thank Norskjell AS for supplying the spat used in the experiments, the diving team from the Institute for Marine Research for collecting the sea stars, and the staff at Scalpro AS for their assistance with experimental set up.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Magnesen, T., Redmond, K.J. Potential predation rates by the sea stars Asterias rubens and Marthasterias glacialis, on juvenile scallops, Pecten maximus, ready for sea ranching. Aquacult Int 20, 189–199 (2012). https://doi.org/10.1007/s10499-011-9451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-011-9451-y