Abstract
Quantifying predator–prey interactions and gaining insights into predator behavior are crucial for optimizing restoration strategies. However, such knowledge is often lacking for marine invertebrates. We examined potential impacts of predation by channeled Busycotypus canaliculatus and knobbed whelks Busycon carica on natural and planted populations of bay scallops in the Peconic Bays, New York, through laboratory and field investigations. In lab experiments, mean predation rates exhibited by small channeled whelks were low: 0.06 and 0.005 scallops d−1 for adult and juvenile scallops, respectively. Predation rates of small knobbed whelks on juvenile scallops were 22 × higher. Eighty-six percent (86%) of scallops consumed by channeled whelks had undamaged shells, while 73% eaten by knobbed whelks had notched ventral margins. In field plots where scallop densities were manipulated via removals/plantings, whelks consumed ~ 2% of ~ 19,100 planted juveniles, whereas crabs and presumably finfish consumed > 40% overall. Acoustic telemetry revealed that tagged channeled whelks moved shorter distances and spent more time in plots planted with scallops versus those without scallops. Whelks spent more time in low versus high-density plots, but consumed far more scallops in the latter. In trials without scallops, whelk movement rates were 5 × higher, presumably due to increased exploratory behavior. Overall, whelks were most active during crepuscular hours and during periods of increasing wind speeds. Our results, combined with population abundance data, suggest that whelks (especially B. carica) are drawn to planted bay scallop aggregations, but probably contribute to relatively low overall mortality in the context of restoration efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation plays a central role in controlling the structure of aquatic and terrestrial communities (Paine 1966; Connell 1970; Janzen 1970) and is generally considered to be the primary cause of mortality of juvenile marine invertebrates (Jensen and Jensen 1985; Eggleston and Armstrong 1995; Hunt and Scheibling 1997; Gosselin and Qian 1997; Newell et al. 2000). Quantifying spatial and temporal variability of predator–prey dynamics and how predator behavior changes with prey availability, prey attributes and environmental factors is vital to better understanding community dynamics (Martin et al. 2015; Bastille-Rousseau et al. 2017). Resource availability across an animal’s landscape, especially at finer resolutions, is one of the primary factors that influence animal behavior. In particular, heterogeneous landscapes with spatially disparate resources can result in shifts in predator migration modes (Jonzén et al. 2011) and behavior can subsequently shape community structure and species coexistence (Hilborn 1975; Holt 1984). Predator movements, and foraging behavior and ability (Hammerschlag et al. 2006; Grigaltchik et al. 2012), can also be mediated by diel, seasonal or episodic environmental factors, including temperature (Sanford 2002), light intensity (Einfalt et al. 2012), atmospheric phenomena (Cherry and Barton 2017), turbidity (Lunt and Smee 2014), tidal exposure (Zamon 2001) and current velocity (Robinson et al. 2011). In general, these processes are less well known for marine taxa, especially in the field.
In the context of shellfish restoration, understanding dynamics of predator–prey size relationships (Hughes and Seed 1981; Arnold 1984; Boulding 1984) and behavior of predators, (e.g. if they are drawn toward high-density plantings: Boulding and Hay 1984; Barbeau et al. 1996), could improve the success of restoration efforts. Field grow out of juvenile shellfish is the most common approach followed in commercial aquaculture and restoration efforts (Goldberg et al. 2000; Castagna 2001). Therefore, when animals are planted without protective enclosures or exclosures, appropriate sizes must be seeded to increase the probability of survival, such as with Mercenaria mercenaria (Castagna 1984), Ruditapes philippinarum (Cigarría and Fernandéz 2000), Mya arenaria (Beal et al. 1995) and Patinopecten yessoensis (Uki 2006).
We have worked to restore northern bay scallops, Argopecten irradians irradians (Lamarck 1819), in the Peconic Bays of eastern Long Island, NY, USA, over a period of 30 + years (Tettelbach and Wenczel 1993; Tettelbach et al. 2013, 2015) after populations were decimated by the brown tide (Aureococcus anophagefferens) algal blooms in the mid-1980’s (Cosper et al. 1987). Our strategy has been to plant millions of juvenile, hatchery-reared scallops that ultimately serve as broodstock. In recent years, we have focused on deploying scallops in nets, but we also conduct extensive unprotected (free) plantings of scallops directly to the bay bottom (Tettelbach and Smith 2009). The latter approach is riskier but much less expensive. The target size (35–40 mm) for free planting is one that affords a partial refuge from predation by the majority of crab species/sizes with which northern bay scallops co-occur (Tettelbach 1986). However, sea stars (Tettelbach and Wenczel 1993), oyster drills (Ordzie and Garofalo 1980) and finfish (Peterson et al. 2001) have been documented to cause extensive mortality of juvenile and adult bay scallops in some areas.
Channeled, Busycotypus canaliculatus (Linnaeus 1758), and knobbed whelks, Busycon carica (Gmelin 1791) also may be important predators of Peconic bay scallops, as suggested by tethering experiments (Carroll et al. 2010; Mladinich 2017) and our direct field observations. These two commercially harvested gastropods (Udelson 2012; Peemoeller and Stevens 2013; Fisher and Rudders 2017; Angell 2018) are regarded as important predators of clams and oysters (Magalhaes 1948; Peterson 1982; Walker 1988; Kraeuter 2001), but much of the basic ecology of these species is not well understood.
In this study, the potential impact of predation by channeled and knobbed whelks on Peconic bay scallops was examined, in the context of restoration strategies, by employing manipulative experiments and by drawing on predator abundance data (Tettelbach et al. 2015). Feeding rates were estimated from laboratory and field studies. Predator–prey size relationships were also examined via opportunistic field observations. Acoustic telemetry was used to investigate how channeled whelk behavior and movement were affected by environmental factors (e.g. tide direction, time of day) and scallop densities to provide novel insight into the underlying mechanisms that drive predatory behavior. We hypothesized that tagged whelks would: (1) be drawn to scallops planted at densities well above ambient levels, (2) spend more time in patches of high vs low scallop densities, and (3) exhibit more encamped (less exploratory) behavior at higher scallop densities.
Materials and methods
Estimation of whelk predation rates on bay scallops
Laboratory experiments
Laboratory experiments were conducted during July–December of 2012–2014, to quantify rates at which scallops of two sizes, stocked at two densities, were preyed upon by channeled and knobbed whelks of representative sizes. Small (0 + year = juvenile) scallops used in experimental trials ranged from 21 to 30 mm shell height (SH), (25.7 ± 0.14, n = 163–27.5 ± 0.13 mm, n = 162) while large (1 + year = adult) scallops ranged from 49 to 62 mm SH (55.2 ± 0.19, n = 162–58.0 ± 0.17 mm, n = 162). These represent the minimum and maximum sizes of scallops we routinely free-plant to the bottom (Tettelbach and Wenczel 1993). Scallop densities were 10 m−2 (low) and 75 m−2 (high). Respectively, these represent a relatively high natural population density and the minimum free-planting density that we currently target (Tettelbach et al. 2013, 2015). Two sizes of channeled whelks were used. Small ranged from 113 to 139 mm shell length (SL) (131.4 ± 0.77 mm, n = 53) and jumbo ranged from 188 – 218 mm SL (205.5 ± 4.96, n = 6) (Table S1). Predation by knobbed whelks was examined in October–November 2014 after field experiments suggested they might be the more important scallop predator (see below). Small knobbed whelks ranged from 106 to 145 mm SL (126.4 ± 2.59, n = 16). These were presented with small scallops that ranged from 25 to 30 mm SH (27.2 ± 0.13, n = 270). The same two scallop densities were used, as above. Predation rates were considered maximal since no alternative prey were available and the probability of scallops avoiding predation was low.
Laboratory experiments were conducted in plastic fish totes (66 cm L × 47.6 cm W × ~ 27 cm D) held within fiberglass raceways at the Cornell Cooperative Extension lab in Southold, New York, USA. Temperature of the air and flowing seawater from Cedar Creek (adjacent to the lab) were both at ambient levels. Tanks were independently supplied with water at a flow rate of 5.8 ± 0.73 l s−1 (n = 91). Totes were fitted with string inside the perimeter of all sides to prevent whelk escapement. Prior to starting a given predation trial, totes were cleaned with fresh water, rinsed, and then filled with beach sand (sieved to 6–8 mm, then dried) to a depth of 18 cm. This depth of sand was found to be necessary for successful predation on bay scallops by channeled whelks in the laboratory (B. Udelson, unpub. data). After flow rates were standardized, a single whelk was added to each tote and allowed to acclimate for ~ 24 h. Usually, individuals were completely buried in the sand within 10 min. Whelks, which had been captured in pots by a commercial fisherman in Gardiners Bay and/or collected by divers in the eastern Peconic Bays, were starved for 2 weeks (Rilov et al. 2002) in a flowing seawater tank prior to use in experiments, for which they were used just once. Scallops were obtained from nets in Orient Harbor (Tettelbach and Smith 2009) or collected by divers from nearby natural populations just before the start of each experimental trial. Scallops were spread relatively evenly over the surface of the sediment at 3 and 24 per tote, respectively for the low and high-density treatments. Whelks were allowed to feed on scallops for 6–7 d. For a given experimental trial, up to 12 tanks were run simultaneously for a given scallop size, depending on whelk and scallop availability: 5 replicates and 1 control (scallops with no whelk) for each of the two density treatments. Six and two trials, respectively, were run for channeled and knobbed whelks (Table S1).
At the time scallops were introduced to the totes, dissolved oxygen (DO), salinity (S) and temperature (T) were measured with a YSI Pro 2030 multi-probe meter and/or a Hach meter (HQ 40d). A submersible Tidbit® automatic temperature recorder was also placed in one of the tanks (except in 2012) to measure water temperature every 30 min. Totes were inspected every 1–2 d to monitor scallop mortality. Water quality measurements were also made at this time. T, S, and DO through all lab experiments ranged from 10.9 to 26.8 ºC, 26.4 to 30.6, and 4.59 to 6.37 mg l−1, respectively.
When dead scallops were first seen, their SH, orientation and position in totes were noted. Shells that contained most or all of the tissues and/or had a strong odor were considered to have died from causes other than predation. At the end of a given experimental trial, SH of consumed scallops (mostly or completely devoid of tissue and without a strong odor) were measured to the nearest mm and damage (if any) to each shell was characterized as: shells chipped, cracked or notched (see below). After experiments were completed, gender of whelks was determined on the basis of whether a penis was present or absent (Gendron 1992; Castagna and Kraeuter 1994; Power et al. 2009; Udelson 2012). Overall male:female ratios for channeled and knobbed whelks used in lab experiments were 30:29 and 13:3, respectively.
Field experiments
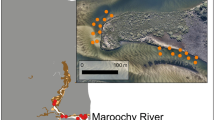
Rates of predation on scallops by channeled and knobbed whelks were estimated in October 2013 following three experimental plantings in a 20 m × 20 m grid that was divided into four 10 m × 10 m cells deployed off Cedar Beach, Southold (Fig. 1). Acoustic telemetry of tagged whelks was carried out here concomitantly (see below). Depth of the site was 2–3 m at MLW. Percent cover of submerged aquatic vegetation (SAV) was very low (≤ 1%) and the sandy substrate was representative of bottom types on which we regularly observe whelks and scallops throughout the Peconic Bays. Sediment type and (qualitative) flow rate was comparable to those used in laboratory experiments.
Inset of acoustic telemetry array and experimental plots (outlined in green squares) for field trials in the Peconic Bay, Long Island, NY. The black star denotes the center of the experimental grids where whelks were released. The red crosses denote acoustic receiver locations (colour figure online)
At the completion of each field experiment, we derived an estimate of the numbers of scallops eaten by whelks and other predators by examining damage to cluckers (recently dead scallops without tissue, but with shell valves still articulated) that were collected by divers from inside each of the four 10 m × 10 m grid cells and from beyond the perimeter of the grid (out to ~ 3 m to the west and east, and ~ 5 m to the north and south). Predation was attributed to different groups on the basis of characteristic traces (Tettelbach 1986; Peterson et al. 1989). Cracks (large chips removed), smaller jagged chips or bore holes at the juncture of scallop shell valves reflected crustacean attacks (Elner 1978; ap Rheinallt and Hughes 1985; Tettelbach 1986) while relatively large (≥ 5 mm) rough-edged hole punches in the upper middle section of scallop shells were attributed to spider crabs, Libinia spp (Tettelbach, unpub data). Small, straight-sided holes and bevel-edged holes, respectively, were due to oyster drills and naticid snails (Carriker 1951). Notched shell edges (thin [≤ 2 mm], straight-sided chips along the ventral shell margin and often only from just one of the valves,) were characteristically caused by knobbed whelks (as seen in our present lab experiments). Cluckers with no visible shell damage reflect predation by sea stars (Barbeau et al. 1994) or channeled whelks (this study). Assignment of predation to different groups is complicated by the fact that a given species may employ various tactics relative to the size of the prey and thus leave different traces (Elner 1978; ap Rheinallt and Hughes 1985; Tettelbach 1986). Despite these limitations, analysis of predatory traces permitted insights into the relative contribution of different predators to losses of planted scallops.
Whelk movement/behavior in the field in response to scallop plantings
Acoustic telemetry was used to examine the movement of channeled whelks in field predation trials. Concurrent to lab experiments, a total of five field trials were run at the site off Cedar Beach, between 7 October–2 November 2013: two in which all scallops were removed from the grid (controls) immediately before tagged whelks were deployed (first and last trials) and three in which hatchery-reared scallops were planted at low (10 ind m−2) or high (75 ind m−2) densities in each of two 10 m × 10 m grid cells (Fig. 1, Table 1). Prior to the first trial on 7 October, natural scallop densities were determined from counts made by divers in the eastern and western halves of the grid. Juvenile and adult densities were found to be 1.76 ± 0.31 ind m−2 (n = 2) and 0.17 ± 0.08 ind m−2 (n = 2), respectively. As the predominant direction of tidal flow was N-S, and because we wanted to factor out other potential differences in bottom type, depth and availability of alternate prey among the grid cells; divers changed the position of the low and high-density scallop treatments in successive trials by moving scallops between cells and by adding additional scallops when necessary (Table 1). Densities of potential alternate molluscan prey species for the suite of expected predators (Tettelbach et al. 2015) were quantified in five 1 m2 quadrats within each grid cell on 7 October; infaunal bivalves were counted in situ after fanning away the top 4–5 cm of sediment while epifaunal species (Crepidula fornicata, Anomia simplex and Anadara transversa) were collected and subsequently counted on the boat. Epifaunal individuals were not replaced into the grid. No significant differences (P > 0.19) were found between grid cells for respective species. Densities of Crepidula, Anomia, Anadara and Mercenaria were 113 ± 13.9 (n = 20), 1.1 ± 0.7 (n = 10), 0.1 ± 0.1 (n = 10) and 0 (n = 20) ind m−2, respectively. These are all low compared to many other Peconic sites that we have collected samples from (Tettelbach, unpub. data). Water temperature was recorded continuously every 30 min with a Tidbit® and salinity readings were taken each day in the field. Sediment grain size analyses were conducted on samples taken at the start of field experiments. Of nine samples taken in three different grid sectors, sand comprised 85–96% of the grain size fractions.
Monitoring of whelk position and time spent in respective grid cells was done using Vemco® 69 kHz (V9-1L) acoustic tags and five time-synchronized VR2W receivers placed in a pentagonal array (Fig. 1, Supplemental material). For each experimental trial, 10 whelks were individually fitted with tags that transmitted unique identification codes every 120 s; and the spatial position of tagged animals within/beyond the receiver array was calculated via hyperbolic positioning (Smith 2013). In this way, we were able to determine: (1) rates of movement; (2) how much time whelks spent in low and high-density planted grid cells, and (3) diurnal differences in movement. Expected transmission range for Vemco tags was estimated at 200–300 m through calibration trials so that positions of whelks that moved well beyond the 20 × 20 m experimental grid and into the natural scallop density area could be determined.
Whelks and scallops used in field experiments were comparable to the smaller respective size groups used in the lab; tagged whelks ranged in size from 104 to 140 mm SL, with most between 130–137 mm SL (Tables S1, S3). Shell height of planted scallops was 28.1 ± 0.2 mm (n = 149) on 16 October and 31.1 ± 0.2 (n = 100) on 30 October. Similar to the laboratory experiments, whelks were starved for 2 weeks prior to release, and ≤ 4 days prior to field deployments, individuals were each fitted with unique acoustic and ‘burial’ tags (Tettelbach et al. 1990). The latter allowed us to locate whelks if/when they were buried in the sediment (which was the case for virtually all tagged whelks during the daytime). Acoustic tags were secured to the dorsum of each whelk shell by first using superglue to attach a Velcro strip, then inserting the acoustic transmitter into a Velcro sleeve which was attached to the first Velcro piece (Brousseau et al. 2004). The proximal end of ‘burial’ tags was glued to the dorsum of whelk shells while a cork was glued to the distal end. Retention of both types of tags was tested in the lab prior to field deployments; none were lost there or during any field experiments.
Just prior to deploying scallops and tagged whelks into the field plot, wild scallops and resident whelks were removed by divers during parallel transects across each of the four 10 m × 10 m grid cells; counts and measurements were recorded on the boat (Table S2). Mean densities of wild juvenile and adult scallops on 7 October were 1.76 ± 0.32 m−2 (n = 2) and 0.17 ± 0.08 m−2 (n = 2), respectively. Respective average densities of resident channeled and knobbed whelks, observed on 16 October (just prior to the first scallop planting), in and just beyond the 20 m × 20 grid were 3/676 m−2 = 0.004 m−2 and 32/676 m−2 = 0.047 m−2. These densities are comparable to respective estimates from 2005 to 2015 transect surveys in nearby Orient and Northwest Harbor (Tettelbach unpub data). Sizes of resident whelks (Table S2) were somewhat larger than our tagged animals. Wild scallops also were larger (Table S2) than planted ones and had fewer growth checks, so it was easy to differentiate the two groups.
For the first of the field predation experiments, a total of 17,000 scallops, which had been obtained earlier that day from nets in Orient Harbor, were haphazardly distributed from the boat into each of the four grid cells, at the targeted densities. Divers then manually moved scallops that had drifted outside the grid into the nearest cell. Counts of scallops were taken within 6–8 haphazardly placed 1–m2 quadrats in each of the sectors to document actual planted scallop densities. For subsequent experiments, in which low- and high-density treatments were rotated among grid cells, a total of 4681 scallops from beyond the perimeter were relocated to adjacent sectors (Table 1). For the experiment begun on 28 October, an additional 2100 hatchery-reared scallops were planted into grid cell 2. Thus, an overall total of 19,100 scallops was planted during field experiments.
Within 1.75 h of when scallop densities had been achieved for respective grid sectors in each of the experimental trials, 10 tagged whelks were manually placed in a circle around the grid center, with the anterior end of each facing away from the central acoustic receiver. Originally, we had planned to run experiments for 6 d, but these were shortened to 2–3 d after it was determined that whelks in the first control trial had moved beyond the range of the experimental array in less than 5 h (and had to be manually located with a hydrophone hung from the boat). At the end of all other field trials, divers systematically swam transects in each of the 4 grid cells to collect tagged whelks and scallop cluckers. Searching was done beyond sector perimeters when necessary. Since all 10 tagged whelks were successfully recovered following each trial, reuse of all transmitters on different whelks was possible for subsequent trials. After the completion of all five telemetry experiments, data were downloaded from the acoustic receivers and sent to Vemco for position calculations (Smith 2013). Although we have data for longer time periods, we used only the first 48 h for analyses of spatial position as we had the highest degree of confidence in these position estimates.
Opportunistic observations of predator–prey size relationships
Whenever we encountered bay scallops being preyed upon by whelks in the field, either in our planted area off Cedar Beach, Southold or during dives in other areas in the Peconic Bays, whelk SL and scallop SH were measured to the nearest mm and observations were made on prey capture and handling.
Statistical analyses
Rates of predation by whelks (channeled and knobbed) on scallops in laboratory experiments were first examined with respect to water temperature, via linear regression. Where data were not statistically different, they were pooled and compared via 2-way ANOVA to examine differences in predation rates versus scallop density and size. For each of the three field predation trials, numbers of cluckers within the sectors planted at high and low scallop densities (n = 2 for each trial) were compared via G tests, where the expected values were the total number of cluckers recovered divided by 4. In all statistical analyses, qqplots and Shapiro–Wilk tests were used to evaluate normality assumptions. If data did not meet these assumptions, we either log-transformed the data, employed a generalized linear model, or used non-parametric statistical tests.
Hidden Markov model setup
To understand the influence of environmental factors and prey availability on whelk behavioral patterns across space and time, we employed discrete-time Hidden Markov models (HMM) to analyze acoustic telemetry data separately for each of the five trials. HMM is a class of time series models that is likelihood-based and is considered a special case of State Space Models (Patterson et al. 2009). HMMs consist of two components to describe animal behavior: observable states and a non-observable (hidden) state. Inference on the unobservable state are made from either relocation data or movement metrics (i.e. turning angle and step length) from the Markov process (Langrock et al. 2012; Whoriskey et al. 2017). HMMs are best suited for telemetry data with negligible error, can identify behavioral states and incorporate both continuous and categorical covariates (Michelot et al. 2016), and have successfully been used on a variety of marine animals (Papastamatiou et al. 2018; Ogburn et al. 2018). Covariates included in each HMM were grid (GR), time of day (TOD), atmospheric pressure (AP), tide height (TH), tide direction (TD), scallop density (SD), hour of day (DC), wind speed (WS), wind direction (WD) and activity state (AS: high or low). Tide data and weather data were obtained from the closest nearby location to the study site with the rtide (Thorley et al. 2017) and rnoaa (Chamberlain 2020) R packages, respectively. The activity state covariate was constructed to account for individual heterogeneity in dispersal capacity (Palmer et al. 2014). To assign an individual’s activity level for each trial, we used the empirical cumulative distribution function on total distance traveled; and individuals that exceeded the median value were considered to have high activity/dispersal and vice versa. The Akaike information criterion (AIC) was used to determine the best fitting model for whelk movement in each trial (Burnham and Anderson 2002). Additional details on HMM setup and fitting procedures can be found in the Supplemental Materials.
We tested the null hypotheses of no differences between control (no scallops) and experimental (scallops present) trials for (1) time spent within vs. outside the experimental plot, (2) total distance travelled (encamped vs exploratory behavior) and (4) day and night movement distances with Wilcoxon rank-sum tests. To determine if the encampment of whelk differed among scallop densities (natural, low planted, high planted: hypothesis (3)), we used a Kruskal–Wallis test (Hollander et al. 2013). A Post-hoc multiple comparisons Conover test was used to determine if there were pairwise differences in whelk residency at different scallop densities (Conover and Iman 1979). A Holm correction was used to control for familywise type 1 error with an assumed familywise error rate of ɑ = 0.05 (Holm 1979). The Conover-test was conducted in the conover.test R package (Dinno 2017). All statistical and modelling analyses were conducted in R (R core team 2020).
Results
Estimation of whelk predation rates on bay scallops
Laboratory experiments
Rates of bay scallop predation by small channeled whelks in the 6 lab experiments were low: 0.054 ± 0.033 scallops eaten whelk−1 day−1 (n = 24) and 0.0057 ± 0.0057 scallops eaten whelk−1 day−1 (n = 29) for large and small scallops, respectively (Table S1). During the 27 August–3 September 2013 trial, the rate of predation on large scallops by jumbo whelks (0.119 ± 0.044 scallops eaten whelk−1 day−1 (n = 6)) was higher than by smaller whelks (0.071 ± 0.071 scallops eaten whelk−1 day−1 (n = 4), respectively: Table S1), but a two-sample t-test comparing these rates showed no statistical difference (t8 = 0.605, P = 0.562). For small knobbed whelks, the overall predation rate on small scallops was 0.125 ± 0.054 scallops eaten whelk−1 day−1 (n = 16). This was 22 × higher than that by small channeled whelks. The highest individual consumption rates were 5 scallops eaten over a 7 d period (0.71 scallops d−1), for a 131 mm SL channeled whelk feeding on large scallops (late July–early August 2012) and a 136 mm SL knobbed whelk feeding on small scallops (late October 2014).
Neither scallop size nor density was shown to affect the rate of predation by channeled whelks in the laboratory according to the Scheirer Ray Hare test (scallop size: H1,55 = 0.66, P = 0.41; scallop density: H1,55 = 1.12, P = 0.28; scallop size x scallop density: H1,55 = 0.91, P = 0.91). Nevertheless, the total number of large scallops eaten by channeled whelks in all trials (n = 9) was greater than the number of small scallops (n = 1) (Table S1). Although these differences in predation rates may reflect higher water temperatures at which experiments were run for large scallops, the much higher rate of byssal attachment by smaller scallops to the walls of totes, in both control and experimental treatments, may have precluded whelks from preying on these individuals thus effectively reducing the numbers available to be preyed upon. Frequencies of byssal attachment per trial in 2013 ranged from 15.6% (25/160) on 23–30 October to 36.9% (59/160) on 24–30 September. In 2014, these ranged from 12.8% (17/133) on 31 October–6 November 2014 to 16.5% (22/133) on 18–24 October. No large scallops were attached to tote walls during any of these trials.
Overall rates of predation were generally higher when water temperatures were higher, except during the 22–26 August 2012 trial when the water temperature was ~ 26.8 ºC (no scallops consumed). Given that the data violated normality assumptions, we employed a zero-inflated Poission regression to examine the influence of minimum and median water temperature on channeled whelk predation rate (# scallops eaten per whelk during a given trial). For all of the trials except the one run from 22 to 26 August 2012, minimum water temperature (Zero-inflated Poisson regression, Z = 3.36, N = 43, P < 0.001) and median water temperature (Zero-inflated Poisson regression, Z = 3.46, N = 43, P < 0.001) significantly influenced channeled whelk predation rate. Since there were only two trials for knobbed whelks, a linear regression was not done. However, the data strongly suggest that predation rates by this species were also greater at higher temperatures: 0.208 ± 0.10 scallops eaten whelk−1 day−1 (n = 8) vs 0.042 ± 0.027 scallops eaten whelk−1 day−1 (n = 8), respectively, at 14.2–16.3 ºC versus 8.3–14.3 ºC.
Different types of damage to scallop shells were inflicted by channeled and knobbed whelks, in laboratory experiments as well as in the field (see below). Most (12/14 ind. = 86%) shells of scallops eaten in the lab by channeled whelks were not damaged; the others (2/14 = 14%) were cracked (Table S1). Knobbed whelks most commonly (8/11 = 73%) notched the ventral margins of predated scallop shells (Fig. S1, Table S1). Of the scallops preyed upon by channeled and knobbed whelks, respectively, hinges were disarticulated in 3/14 (21%) and 5/11 individuals (45%).
Field experiments
An overall total of 1067 hatchery-reared cluckers were recovered over the course of the three field predation experiments (ending 18, 24 and 30 October 2013: Table S4). These included 879 from inside the four grid cells (including 8 planted scallops seen being eaten by knobbed whelks) and 188 from just beyond the grid perimeter (the latter were only quantified after the last experiment). The total of 1067 cluckers, out of 19,100 planted scallops, represents a minimum predation rate of 5.6%. The total number of live scallops recovered on 30 October, following the last field predation trial, was 10,404; thus, the maximum rate of losses due to predation was inferred to be 45.5%. In contrast, only two wild cluckers were found while clearing the grid of scallops before starting the first predation experiment on 16 October 2013.
Higher numbers of cluckers were recovered inside the cells planted with high vs low densities of scallops in each of the three field predation trials (χ23 = 26.78, 72.25, 12.19; P < 0.0005). Similarly, on 30 October, pooled counts of cluckers from inside and adjacent to high-density grid cells were higher than those for low-density sectors (χ23 = 14.96; P < 0.0005).
Of 661 cluckers which were examined to characterize traces of predation (Table 2) the predominant types of shell damage were: cracked (36.6%), no visible shell damage (23.0%), chipped (18.8%), pried (9.8%) and notched (9.5%). Predation by drills (Urosalpinx cinerea and Eupleura caudata) was relatively unimportant at the site of our field predation experiments, accounting for only 0.8% of clucker damage.
Opportunistic observations of predator–prey size relationships
A total of 20 whelks preying on scallops were observed directly between 2011 and 2015: four by channeled whelks (one 0 + year scallop and three 1 + yr individuals) and 16 by knobbed whelks (eight 0 + year and eight 1 + year scallops) (Fig. 2, Table S6). A linear regression of predator–prey size was statistically significant (R2 = 0.26, F1,18 = 6.19, P = 0.023 (Fig. 2). For seven predation events which we examined closely, knobbed whelks completely engulfed the shells of four scallops (two juvenile, two adults) as did two channeled whelks (one juvenile, one adult). In another instance, a channeled whelk inserted its outer shell lip between the ventral margins of an adult scallop shell, while the hinge of the shell was held firmly in place by the foot and perhaps the operculum.
Scallop SH (mm) vs. whelk SL (mm) relationships based on direct opportunistic observations in the Peconic Bays, NY, 2011–2015. Observations for knobbed whelks (n = 16) were pooled with those for channeled whelk due to low sample size (n = 4) for the latter species. The linear regression formula is y = − 10.4 + 0.349x, r2 = 0.26, F1,18 = 6.19, n = 20, P = 0.023. Shaded regions denote standard error
Whelk movement and behavioral response to scallop field plantings
Visual observations by divers revealed that tagged whelks exhibited similar movement (searching) behaviors within the first 5–10 min after deployment, where most oriented into the prevailing current. Untagged whelks moved in a similar fashion, whereby the shell and proboscis were rotated back and forth across an arc of ~ 90° while the foot moved forward in a mostly linear direction. In the first field predation trial (16 October 2013), large numbers of wild untagged whelks were seen inside the grid within 0.5 h after scallops were planted from the boat. This occurred despite having just removed all visible animals from the area. At this time, nine whelks (one channeled, eight knobbed) were seen in the process of preying on scallops. Several scallop cluckers which had just been eaten by whelks, as evidenced by the heavy mucus coating on the shell, were also noted.
Acoustic telemetry field trials yielded very high resolution tracking results for individually tagged whelks, which allowed us to continuously track all animals for 48 h. Thus, we were able to determine that tagged whelks displayed different movement and occupancy patterns in control trials (no scallops) compared to those with planted scallops (Fig. 3). When no scallops were present, whelks moved out of the experimental grid quickly, often within 4–5 h after their initial release. Thus, the total amount of time that whelks spent inside the grid was significantly lower in control trials versus those with planted scallops (Wilcoxon rank-sum, T = 134, N = 50, P < 0.001: Fig. 3). Path analysis showed that, on average, the total distance traveled by tagged whelks (sum of the segment trajectories for individuals) was considerably higher in control trials (447 m) compared to those when scallops were present (90.7 m): Wilcoxon rank-sum, T = 556, N = 50, P < 0.001 (Fig. 4). Displacement (distance travelled by whelks between the first and last points of individual trajectories) over the first 48 h was 43.3 ± 8.0 m (n = 20) and 20.1 ± 3.2 m (n = 30), respectively, for whelks in control and planted trials. Similarly, rates of whelk movement were higher in control trials (10.55 ± 1.52 m h−1, n = 20) versus those with planted scallops (3.37 ± 0.23 m h−1, n = 30): Wilcoxon rank-sum; T = 52, N = 50, P < 0.001. These results corresponded to those for behavioral state, where whelks in control trials spent more time exhibiting exploratory behavior (10.8–84.8%; average = 33.3%, n = 20) compared to whelks in predation trials (0.80–29.4%; average = 12.1%, n = 50) (Fig. S2).
Length of time spent by whelks inside (red) and outside (blue) of the acoustic telemetry experimental plot for control (no bay scallops present) and predation trials (scallops present), 7 October – 2 November 2013. Average time whelk spent inside vs outside the grid were significantly different in both control (Wilcoxon rank-sum test; T = 109, n = 20, P < 0.01) and predation treatments (Wilcoxon rank-sum test; T = 589.5, n = 30, P < 0.001). Boxplots represent median (solid horizontal line), 25th and 75th interquartile ranges (outer edge of boxes) and whiskers representing the lower and upper extremes outside of the interquartile ranges. Points outside of the whiskers are outliers (colour figure online)
Distance (m) travelled by whelks along the path trajectories for control (no bay scallops present) and predation trials (scallops present), 7 October – 2 November 2013 (Wilcoxon rank-sum; T = 548, n = 50, P < 0.001). Refer to Fig. 3 for description of the values box-and-whisker plots represent
In general, whelks remained in an encamped behavioral state for most of a given day, thus behavioral state probabilities only varied in a moderate way with time of day. This was true in all field trials except in trials 3 and 4 (Fig. 5C, D). Whelks were seen to be most active during early evening/twilight hours (1500–2000 h) and least active from 0000 to 1000 h, in all trials (Fig. 5). Marked diel differences in total distances traveled were exhibited by tagged whelks in both control (Wilcoxon rank-sum, T = 83, N = 40, P < 0.001) and predation (Wilcoxon rank-sum, T = 190, N = 60, P < 0.001) trials (Fig. 6). Whelks traveled greater distances in daytime versus nighttime hours.
Time of day (EST local time) dependent stationary state transition probabilities for channeled whelk in acoustic telemetry trials. Figures A and E represent control trials 1 and 5, respectively. Figures B, C and D, represent experimental trials 2, 3, and 4, respectively. Error bars denote 95% confidence intervals. Stationary state probabilities represent the covariate of interest’s probability in multiple states when other covariates are averaged and held constant (colour figure online)
Distances (m) traveled by tagged whelks during day (blue) versus night (red), for field trials where all scallops were removed from the acoustic telemetry plot (control) and when planted scallops were present; 7 October – 2 November 2013. Average total distance traveled by whelks differed between day and night periods in both control (Wilcoxon rank-sum; T = 105, n = 40, P < 0.01: **) and treatment (Wilcoxon rank-sum; T = 192, n = 60, P < 0.001:***. trials.) Refer to Fig. 3 for description of the values box-and-whisker plots represent
The average amount of time whelks spent within each scallop density plot varied considerably among predation trials (Fig. 7). In trials 2 and 4, whelks exhibited no differences in time spent among all scallop densities (Fig. 7A, C); however, in trial 3, whelks spent the majority of their time in grid cells with low versus natural (i.e. outside the grid) or high densities of planted scallops (Fig. 7B). For all three experimental trials, encamped behavior was quite common in both the low and high planted scallop densities (Fig. 8). However, in trial 3, there was some evidence of whelks spending more time in lower density scallop grids and also a higher probability of displaying exploratory behaviors (Fig. 8).
Box plots showing the length of time whelk spent in each scallop treatment across experimental trials over the course of 48 h. Trials 2, 3, and 4 are represented in plots A, B and C, respectively. Significance values (ns, *, **, ***, **** = P > 0.05, P < 0.05, P < 0.01, P < 0.001, P < 0.0001, respectively) are for Conover post hoc pairwise comparison tests adjusted for multiple comparisons with the Holm method. The natural scallop treatment density denotes ambient densities outside the experimental grid boundaries. Refer to Fig. 3 for description of the values box-and-whisker plots represent
Scallop density (ind m−2) dependent stationary state transition probabilities. The “N” scallop density denotes the average ambient scallop densities in the Peconic Bay outside of the experimental grids. Figures A, B, C represent experimental trials 2, 3, and 4. Error bars denote 95% confidence intervals
Considerable differences of supported covariates that influenced whelk behavior were evident between control and experimental trials (Table 3). In all trial HMM models, the covariates time of day, some tidal components (tide direction, tidal water height or both) and a spatial component (either grid or scallop density plot) were highly supported with the exception of the first experimental trial (Table 3). Of note, the top 2– 3 models containing these covariates within each trial had high cumulative AIC weight (> 0.98 and ΔAIC > 9) (Table 3). Covariates that were strongly supported in the control trials (1 and 5) but not in the experimental trials were activity state, wind direction, and wind speed. In most predation experimental trials (except trial 2), strong support was present for whelks exhibiting diel movement behavior (most active around 1400–2000 h), while diel behavior was not supported in the controls. Scallop densities and time of day appeared to have the strongest support in trial 2 (Table 3) and were the only covariates included in the top two models (collective AIC weight = 0.85).
Discussion
Predation of bay scallops by channeled and knobbed whelks
Whelks of the genus Busycon are known to prey upon bivalves with tightly closing shells (e.g. clams, oysters) whereas thinner-shelled Busycotyopus prefer to feed on bivalves with a larger shell gape, as well as carrion (Edwards and Harasewych 1988). Our observations confirm that channeled and knobbed whelks both prey upon live juvenile and adult bay scallops. The suggested preference of channeled whelks for larger scallops in lab experiments may be an artifact that reflects active swimming of juveniles at 25–30 mm SH (Tettelbach 1986), when peak levels of octopine dehydrogenase, the enzyme responsible for fueling bay scallop swimming, are seen (Garcia-Esquivel and Bricelj 1993). This trend should be investigated further. Nevertheless, despite their swimming abilities, many juvenile scallops succumbed to predation < 30 min after they were released into field plots.
Specifics of prey capture and handling of bay scallop prey by channeled and knobbed whelks, however, are still not clear. Most often, we observed whelks engulf the entire scallop shell with their foot, but on one occasion a channeled whelk inserted its shell lip between the valves of its prey. The latter is a common method employed by Busycon carica to open Mercenaria shells, but has rarely been observed with Busycotypus canaliculatus (Magalhaes 1948). Prescott (1990) observed that knobbed whelks ate adult bay scallops (Argopecten irradians concentricus) in the laboratory, but did not observe any type of shell damage. In our lab experiments, most shells of scallops eaten by knobbed whelks were characteristically notched; this was never observed for Busycotypus. Although not observed directly, this type of damage may result from the ‘hammering’ method, by which pieces of Mercenaria shell margins are broken off prior to insertion of the proboscis (Magalhaes 1948).
Reduced rates of predation at lower water temperatures in our experiments parallel observations by baymen, who typically stop fishing for channeled whelks when they cease feeding in late November (P. Wenczel, pers. comm.). Relatively high rates of predation by whelks in the late August–early September 2013 trial were surprising, as this is the time of year when Busycotypus do not readily enter conch pots in the Peconic Bays (P. Wenczel, pers. comm). This is likely because they are mating and/or laying eggs instead of feeding (Edmundson 2016). Reduced whelk catches in late August may also reflect higher water temperatures, which may deter feeding activities, as corroborated by the lack of predation in our 22−26 August 2012 trial.
Of 19,100 scallops planted in the field, 60% (11,471) were accounted for by the end of our experiments. 5.6% (1067 of the total planted) were cluckers, which represents the minimum rate of predation. The loss of the other 40% of planted scallops may have been due to dispersal or predation. Relatively high rates of dispersal were illustrated by the transport of large numbers of cluckers outside the planted sectors (especially to the South) and by the rates of immigration of wild scallops into the grid. However, extensive searching beyond the grid perimeter, even out to a distance of ~ 15–20 m to the South (where the number of planted scallops and cluckers was very low), suggests that we recovered the majority of cluckers. Thus, much or perhaps all of the missing 40% of planted scallops (7629) may have been lost to predation, whereby shells were crushed to smaller bits or shells were removed from the area. Scup, Stenotomus chrysops, are abundant throughout the Peconic Bays and were observed within the planting area. Since they swallow scallops whole (Weinstock 2010; Mladinich 2017) they (or other fishes) may be responsible for some of these scallop losses. If the missing 40% of planted scallops is added to the confirmed rate of predation (recovered cluckers = 5.6%), maximum cumulative predation amounts to 45.6% over the course of three field experiments: roughly 3–5% predation d−1. This aligns fairly well with empirical predation rates determined by Tettelbach (1986) for similar sizes of bay scallops planted in Connecticut over 1 week periods in the fall.
Since ~ 67% of recovered cluckers had shells that were cracked, chipped, pried or had hole punches, signatures most closely aligned with crustacean predation, we conclude that crabs were probably the most important cause of scallop mortality (Tettelbach 1986; Peterson et al. 1989; Prescott 1990). Cracked shells (36% of cluckers examined) are most likely the result of predation by large crabs such as blue crabs, Callinectes sapidus, and large male spider crabs, Libinia emarginata. Both crab species are common in the Peconic Bays and can consume scallops at high rates (Tettelbach 1986; Carroll et al. 2010).
Signatures of predation attributed to whelks in the field and observed rates of predation in the lab were both relatively low, suggesting that the overall importance of whelk predation on planted scallops is also relatively low. Whelk predation was inferred when cluckers recovered from field experiments exhibited notches (n = 101) or no shell damage (n = 245): 9.5% and 23% of cluckers, respectively. The only other known predator of adult and large juvenile Peconic bay scallops that leaves no trace of shell damage is the common sea star, Asterias forbesi. However, this species has not been observed in the central and eastern Peconic Bays over the last 15 + years (Tettelbach et al. 2015). As whelks sometimes also left other traces of predation in lab experiments (9% chipped, 9% cracked), we inferred that another 2.6% of cluckers (n = 28) recovered from the field resulted from whelk predation. Added together, the cluckers that can be attributed to whelk predation represent 4.3% of total presumed scallop losses (374/8709) or 2% of all planted scallops (374/19,100). This may somewhat underestimate overall whelk predation, as 32% of scallops eaten by both species in lab experiments had disarticulated valves and thus would not have been counted amongst cluckers in field surveys. If the rate of predation on scallops planted in the field is calculated on the basis of the numbers of acoustic tagged (n = 30) and observed wild whelks (total = 157), this works out to a predation rate of 0.13 scallops eaten whelk−1 day−1 (= 374 scallops eaten/157 whelks/18 days). This is comparable to observed predation rates by knobbed whelks in our laboratory experiments.
Busycon carica appears to represent a greater threat than Busycotypus canaliculatus to planted and natural bay scallop populations in the Peconic Bays because of its much higher abundance (~ 9–10 × higher than channeled whelks: Udelson et al. in prep) and higher (24x) rate of predation on scallops in the lab (this study). The importance of predation by knobbed whelks is also suggested by our observations of high frequencies of notched scallops in the Peconic Bays at some sites. In Northwest Harbor, south of Cedar Island Lighthouse, where knobbed whelks are particularly abundant (Tettelbach et al. 2015), notched scallops comprised 33.3% (7/21) and 39.1% (43/110) of adult and juvenile scallop cluckers, respectively, in dive surveys from Fall 2015–Spring 2018. Thus, at some sites, whelk predation may be more important than that what we observed in the present field experiments.
Whelk movement and behavior in response to scallop plantings
Intensive predation of scallops by channeled and knobbed whelks right after the first planting suggests that emergence of buried whelks and foraging activity were both stimulated; mucus trails confirmed that whelks also immigrated into the area. The orientation of most whelks into the direction of the prevailing current, along with a sweeping, side to side motion, were the same behaviors exhibited by Busycon carica in flume experiments (Ferner and Weissburg 2005). This strategy is likely advantageous in that scallops may not detect the odor plume of an approaching whelk until they are almost in physical contact, reducing the window of opportunity for scallops to escape.
While the lack of sudden attraction of other large predators (crabs, finfish) to the seeded scallops is similar to what we observed in previous plantings (Tettelbach et al. 2011), others have observed a marked attraction of crabs (Boulding and Hay 1984; Barbeau et al. 1996) or sea stars (Tettelbach and Wenczel 1993) to high-density scallop plantings. These differences may certainly reflect predator/prey densities or environmental factors (e.g. water temperature), but a better understanding of potential differences in predator response is important within the context of restoration efforts. For these efforts, decisions regarding planting density may be central to their success (Tettelbach and Wenczel 1993; Tettelbach et al. 2013, 2015).
The greater overall amount of time spent by tagged whelks in the grid after it was planted with scallops versus controls (no scallops) was expected, as were the shorter distances traveled and lower movement rates by whelks in these trials (The Nature Conservancy 2018). It is important to note that flight responses have been well documented in marine invertebrates after tagging (Cote et al. 2020), but we found minimal evidence of flight responses immediately following release based on step lengths and visual movement tracks (Fig. S3). These patterns likely reflected more directed searching for prey and lower encounter rates in the absence of scallops (The Nature Conservancy 2018). However, there was surprisingly a lack of concomitant differences in exploratory behavior probability exhibited by whelks in predation trials. These metrics may reflect the findings of Ferner and Weissburg (2005), who determined that Busycon carica was able to successfully locate prey odor plumes at distances of ≥ 1.5 m in flume experiments. Searching behaviors (e.g. side to side scanning) were reduced at higher flow velocities and in the presence of obstructions so that whelks reached the prey odor source more quickly.
While tagged whelks spent more time encamped inside the grid when planted scallops were present, compared to control trials with no scallops, whelks typically exhibited a higher probability of an encamped behavioral state in the cells planted at lower (6.7–17.5 ind m−2) rather than higher (51.5–72.0 ind m−2) scallop densities, which is contrary to most animal movement studies. Encamped movement patterns are commonly characterized in landscapes where prey/resource abundance is high; thus, animal step lengths are short, with sharp turning angles, as animals perform fewer large-scale movements (Zollner and Lima 1999; Morales et al. 2004). Even at lower planting densities, which were ~ 4 × those of the ambient wild scallop population, predator satiation and thus reduced foraging activity might have been expected on the basis of low daily rates of prey consumption in lab experiments. The post-foraging lag time for appetite to return was determined to be 25–39 h for another temperate whelk species, Buccinum undatum (Evans et al. 1996). While we did not examine scallop predation by whelks from a functional response standpoint, the unexpectedly higher amount of time spent by tagged individuals in grid cells planted at lower scallop densities may reflect behavioral processes related to the modified Type III functional response described for sea stars, Asterias vulgaris, feeding on mobile sea scallops, Placopecten magellanicus (Wong et al. 2006). These authors found that handling time and proportion of time spent searching for prey did not vary with prey density but suggested that the decreased foraging efficiency of sea stars observed at high prey densities may have resulted from conflicting stimuli from multiple nearby sea scallops (Wong et al. 2006). Interference competition from other predators (Gotelli 2008) within our high density sectors is another possible explanation for greater observed encampment at high versus low bay scallop densities. The potential presence of higher numbers of blue crabs (known predators of whelk and scallops) and spider crabs at higher scallop densities may have resulted in whelk burial/avoidance (Harding 2003; Hernández Cordero and Seitz 2014). This may have contributed to more encamped behaviors for longer periods.
Different patterns may have emerged in field trials 2, 3 and 4 based on the change in the placement of high- and low-density scallop densities across grids among trials coupled with the influence of tidal currents. For instance, tide direction and scallop densities were two covariates that were strongly supported across all three experimental trials based on AIC. Depending on the direction of tidal currents, current flow, and the dispersal of chemical cues (e.g. scallop mucus, pheromones) that whelks may have relied on, this could change their behavior (turning angle and step lengths) and in turn affected the probability of remaining in a given behavioral state. Ferner and Weissburg (2005) found that knobbed whelks were able to reach sources of chemical stimuli much faster in fast and turbulent flows compared to low flow and velocity conditions during controlled flume experiments in the lab. In addition, the presence of predators could have impacted whelk behavior. For example, Ferner (2006) also demonstrated that whelks avoided traps with stone crabs (Menippe mercenaria) under favorable conditions for foraging, such as high turbulent flow and high dispersal of chemical stimulants. Overall, we think a combination of these factors may have impacted whelk behavior in experimental trials.
Average overall movement rates of channeled whelk in field predation trials (45.8 m d−1) and in control trials (223.5 m d−1) were much higher than those reported by Edmundson (2016) in Lake Tashmoo, a small embayment in Massachusetts. In this study, movement rates of 12 m d−1 from late October to early November were observed for channeled whelks also tracked with Vemco acoustic tags. In other, longer-term studies that employed traditional (non-acoustic) tags, daily movement rates were also lower than those for our short-term studies: 12 m d−1 for channeled whelks in Narragansett Bay, Rhode Island (Sisson 1972), and 18 and 0.7 – 7 m d−1, respectively, for knobbed whelks at Beaufort, North Carolina (Magalhaes 1948) and Wassaw Sound, Georgia (Shalack 2007). The latter studies may have underestimated whelk movement rates because they were based on linear distances (start to end positions). However, because they encompassed periods of winter inactivity, annual rates of movement would expectedly be lower than those observed during warmer times of the year. In the study by Edmundson (2016), channeled whelks only moved an average of ~ 1 m d−1 over a 1 yr period. Nevertheless, total distances traveled by channeled whelks in other longer term (~ 10 mo) studies in larger bodies of water were considerable: up to 1.6 and 4.2 km, respectively, in Great South Bay, New York (Lynn 2018) and Narragansett, RI (Sisson 1972). These observations and suggestions of the seasonality of channeled whelk movement, particularly in the spring and fall have come from many years of field observations by baymen (F. Sloup, P. Wenczel, pers. comm) and deserve further study.
The above observations, and those from the present study, provide important insights into the timing and range of movement of mobile gastropods. These, in turn, may have management implications. For instance, Glazer et al. (2003) determined that the aggregated home range of queen conch (Aliger gigas) was twice as large as a designated no-take reef in the Florida Keys, resulting in incomplete protection. An acoustic telemetry study of giant triton snails (Charonia tritonis) revealed their average daily movement rate (234.2 m d−1) was 23 × the maximum daily movement rate of their prey, the crown-of-thorns starfish (Acanthaster planci), suggesting tritons could successfully control population outbreaks of the latter species in sections of the Great Barrier Reef (Schlaff et al. 2020). Additional data on the movement rates of channeled and knobbed whelks for longer durations than our trials and across seasons would provide a more comprehensive picture of spatio-temporal variability in whelk-scallop interactions, movement and behavior (Sperry et al. 2008) and could help inform the timing of scallop plantings to improve survival and hence, the success of restoration programs.
Average rates of movement by whelks in our control trials (223.5 m d−1) were considerably higher than in predation trials. This may have reflected greater searching in the absence of scallop prey, as discussed above. However, in the first control trial, greater dispersion and directed movement of tagged whelks to deeper offshore waters (to the northeast), perhaps to avoid increased wave energy and exposure, may well have been precipitated by high winds at the time of planting. Thus, wind direction and wind speed were well-supported predictors of whelk movement in the best models for control trials, although they were not in experimental trials.
Effects of atmospheric phenomena on animal behavior have been described for a variety of taxa, but are much more well-known for vertebrates than invertebrates (Massie et al. 2019; Strickland et al. 2020). For example, rock blackfish (Girella elevata) in shallow subtidal habitats responded sharply to increased wind speed by moving to deeper depths, likely to avoid greater wave height (Stocks et al. 2015). Blacktip sharks were capable of anticipating incoming hurricanes along the Florida Gulf Coast with as little as a 5 mb drop in barometric pressure, without large changes in wind speed, and temporarily migrated to deeper waters to avoid storm exposure (Heupel et al. 2003). Other fish, such as summer flounder, responded to declines in average atmospheric pressure of 4 mb week−1 by increasing emigration from estuaries to deeper waters on the continental shelf off New Jersey (Sackett et al. 2007). Amongst insects, rapid changes in barometric pressure (30 mb h−1) resulted in reduced flight initiation frequency in polyphagous wasps, Trichogramma spp. (Fournier et al. 2005). Pellegrino et al. (2013) found that curcurbit beetles (Diabrotica speciosa) showed reduced locomotive activity when exposed to decreasing barometric pressure at rates as low as 0.4 mb h−1.
Atmospheric pressure was not a strong predictor of whelk behavioral switching in any trial, which was surprising considering that whelks in the control trials (1, 5) experienced average ranges of 20.5 mb: 2 × higher than those during predation trials (2, 3, 4). However, it is important to note that the average distance traveled was nearly 4 × higher in control trials when atmospheric pressure was declining, indicating that whelk locomotive activity may be partially responsive to changes in barometric pressure. Although the specifics of how whelks sense atmospheric pressure are not known, both marine invertebrates and vertebrates without swim bladders have been shown to detect small changes (5–10 mb) in hydrostatic pressure via vestibular hair cells (Fraser and MacDonald 1994; Fraser et al. 2003). Our novel results strongly suggest that gastropods respond to weather events, but this certainly deserves further study.
The importance of time of day in explaining channeled whelk movement patterns, as supported in HMMs for both control and treatment trials, with the highest observed activity in early evening/twilight hours, corroborates the findings of Magalhaes (1948). While we cannot pinpoint the mechanism(s) behind the crepuscular activity pattern within the confines of our dataset, predator avoidance (Lima and Dill 1990) is a plausible explanation. Blue crabs (see above) are most likely to exhibit agonistic and defensive behaviors during this time (Clark et al. 1999). Diurnal patterns in dissolved oxygen (DO) levels may also potentially influence whelk activity patterns. Greater locomotion of another large gastropod, queen conch (Aliger gigas), is suggested to coincide with higher DO concentrations in the late afternoon/evening (Dujon et al. 2019).
The behavioral responses of predators to external surroundings and prey density have crucial ecological and management implications (Schmitz and Barton 2014). We have provided novel insight into predator–prey interactions among three commercially important marine invertebrate species in both the field and lab. Despite the relatively low scallop predation rates observed for both channeled and knobbed whelks in the lab, field experiments revealed that whelks were probably responsible for 4.5% of scallop mortality and that 45% of all planted scallops were lost after 14 days in the field—plausibly the result of predation. Our work suggests that both channeled and knobbed whelks may be drawn to planted scallop aggregations. Given this, and the fact that both whelk species can consume juvenile as well as adult scallops, these behaviors should be factored into site selection for bay scallop restoration efforts. In addition, where channeled whelk densities are high, deployment of pots prior to scallop planting is advised. Since knobbed whelks are not caught at high rates in conventional whelk pots (F. Sloup, P. Wenczel, pers. comm) manual removals of this species by divers at the time of planting is recommended.
Availability of data and material
Data is held by the authors and is available upon request from the corresponding author and will also be available on the Peconic Estuary Partnership’s website (www.peconicestuary.org).
Code availability
The R-code generated during the current study is available from the corresponding author on reasonable request.
References
Angell TE (2018) Age, growth and sexual maturity of the channeled whelk Busycotypus canaliculatus (Linnaeus, 1758) and knobbed whelk Busycon carica (Gmelin, 1791) in Narragansett Bay, Rhode Island. J Shellfish Res 37:207–219. https://doi.org/10.2983/035.037.0119
Arnold WS (1984) The effects of prey size, predator size, and sediment composition on the rate of predation of the blue crab, Callinectes sapidus Rathbun, on the hard clam, Mercenaria mercenaria (Linné). J Exp Mar Biol Ecol 80:207–219. https://doi.org/10.1016/0022-0981(84)90150-3
Barbeau MA, Scheibling RE, Hatcher BG, Taylor LH, Hennigar AW (1994) Survival analysis of tethered juvenile sea scallops Placopecten magellanicus in field experiments: effects of predators, scallop size and density, site and season. Mar Ecol Prog Ser 115:243–256
Barbeau MA, Hatcher BG, Scheibling RE, Hennigar AW, Taylor LH, Risk AC (1996) Dynamics of juvenile sea scallop (Placopecten magellanicus) and their predators in bottom seeding trials in Lunenburg Bay, Nova Scotia. Can J Fish Aquat Sci 53:2494–2512. https://doi.org/10.1139/f96-202
Bastille-Rousseau G, Gibbs JP, Yackulic CB (2017) Animal movement in the absence of predation: environmental drivers of movement strategies in a partial migration system. Oikos 126:1004–1019. https://doi.org/10.1111/oik.03928
Beal BF, Lithgow CD, Shaw DP, Renshaw S, Ouellette D (1995) Overwintering hatchery-reared individuals of the soft-shell clam, Mya arenaria L.: a field test of site, clam size, and intraspecific density. Aquaculture 130:145–158. https://doi.org/10.1016/0044-8486(94)00221-9
Boulding EG (1984) Crab-resistant features of shells of burrowing bivalves: decreasing vulnerability by increasing handling time. J Exp Mar Biol Ecol 76:201–223
Boulding EG, Hay TK (1984) Crab response to prey density can result in density-dependent mortality of clams. Can J Fish Aquat Sci 41:521–525. https://doi.org/10.1139/f84-063
Brousseau LJ, Sclafani MS, Smith DR, Carter DB (2004) Acoustic-tracking and radio-tracking of horseshoe crabs to assess spawning behavior and subtidal habitat use in Delaware Bay. N Am J Fish Man 24:1376–1384. https://doi.org/10.1577/1548-8675
Burnham KP, Anderson DR (2002) A practical information-theoretic approach. Model selection and multimodel inference, 2nd edn. Springer, New York
Carriker MR (1951) Observations on the penetration of tightly closing bivalves by Busycon and other predators. Ecology 32:73–83. https://doi.org/10.2307/1930973
Carroll JM, Peterson BJ, Bonal D (2010) Comparative survival of bay scallops in eelgrass and the introduced alga, Codium fragile, in a New York estuary. Mar Biol 157:249–259. https://doi.org/10.1007/s00227-009-1312-0
Castagna M (1984) Methods of growing Mercenaria mercenaria from postlarval-to preferred-size seed for field planting. Aquaculture 39:355–359. https://doi.org/10.1016/0044-8486(84)90277-1
Castagna M (2001) Aquaculture of the hard clam, Mercenaria mercenaria. In: Kraeuter JN, Castagna M (eds) Biology of the hard clam. Elsevier, New York, pp 675–699
Castagna M, Kraeuter JN (1994) Age, growth rate, sexual dimorphism, and fecundity, of knobbed whelk Busycon carica (Gmelin 1791) in Virginia. J Shellfish Res 13:581–585
Chamberlain S (2020) rnoaa: 'NOAA' Weather Data from R. R package version 1.2.0. https://CRAN.R-project.org/package=rnoaa. Accessed 10 October 2020.
Cherry MJ, Barton BT (2017) Effects of wind on predator-prey interactions. Food Webs 13:92–97. https://doi.org/10.1016/j.fooweb.2017.02.005
Cigarría J, Fernández JM (2000) Management of Manila clam beds: I. Influence of seed size, type of substratum and protection on initial mortality. Aquaculture 182:173–182. https://doi.org/10.1016/S0044-8486(99)00257-4
Clark ME, Wolcott TG, Wolcott DL, Hines AH (1999) Foraging and agonistic activity co-occur in free-ranging blue crabs (Callinectes sapidus): observation of animals by ultrasonic telemetry. J Exp Mar Biol Ecol 233:143–160. https://doi.org/10.1016/S0022-0981(98)00129-4
Connell JH (1970) A predator-prey system in the marine intertidal region. I. Balanus glandula and several predatory species of Thais. Ecol Monogr 40:49–78. https://doi.org/10.2307/1942441
Conover WJ, Iman RL (1979) On multiple-comparisons procedures. Los Alamos Sci. Lab. Tech. Rep. LA-7677-MS, pp 1–14
Cosper EM, Dennison WC, Carpenter EJ, Bricelj VM, Mitchell JG, Kuenstner SH, Colflesh D, Dewey M (1987) Recurrent and persistent brown tide blooms perturb coastal marine ecosystem. Estuaries 10:284–290. https://doi.org/10.2307/1351885
Cote D, Morris CJ, Regular PM, Piersiak MG (2020) Effects of 2D Seismic on snow crab movement behavior. Fish Res 230:105661. https://doi.org/10.1016/j.fishres.2020.105661
Dinno A (2017) conover.test: Conover-Iman test of multiple comparisons using rank sums. https://CRAN.R-project.org/package=conover.test. Accessed 10 October 2020
Dujon AM, Stieglitz TC, Amice E, Webber DM (2019) Snail leaps and bounds: drivers of the diel movement pattern of a large invertebrate, the Caribbean queen conch (Lobatus gigas), in a marginal inshore habitat. Can J Zool 97:436–445. https://doi.org/10.1139/cjz-2018-0106
Edmundson SA (2016) Channeled whelk (Busycotypus canaliculatus) ecology in relation to the fishery in Vineyard and Nantucket Sounds, Massachusetts. Dissertation, University of New Hampshire
Edwards AL, Harasewych MG (1988) Biology of recent species of the subfamily busyconinae. J Shellfish Res 7(3):467–472
Eggleston DB, Armstrong DA (1995) Pre- and post-settlement determinants of estuarine Dungeness crab recruitment. Ecol Monogr 65:193–216. https://doi.org/10.2307/2937137
Einfalt LM, Grace EJ, Wahl DH (2012) Effects of simulated light intensity, habitat complexity and forage type on predator-prey interactions in walleye Sander vitreus. Ecol Freshw Fish 21:560–569. https://doi.org/10.1111/j.1600-0633.2012.00576.x
Elner RW (1978) The mechanics of predation by the shore crab, Carcinus maenas (L.), on the edible mussel, Mytilus edulis (L.). Oecologia 36:333–344. https://doi.org/10.1007/BF00348059
Evans PL, Kaiser MJ, Hughes RN (1996) Behaviour and energetics of whelks, Buccinum undatum (L.), feeding on animals killed by beam trawling. J Exp Mar Biol Ecol 197:51–62. https://doi.org/10.1016/0022-0981(95)00144-1
Ferner MC (2006) Environmental modification of chemosensory interactions between predators and prey: the world according to whelks. Dissertation, Georgia Institute of Technology
Ferner MC, Weissburg MJ (2005) Slow-moving predatory gastropods track prey odors in fast and turbulent flow. J Exp Biol 208:809–819. https://doi.org/10.1242/jeb.01438
Fisher RA, Rudders DB (2017) Population and reproductive biology of the channeled whelk, Busycotypus canaliculatus, in the U.S. mid-Atlantic. J Shellfish Res 36:427–444. https://doi.org/10.2983/035.036.0215
Fournier F, Pelletier D, Vigneault C (2005) Effect of barometric pressure on flight initiation by Trichogramma pretiosum and Trichogramma evanescens (Hymenoptera: Trichogrammatidae). Environ Entomol 34:1534–1540. https://doi.org/10.1603/0046-225x-34.6.1534
Fraser PJ, Macdonald AG (1994) Crab hydrostatic pressure sensors. Nature 371:383–384. https://doi.org/10.1038/371383b0
Fraser PJ, Cruickshank SF, Shelmerdine RL (2003) Hydrostatic pressure effects on vestibular hair cell afferents in fish and crustacea. J Vest Res 13:235–242
Garcia-Esquivel Z, Bricelj VM (1993) Ontogenetic changes in microhabitat distribution of juvenile bay scallops, Argopecten irradians irradians (L.) in eelgrass beds, and their potential significance to early recruitment. Biol Bull 185:42–53. https://doi.org/10.2307/1542129
Gendron L (1992) Determination of the size at sexual maturity of the waved whelk Buccinum undatum in the Gulf of St. Lawrence as basis for the establishment of a minimum catchable size. J Shellfish Res 11:1–7
Glazer RA, Delgado GA, Kidney JA (2003) Estimating queen conch (Strombus gigas) home ranges using acoustic telemetry: implications for the design of marine fishery reserves. Gulf Car Res 14:79–89
Gmelin JF (1791) Vermes. In: Gmelin JF (ed) Caroli a Linnaei systema naturae per regna tria naturae, Ed. 13. Tome 1(6). GE Beer, Leipzig, pp 3021–3910
Goldberg R, Pereira J, Clark P (2000) Strategies for enhancement of natural bay scallop, Argopecten irradians irradians, populations; A case study in the Niantic River estuary, Connecticut, USA None. Aquac Int 8:139–158. https://doi.org/10.1023/a:1009242429529
Gosselin L, Qian P (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282. https://doi.org/10.3354/meps146265
Gotelli NJ (2008) A primer of ecology, 4th edn. Sinauer Associates, Sunderland, pp 99–124
Grigaltchik VS, Ward AJ, Seebacher F (2012) Thermal acclimation of interactions: differential responses to temperature change alter predator–prey relationship. Proc R Soc B 279:4058–4064
Hammerschlag N, Martin RA, Fallows C (2006) Effects of environmental conditions on predator–prey interactions between white sharks (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus) at Seal Island, South Africa. Environ Biol Fish 76:341–350. https://doi.org/10.1007/s10641-006-9038-z
Harding JM (2003) Predation by blue crabs, Callinectes sapidus, on rapa whelks, Rapana venosa: possible natural controls for an invasive species? J Exp Mar Biol Ecol 297:161–177. https://doi.org/10.1016/j.jembe.2003.07.005
Hernández Cordero AL, Seitz RD (2014) Structured habitat provides a refuge from blue crab, Callinectes sapidus, predation for the bay scallop, Argopecten irradians concentricus (Say 1822). J Exp Mar Biol Ecol 460:100–108. https://doi.org/10.1016/j.jembe.2014.06.012
Heupel MR, Simpfendorfer CA, Hueter RE (2003) Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. J Fish Biol 63:1357–1363. https://doi.org/10.1046/j.1095-8649.2003.00250.x
Hilborn R (1975) The effect of spatial heterogeneity on the persistence of predator-prey interactions. Theor Pop Biol 8:346–355. https://doi.org/10.1016/0040-5809(75)90051-9
Hollander M, Wolfe DA, Chicken E (2013) Nonparametric statistical methods. John Wiley and Sons, Hoboken
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Holt RD (1984) Spatial heterogeneity, indirect interactions, and the coexistence of prey species. Am Nat 124:377–406. https://doi.org/10.1086/284280
Hughes RN, Seed R (1981) Size selection of mussels by the blue crab Callinectes sapidus: energy maximizer or time minimizer? Mar Ecol Prog Ser 6:83–89
Hunt H, Scheibling R (1997) Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar Ecol Prog Ser 155:269–301. https://doi.org/10.3354/meps155269
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528. https://doi.org/10.1086/282687
Jensen KT, Jensen JN (1985) The importance of some epibenthic predators on the density of juvenile benthic macrofauna in the Danish Wadden Sea. J Exp Mar Biol Ecol 89:157–174. https://doi.org/10.1016/0022-0981(85)90124-8
Jonzén N, Knudsen E, Holt JD, Saether BE (2011) Uncertainty and predictability: the niches of migrants and nomads. In: Milner-Gulland E, Fryxell JM, Sinclair ARE (eds) Animal migration: a synthesis. Oxford University Press, Oxford, pp 91–109
Kraeuter JN (2001) Predators and predation. In: Kraeuter JN, Castagna M (eds) Biology of the hard clam. Elsevier, Amsterdam, pp 441–589
Lamarck JPBA (1819) Histoire naturelle des animaux sans vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s'y rapportent; précédée d'une introduction offrant la détermination des caractères essentiels de l'animal, sa distinction du végétal et des autres corps naturels, enfin, l'exposition des principes fondamentaux de la zoologie. Tome sixième. Ire. partie. j-vj:1−343
Langrock R, King R, Matthiopoulos J (2012) Flexible and practical modeling of animal telemetry data: hidden Markov models and extensions. Ecology 93:2336–2342. https://doi.org/10.1890/11-2241.1
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zoo 68:619–640
Linnaeus C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata, 10th edn. Laurentius Salvius, Holmiae, p 824
Lunt J, Smee DL (2014) Turbidity influences trophic interactions in estuaries. Limnol Oceanogr 59:2002–2012
Lynn RC (2018) Maturity, growth rates and movement patterns of the channeled whelk, Busycotypus canaliculatus, in the Great South Bay, New York. Thesis. LIU-Post
Magalhaes H (1948) An ecological study of snails of the genus Busycon at Beaufort, NC. Ecol Monogr 18:377–409. https://doi.org/10.2307/1948577
Martin J, Benhamou S, Yoganand K, Owen-Smith N (2015) Coping with spatial heterogeneity and temporal variability in resources and risks: adaptive movement behaviour by a large grazing herbivore. PLoS ONE 10:e0118461. https://doi.org/10.1371/journal.pone.0118461
Massie JA, Strickland BA, Santos RO, Hernandez J, Viadero N, Boucek RE, Willoughby H, Heithaus MR, Rehage JS (2019) Going downriver: patterns and cues in hurricane-driven movements of common snook in a subtropical coastal river. Est Coast 43:1158–1173. https://doi.org/10.1007/s12237-019-00617-y
Michelot T, Langrock R, Patterson TA (2016) moveHMM: an R package for the statistical modelling of animal movement data using hidden Markov models. Meth Ecol Evol 7:1308–1315. https://doi.org/10.1111/2041-210x.12578
Mladinich KM (2017) Crepidula fornicata shell beds as a potential spatial refuge for bay scallops, Argopecten irradians irradians, in the Peconic Bays, New York. Thesis, LIU-Post
Morales JM, Haydon DT, Frair J (2004) Extracting more out of relocation data: building movement models as mixtures of random walks. Ecology 85:2436–2445. https://doi.org/10.1890/03-0269
Newell RIE, Alspach GS, Jacobs D, Kennedy VS (2000) Mortality of newly metamorphosed eastern oysters (Crassostrea virginica) in mesohaline Chesapeake Bay. Mar Biol 136:665–676. https://doi.org/10.1007/s002270050726
Ogburn MB, Bangley CW, Aguilar R, Fisher RA, Curran MC, Webb SF, Hines AH (2018) Migratory connectivity and philopatry of cownose rays Rhinoptera bonasus along the Atlantic coast, USA. Mar Ecol Prog Ser 602:197–211
Ordzie CJ, Garofalo GC (1980) Predation, attack success, and attraction to the bay scallop, Argopecten irradians (Lamarck) by the oyster drill, Urosalpinx cinerea (Say). J Exp Mar Biol Ecol 47:95–100. https://doi.org/10.1016/0022-0981(80)90141-0
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Palmer SC, Coulon A, Travis JM (2014) Inter-individual variability in dispersal behaviours impacts connectivity estimates. Oikos 123:923–932. https://doi.org/10.1111/oik.01248
Papastamatiou YP, Watanabe YY, Demšar U (2018) Activity seascapes highlight central place foraging strategies in marine predators that never stop swimming. Mov Ecol. https://doi.org/10.1186/s40462-018-0127-3
Patterson TA, Basson M, Bravington MV, Gunn JS (2009) Classifying movement behaviour in relation to environmental conditions using hidden Markov models. J Anim Ecol 78:1113–1123. https://doi.org/10.1111/j.1365-2656.2009.01583.x
Peemoeller BJ, Stevens B (2013) Age size and sexual maturity of channeled whelk (Busycotypus canaliculatus) in Buzzards Bay, Massachusetts. Fish Bull 111:265–278. https://doi.org/10.7755/FB.111.3.5
Pellegrino AC, Peñaflor MFGV, Nardi C, Bezner-Kerr W, Guglielmo CG, Bento JMS, McNeil JN (2013) Weather forecasting by insects: modified sexual behaviour in response to atmospheric pressure changes. PLoS ONE 8:75004. https://doi.org/10.1371/journal.pone.0075004
Peterson CH (1982) Clam predation by whelks (Busycon spp.): experimental tests of the importance of prey size, prey density and eelgrass cover. Mar Biol 66:159–170. https://doi.org/10.1007/BF00397189
Peterson CH, Summerson HC, Fegley SR, Prescott RC (1989) Timing, intensity and sources of autumn mortality of adult bay scallops Argopecten irradians concentricus Say. J Exp Mar Biol Ecol 127:121–140. https://doi.org/10.1016/0022-0981(89)90179-2
Peterson CH, Fodrie JF, Summerson HC, Powers SP (2001) Site-specific and density-dependent extinction of prey by schooling rays: generation of a population sink in top-quality habitat for bay scallops. Oecologia 129:349–356
Power AJ, Sellers C, Walker R (2009) Growth & sexual maturity of the knobbed whelk, B. carica (Gmelin 1791) from a commercially harvested population in Georgia. Pap Univ GA Mar Ext Serv 4:1–29
Prescott RC (1990) Sources of predatory mortality in the bay scallop Argopecten irradians (Lamarck): interactions with seagrass and epibiotic coverage. J Exp Mar Biol Ecol 144:63–83
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rheinallt T, Hughes RN (1985) Handling methods used by the velvet swimming crab Liocarcinus puber when feeding on mollusks and shore crabs. Mar Ecol Prog Ser 25:63–70
Rilov G, Gasith A, Benayahu Y (2002) Effect of exotic prey on feeding pattern of a predatory snail. Mar Environ Res 54:85–98
Robinson EM, Smee DL, Trussell GC (2011) Green crab (Carcinus maenas) foraging efficiency reduced by fast flows. PLoS ONE 6(6):e21025. https://doi.org/10.1371/journal.pone.0021025
Sackett DK, Able KW, Grothues TM (2007) Dynamics of summer flounder, Paralichthys dentatus, seasonal migrations based on ultrasonic telemetry. Est Coast Shelf Sci 74:119–130. https://doi.org/10.1016/j.ecss.2007.03.027
Sanford E (2002) Water temperature, predation, and the neglected role of physiological rate effects in rocky intertidal communities. Integr Comp Biol 42:881–891. https://doi.org/10.1093/icb/42.4.881
Schlaff A, Menéndez P, Hall M (2020) Acoustic tracking of a large predatory marine gastropod, Charonia tritonis, on the Great Barrier Reef. Mar Ecol Prog Ser 642:147–161. https://doi.org/10.3354/meps13291
Schmitz OJ, Barton BT (2014) Climate change effects on behavioral and physiological ecology of predator–prey interactions: implications for conservation biological control. Biol Control 75:87–96. https://doi.org/10.1016/j.biocontrol.2013.10.001
Shalack J (2007) Movement and behavior of whelks (Family Melongenidae) in Georgia coastal waters. Thesis, University of Georgia
Sisson RT (1972) Biological and commercial fisheries related research on the channeled whelk, Busycotypus canaliculatum (Linné) in Narragansett Bay, RI. Thesis, University of Rhode Island
Smith F (2013) Understanding HPE in the VEMCO Positioning System (VPS). Document #: DOC-005457–01, 31 pp. Accessed 20 September 2020
Sperry JH, Peak RG, Cimprich DA, Weatherhead PJ (2008) Snake activity affects seasonal variation in nest predation risk for birds. J Avian Biol 39:379–383. https://doi.org/10.1111/j.0908-8857.2008.04451.x
Stocks JR, Gray CA, Taylor MD (2015) Intra-population trends in the maturation and reproduction of a temperate marine herbivore Girella elevata across latitudinal clines. J Fish Biol 86:463–483. https://doi.org/10.1111/jfb.12563
Strickland BA, Gastrich K, Mazzotti FJ et al (2020) Variation in movement behavior of alligators after a major hurricane. Anim Bioelemetry. https://doi.org/10.1186/s40317-020-00193-0
Tettelbach ST (1986) Dynamics of crustacean predation on the northern bay scallop, Argopecten irradians irradians. Dissertation, University of Connecticut
Tettelbach ST, Smith CF (2009) Bay scallop restoration in New York. Ecol Rest 27:20–22. https://doi.org/10.3368/er.27.1.20
Tettelbach ST, Wenczel P (1993) Reseeding efforts and the status of bay scallop Argopecten irradians (Lamarck, 1819) populations in New York following the occurrence of “brown tide” algal blooms. J Shellfish Res 12:423–431
Tettelbach ST, Smith CF, Kaldy JE III, Arroll TW, Denson MR (1990) Burial of transplanted bay scallops Argopecten irradians irradians (Lamarck, 1819) in winter. J Shellfish Res 9:127–134
Tettelbach ST, Barnes D, Aldred J (2011) Utility of high-density plantings in bay scallop, Argopecten irradians irradians, restoration. Aquac Int 19:715–739. https://doi.org/10.1007/s10499-010-9388-6
Tettelbach S, Peterson B, Carroll J (2013) Priming the larval pump: resurgence of bay scallop recruitment following initiation of intensive restoration efforts. Mar Ecol Prog Ser 478:153–172. https://doi.org/10.3354/meps10111
Tettelbach ST, Peterson BJ, Carroll JM, Furman BT, Hughes SW, Havelin J, Smith CF (2015) Aspiring to an altered stable state: rebuilding of bay scallop populations and fisheries following intensive restoration. Mar Ecol Prog Ser 529:121–136. https://doi.org/10.3354/meps11263
The Nature Conservancy (2018) FishPath workshop report: Rhode Island channeled whelk. The Nature Conservancy, Arlington
Thorley J, Fleishman A, Miller L (2017) rtide: tide heights. R Package Version 0.0. 4
Udelson B (2012) Age and size at onset of sexual maturity of the channeled whelk, Busycotypus canaliculatus (Linnaeus, 1758), in the Peconic Bays of Long Island, New York. Thesis, LIU-Post
Uki N (2006) Stock enhancement of the Japanese scallop Patinopecten yessoensis in Hokkaido. Fish Res 80:62–66. https://doi.org/10.1016/j.fishres.2006.03.013
Walker RL (1988) Observations on intertidal whelk (Busycon and Busycotypus) populations in Wassaw Sound, GA. J Shellfish Res 7:473–478
Weinstock AJ (2010) Predation on the northern bay scallop Argopecten irradians irradians (Lamarck, 1819) by scup, Stenotomus chrysops (Linneaeus, 1766): effects of predator/prey size and substrate. Thesis, LIU-Post
Whoriskey K, Auger-Méthé M, Albertsen CM (2017) A hidden Markov movement model for rapidly identifying behavioral states from animal tracks. Ecol Evol 7:2112–2121. https://doi.org/10.1002/ece3.2795
Wong M, Barbeau M, Dowd M, Richard K (2006) Behavioural mechanisms underlying functional response of sea stars Asterias vulgaris preying on juvenile sea scallops Placopecten magellanicus. Mar Ecol Prog Ser 317:75–86. https://doi.org/10.3354/meps317075
Zamon JE (2001) Seal predation on salmon and forage fish schools as a function of tidal currents in the San Juan Islands, Washington, USA. Fish Oceanogr 10:353–366. https://doi.org/10.1046/j.1365-2419.2001.00180.x
Zollner PA, Lima SL (1999) Search strategies for landscape-level interpatch movements. Ecology 80:1019–1030. https://doi.org/10.1890/0012-9658(1999)080[1019:ssflli]2.0.co;z
Acknowledgements
We would like to thank the reviewers and editor for providing suggestions that greatly enhanced the quality of the manuscript. We would like to thank Sherryll Jones and Justin Reyes for their assistance with the laboratory experiments. Barry Udelson provided GIS support for acoustic telemetry GIS analysis. Peter Wenczel assisted with the collection of whelk; he and Frank Sloup provided many insights into their movement and behavior. We thank Philip Wisocki for his helpful discussions. This project was funded through the Suffolk County 477 fund through the Peconic Estuary Program. Kim Shaw, Kim Barbour, Sheri Jewhurst and Alison Branco provided helpful suggestions during the planning stages.
Funding
This project was funded through the Suffolk County 477 fund through the Peconic Estuary Partnership.
Author information
Authors and Affiliations
Contributions
MS and ST designed the experiments, carried out the research, analyzed the data, and wrote and revised the original manuscript. JB analyzed the telemetry data and prepared and revised the original manuscript. JE prepared the telemetry data and revised the manuscript. JH, CH, SWT contributed to experimental design, carried out the research, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests.
Ethical approval
This research was carried out in accordance with all applicable institutional guidelines at the time that the study was conducted. Ethical review and approval were not required for the animal study because the laboratory experiments were conducted at Cornell Cooperative Extensions Suffolk County Marine Environmental Learning Center, Southold New York. Cornell Cooperative Extension of Suffolk County does not have an Institutional Animal Care and Use Committee (IACUC) approval process for research on invertebrates. All work followed American Fisheries Society policies on the Guidelines for Use of Fishes in Research (https://fisheries.org/docs/policy_useoffishes.pdf) and AVMA (American Veterinary Medical Association) Guidelines on Euthanasia (https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf).
Consent to participate
All authors reviewed, edited, and approved the manuscript for submission.
Consent for publication
All authors reviewed, edited, and approved the manuscript for submission.
Additional information
Responsible Editor: P. Ramey-Balci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sclafani, M., Bopp, J., Havelin, J. et al. Predation on planted and wild bay scallops (Argopecten irradians irradians) by busyconine whelks: studies of behavior incorporating acoustic telemetry. Mar Biol 169, 66 (2022). https://doi.org/10.1007/s00227-022-04033-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04033-y