Abstract
Dermanyssus gallinae, the poultry red mite (PRM), is a hematophagous temporary ectoparasite that causes serious economic losses and animal health impairment on laying hen farms worldwide. Control is limited by the parasite’s hidden lifestyle, restrictions on the use of chemical acaricides and the development of resistance against certain drug classes. As a result, research was conducted to explore alternative control methods. In recent years, atmospheric pressure plasma has been increasingly reported as an alternative to chemical acaricides for pest control. This physical method has also shown promising against PRM under laboratory conditions. However, the detailed mechanisms of action have not yet been elucidated. In the present study, the effects of cold atmospheric pressure plasma on PRM were investigated using digital videography and optical coherence tomography (OCT), an imaging technique that visualizes the topography of surfaces and internal structures. Digital videography showed that a redistribution of the contents of the intestinal tract and excretory organs (Malpighian tubules) occurred immediately after plasma exposure. The body fluids reached the distal leg segments of PRM and parts of the haemocoel showed whiter and denser clumps, indicating a coagulation of the haemocoel components. OCT showed a loss of the boundaries of the hollow organs in transverse and sagittal sectional images as well as in the three-dimensional image reconstruction. In addition, a dorso-ventral shrinkage of the idiosoma was observed in plasma-exposed mites, which had shrunk to 44.0% of its original height six minutes after plasma exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The poultry red mite (PRM) Dermanyssus gallinae is a hematophagous temporary ectoparasite affecting birds of various species. Particularly it is prevalent in laying hen husbandry (Flochlay et al. 2017; Nunn et al. 2020). An infestation not only significantly reduces the health and welfare of host animals and personnel, but also causes immense economic damage. Environmental chemical acaricides and acaricidal drugs are mainly used to control PRM infestations (Sommer 2011; Decru et al. 2020). However, some of them are considered carcinogenic, contain neurotoxic substances and have effects on mental health (Ansari et al. 2014; Soulié et al. 2021). Additionally, the use of acaricides is restricted by regulatory requirements and stricter legislation (Decru et al. 2020). Furthermore, the use of chemical acaricides has been increasingly restricted in recent decades due to the increasing resistance of PRM. Resistance to products containing organophosphates, carbamates and pyrethroids has been reported (Liebisch and Liebisch 2003; Marangi et al. 2009; Abbas et al. 2014). Therefore, physical and biological control methods have gained importance (Lima-Barbero et al. 2020).
In recent years, intensive research has been conducted on the development of effective and sustainable methods of controlling PRM infestations. Laboratory experiments and field studies have investigated the use of temperature, showing the effectiveness of heating the hen house to over 45 °C or using hot water high-pressure cleaners (Decru et al. 2020). Lighting programmes influence the behavior of PRM and can reduce the population under laboratory conditions (Zoons 2004; Stafford et al. 2006). However, changes in light regimes may affect animal welfare, and national guidelines for livestock must be observed (Mul and Koenraadt 2009). Diatomaceous earth or synthetic amorphous silica are common biophysical measures against PRM (Maurer and Perler 2006; Kilpinen and Steenberg 2009; Schulz et al. 2014). Ulrichs et al. (2020) demonstrated an efficient control of PRM in a hen house for 46 weeks using silica-based products. In addition, repellent essential oils and plant-based preparations showed promising efficacy in vitro, but had limited success in the field (Kim et al. 2004; Maurer et al. 2009; Locher 2010; Faghihzadeh et al. 2014). Natural enemies such as predatory mites, bacteria and fungi can also be used against the PRM (Nordenfors and Höglund 2000; Roy et al. 2017; Alves et al. 2023), but such biological methods are rather supportive measures as they are significantly influenced by factors like the temperature (Ali et al. 2012; Lesna et al. 2012; Hwang 2023). Novel physical control methods such as high-voltage pulses (Ueno et al. 2023) have shown high efficacy against PRM in experimental studies. Obviously, there is still a need to explore alternative, sustainable and effective control strategies for the field (Hwang 2023).
A possible solution with great potential for effective and sustainable control could also be integrated pest management (IPM) as reported recently (Rüster et al. 2023). IPM involves the combination of preventive measures, monitoring techniques and effective control methods of varying efficacy (Sparagano et al. 2014). The main aim of this approach is to reduce the use of environmental chemical acaricides and acaricidal drugs, which should only be used when alternative methods have not been sufficiently effective (Mul 2017). Therefore, IPM could limit the use of pesticides and reduce or minimize the side effects and risks to human health and the environment (FAO 2020; Hwang 2023).
In recent years, promising studies have been reported on the application of cold atmospheric pressure plasma (CAPP) to several arthropod pests. Physical plasma is already being used in agriculture for seed treatment, crop decontamination, soil remediation and control of various insect pests (Zhang et al. 2014; Ohta 2016; Brandenburg et al. 2019; Carpen et al. 2019; ten Bosch 2022). The red flour beetle (Tribolium castaneum), the Indian meal moth (Plodia interpunctella) and the head lice (Pediculus humanus capitis) can be effectively killed with non-thermal plasma (ten Bosch et al. 2019; Nasr et al. 2020; Sayed et al. 2021). Short-term exposure to CAPP also resulted in effective control of all developmental stages of PRM, including mite eggs (Rüster et al. 2022). Irrespective of the setting parameters (electrical power and exposure time) used, plasma-exposed eggs were unable to develop, i.e. no larval hatch was observed in any of the eggs. In the unexposed control group, normally developed larvae hatched from all mite eggs. After treatment with 10 W power and an exposure time of 1.0 s, high mortality was observed immediately after plasma exposure in larvae (96.6%), protonymphs (92.2%), deutonymphs (85.5%) and adult males (93.3%), while mortality in adult females (42.2%) was initially much lower. However, almost all initially surviving mites died within 60 min of plasma exposure, only a few protonymphs died up to 120 min after plasma exposure (Rüster et al. 2022). However, the mode of action of plasma on mite species is still unknown. To determine the lethal effects of plasma, exposed PRM were examined using optical coherence tomography and a high-resolution video camera. This allows repeated non-contact imaging of the treated PRM to visualize the short-term changes in morphological structures as a result of plasma treatment.
Materials and methods
Poultry red mites
Mites originated from the acaricide-susceptible D. gallinae F0 isolate maintained at the Institute for Parasitology, University of Veterinary Medicine Hannover. This isolate was kept in breeding boxes at room temperature. For feeding, a hen was placed in the box at weekly or biweekly intervals and the mites were allowed to take a blood meal. For the present study, non-engorged (i.e., grey), alive adult females were collected and subjected to examinations within 2 days of collection.
Plasma setup and treatment of PRM
Exposure to cold atmospheric pressure plasma (CAPP) was performed using a dielectric barrier discharge (DBD) system using the Tantec generator V-X20 (Tantec, Lunderskov, Denmark) as described by Rüster et al. (2022). CAPP was produced at room temperature and under atmospheric pressure in ambient air (composition: nitrogen 78.08%, oxygen 20.95%, argon 0.93%, carbon dioxide < 0.04%, small and variable amounts of trace gases, e.g. methane, ozone, various noble gases; Schlesinger and Bernhardt 2020), which was generated between two parallel electrodes (discharge distance = 1 mm) in a capacitor arrangement. In preliminary tests, a power of 30 W and an exposition time of 3.0 s proved to be suitable for the intended imaging. The electrode discharge area was 22 cm2, resulting in power density of 1.36 W/cm2. For the imaging studies, ten alive adult female mites were individually attached on the ground electrode of the DBD with double-sided adhesive tape using a fine brush to restrict mite movement.
Digital videography and optical coherence tomography (OCT)
Ten adult female PRM were initially examined by OCT and videography at the observation time (tobs) of 1 min before CAPP exposure (tobs = 0:00 min). Thus, each mite served as its own untreated control. After exposure to CAPP at tobs = 2:00 min, tobs = 4:00 min and tobs = 6:00 min, the mites were re-examined to observe morphological changes and thus possible effects of the plasma treatment. The timeline of the experiment is shown in Fig. 1.
Live imaging of plasma treated mites with OCT and videography were performed at the department of Clinical Sensoring and Monitoring at the Medical Faculty of the Technische Universität Dresden, Germany. Optical coherence tomography (OCT) is an interferometric imaging technique based on near-infrared light that allows contactless and marker-free visualization of the inner structure of scattering materials like biological tissue with a spatial resolution in the micrometer range (Schnabel et al. 2013, 2014; Jannasch et al. 2021).
The OCT system used in this study consists of a custom build spectrometer and scanner head containing a Michelson interferometer as described in Schnabel et al. 2014 (Fig. 2).
OCT is used to obtain a large number of sectional images that can then be analyzed using the Fiji program, an open source image processing package based on ImageJ2 (Schindelin et al. 2012). First, the OCT data stack is exported as an uncompressed.avi file. Care must be taken to ensure correct scaling so that the distance between voxels is uniform in all three dimensions matching the real morphologic scale (voxel distance ≙ 2.33 μm). The number of sectional images varied depending on the size of the mite. To measure the idiosoma of the mite, it was displayed in orthogonal projection and the center of the mite was determined. The sagittal, transverse and horizontal views were saved as separate files. To measure the length, a line was drawn from the cranial to the caudal end of the mite in the sagittal view. In the center of the length, the height of the mite was determined by a vertical line. Similar procedure was used to measure the width. The center of the mite was measured in cross-section from left to right.
Statistical analysis
The idiosoma dimension data were analyzed using Dunnett’s multiple comparisons test. A p-value ≤ 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism (version 6, GraphPad Software, La Jolla, CA, USA).
Results
Video analysis
Video recordings of each mite were taken one minute before plasma exposure (tobs = 0:00 min) until five minutes after plasma exposure (tobs = 6:00 min). Image sections of these video sequences before plasma exposure at tobs = 0:00 min and after plasma exposure at tobs = 2:00 min, tobs = 4:00 min and tobs = 6:00 min are shown in Fig. 3. The video recordings showed a redistribution of the white milky contents of the Malpighian tubules into the anterior part of the mite body starting immediately after plasma exposure. In addition, a movement in the intestinal tubules was observed immediately after plasma exposure, showing a flow of dark red blood residues from the hindgut towards the midgut up to the anterior part of the mite (Fig. 3c, d). In addition, blood residues and contents of the Malpighian tubules were found in the anterior segments of the legs after plasma exposure (Fig. 3c, d). In addition, parts of the hemolymph clumped, appearing whiter and denser after plasma treatment (Fig. 3a, b; light green arrows).
Comparison of video sequence images of a female D. gallinae individual. (a) Untreated living mite before CAPP exposure (tobs = 0:00 min), (b) living mite after plasma exposure (tobs = 2:00 min), (c) inanimate mite after plasma exposure (tobs = 4:00 min) and (d) inanimate mite min after exposure (tobs = 6:00) to CAPP at 30 W for 3.0 s. Arrows depict the white, denser haemocoel. Ca I-III– caeca IIII, Mg– midgut, Mp– Malpighian tubules, Hg– hindgut. Bar = 0.2 mm
OCT analysis
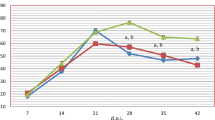
After exposure to CAPP, real-time OCT imaging showed reduced contrast of the internal organs. The clear borders of the Malpighian tubules, which were previously visible, disappeared after exposure to CAPP (Fig. 4). A subsidence of the idiosoma was observed immediately after CAPP irradiation. This effect became more pronounced with time (Fig. 5). A significant reduction in idiosoma height of the plasma-exposed mites to 72.4 ± 19.2%, 52.7 ± 13.5% and 44.0 ± 12.7% compared to the pre-treatment control (tobs = 0:00 min) was recorded for tobs = 2:00 min, tobs = 4:00 min and tobs = 6:00 min, respectively (Fig. 5). In contrast, the measurements of length and width showed only minimal changes (Fig. 5). At the time tobs = 2:00 min and tobs = 4:00 min, no changes in width could be detected, while mites were reduced in width by 1.1% at tobs = 6:00 min. The length of the mites was reduced by 1.9% at tobs = 4:00 min and by 2.1% at tobs = 6:00 min. The shrinkage effect from tobs = 0:00 min to tobs = 6:00 is clearly visible in the 3D OCT reconstruction (Fig. 6).
Comparison of the mean idiosoma dimensions of ten female D. gallinae individuals before (control, tobs = 0:00 min) and after plasma exposure at 30 W for 3.0 s. Bars are presented as mean + standard deviation [µm]. Plasma-exposure has significantly flattened the mites (Dunnett’s multiple comparisons test; * p < 0.005; ** p < 0.0001)
Discussion
The development of resistance to chemical acaricides is a serious problem in the management of PRM infestations (Lima-Barbero et al. 2020; Decru et al. 2020). Nocturnal activity, hiding in places that are difficult to access, and survival of long periods of starvation of up to 34 weeks make PRM difficult to control effectively and sustainably (Nordenfors and Höglund 2000; Kirkwood 1963). Mite control is not only limited by the hidden lifestyle of PRM, but effective control is also limited by the risk of acaricide residues in eggs and meat. As a result, the use of environmental chemical acaricides and acaricidal drugs is severely restricted by strict legislation (Decru et al. 2020). Moreover, consumer demand for pesticide-free products is increasing (Mul 2013), so there is a great need to develop effective and sustainable control methods.
Different studies indicate that CAPP has a killing effect on a wide range of microorganisms and arthropods (Donohue et al. 2006; ten Bosch et al. 2019; Sayed et al. 2021; Rüster et al. 2022; Anuntagool et al. 2023). The potentially inactivating components formed in the plasma can include charged particles, UV radiation, ozone and reactive species (Matthes et al. 2013). However, the exact mode of action of CAPP on microorganisms and arthropods is not well understood. In the present study, high resolution video sequence analysis and OCT showed that morphological changes occurred inside the examined female D. gallinae mites after exposure to CAPP. Like light microscopy, OCT has a resolution in the micrometer range. However, the advantage of OCT is that it can visualize the internal structures of a sample like ultrasonic imaging (Nawaz et al. 2023). In addition, no sample preparation is required, which means that OCT can be used repeatedly on living biological material (Berry 2022). This fast and non-contact method is widely used in medicine to visualize structures of the eye (Berry 2022; Lu et al. 2023). In recent years, it has also proved useful in entomology. OCT has been used to visualize the brain of honey bees (Apis mellifera), the heart of the fruit fly Drosophila melanogaster and the internal structures of the Indian meal moth (Plodia interpunctella) during its developmental cycle (Bizheva et al. 2004; Choma et al. 2006; Wolf et al. 2006). OCT has also been shown to be a suitable tool for phenotypic analysis in embryonic development of insects (Su et al. 2020). Similarly, the process of light adaptation in moths has been studied by visualizing the anatomical structures of the compound eye (Berry 2022). Our investigations showed a redistribution of the gut content up to the pretarsal segments of the limbs. Similarly, a displacement of the contents of the Malpighian tubules into the surrounding haemocoel was observed, suggesting a rupture of organ-confining structures. Similar findings were observed in plasma-exposed head lice (Pediculus humanus capitis) in OCT, showing a rupture between the midgut and the head, resulting in internal damage to the intestine. The lice showed leakage of digested blood from the ventricle into the surrounding hemolymph, which spread to the legs (ten Bosch et al. 2019; Bosch et al. 2022). In German cockroaches (Blattella germanica), exposure to high doses of plasma resulted in abdominal ruptures (Donohue et al. 2008). The cracks could be caused by the plasma-damaged walls of the organs in combination with the plasma- and/or current-induced heating or heating due to dielectric losses and the resulting increase in pressure inside the mite. The pressure destroys the walls of the internal hollow organs, allowing their contents to spill out. In PRM, the structure-limiting membranes are no longer separable after plasma treatment, which may also be caused by a change in the permeability of the peritrophic membrane. Plasma exposure leads to the destruction of internal organs, resulting in the distribution of intestinal contents and contents of the Malpighian tubules in the haemocoel. The PRMs died within the observation period. It is likely that digestive enzymes are released, leading to autolysis of the mite.
The whitish altered and denser hemolymph of the mites exposed to plasma in our study suggests that parts of the hemolymph were coagulated. The changes in the haemocoel could be due to the coagulation of proteins. These findings are similar to the coagulation of the hemolymph due to oxidative stress in cold plasma-treated red flour beetles (Tribolium castaneum) (Ramanan et al. 2018), but local thermal effects might also play a role. Free radicals produced by reactive oxygen species also lead to indirect effects on the DNA, immune system and circulation of insects (Sayed et al. 2021; Ji et al. 2019). In addition, CAPP affected the neuromuscular system of insects through surface electrostatic effects, causing ataxia, loss of vibrotaxis, phototaxis and thigmotaxis (Donohue et al. 2006).
In vivo imaging by OCT allowed real-time detection of the mite body shrinkage immediately after plasma exposure. The plasma-exposed mites appeared desiccated. Dehydration after plasma exposure has previously been observed in German cockroaches (Donohue et al. 2008) and head lice (ten Bosch et al. 2019). In addition to the direct and indirect thermal effects of plasma described above, Donohue et al. (2008) proposed that dehydration can be caused by the breakdown of C-H bonds in the lipid layer of the insect cuticle, which ultimately leads to death. Similarly, exposure to cold plasma resulted in body malformation in red flour beetles (Ramanan et al. 2018) and head lice (ten Bosch et al. 2019). The shrinkage could be due to water loss as a result of increased respiratory or metabolic rates, probably due to the effects on the nervous and neuromuscular systems (Donohue et al. 2006). These desiccation effects can also be observed after the use of inert dusts due to the destruction of the protective wax layer of the mite cuticle, resulting in increased cuticle permeability and fluid loss (Ebeling 1971). Dorsally and ventrally, the idiosoma is stabilised by sclerotized shields (dorsal: dorsal shield; ventral: sternal shield, genitoventral shield, anal shield), presumably making it more resistant to changes in length and width than to changes in height.
PRM is usually controlled with environmental chemical acaricides or acaricidal drugs (Sommer 2011; Decru et al. 2020). The use of pesticides should be conscientious and minimized to reduce the impact on animals, humans and the environment (Decru et al. 2020). In addition, this could also help to prevent the development of resistance and prolong the availability of effective acaricides. Effective long-term methods against PRM are needed to minimize high control costs, animal losses and production losses. There is an urgent need for alternative control methods due to the development of resistance to chemical acaricides and increasing consumer demand for chemical-free food (Soulié et al. 2021). CAPP has shown promising in vitro acaricidal activity (Rüster et al. 2022). Due to the plasma-based mechanical damage to the hollow organs and the supposed multimodal mode of action, resistance is unlikely, in contrast to environmental chemical acaricides or acaricidal drugs (Heinlin et al. 2010). The use of CAPP as an alternative physical control method, if successfully transferred into practice, may help to reduce the use of environmental chemical acaricides and acaricidal drugs to control PRM in laying hen houses (Rüster et al. 2022). As a chemical-free method, CAPP could make a valuable contribution to animal health by reducing the number of infections requiring treatment and improving housing conditions, thereby improving animal welfare. Results to date have shown that even short-term application of CAPP is effective against PRM (Rüster et al. 2022). This cold plasma technology for practical use in occupied laying hen flocks is currently under development. The plasma system is designed as a box that can be used by the mites as a shelter and hiding place during the light phase, based on mite traps with a corrugated cardboard structure (Rüster et al. 2022). The plasma system can be used in close proximity to the host without the chickens coming into direct contact with the plasma technology (Rüster et al. 2022). This could be achieved, for example, by placing the box on the PRM’s path to or from the host, e.g. under the perches. The method could be adapted to current modular housing systems without major structural changes. Further research is needed to clarify the possible influence of stable climatic parameters such as dust pollution, humidity, noxious gases such as ammonia or hydrogen sulfide, etc. on the functionality of the plasma sources (Rüster et al. 2022). In addition, it is necessary to determine how many plasma systems are required depending on the size of the house, the housing system and the number of laying hens in order to achieve a sustainable effect on the mite population. These questions can only be answered in a field study once practical devices for mite control in occupied poultry houses have been produced. In addition, the exact mechanisms of action of CAPP need to be further investigated in order to realize its full potential for pest control.
Data availability
No datasets were generated or analysed during the current study.
References
Abbas R, Colwell D, Iqbal Z, Khan A (2014) Acaricidal drug resistance in poultry red mite (Dermanyssus Gallinae) and approaches to its management. World’s Poult Sci J 70:113–124. https://doi.org/10.1017/S0043933914000105
Ali W, George D, Shiel R, Sparagano O, Guy JH (2012) Laboratory screening of potential predators of the poultry red mite (Dermanyssus Gallinae) and assessment of Hypoaspis miles performance under varying biotic and abiotic conditions. Vet Parasitol 187:341–344. https://doi.org/10.1016/j.vetpar.2012.01.014
Alves LFA, Johann L, Oliveira DGP (2023) Challenges in the biological control of pests in poultry production: a critical review of advances in Brazil. Neotrop Entomol 52:292–301. https://doi.org/10.1007/s13744-022-01021-1
Ansari MS, Moraiet MA, Ahmad S (2014) Insecticides: impact on the environment and human health. In: Environmental deterioration and human health: natural and anthropogenic determinants, pp. 99–123
Anuntagool J, Srangsomjit N, Thaweewong P, Alvarez G (2023) A review on dielectric barrier discharge nonthermal plasma generation, factors affecting reactive species, and microbial inactivation. Food Control 153:109913. https://doi.org/10.1016/j.foodcont.2023.109913
Berry S (2022) The use of optical coherence tomography to demonstrate dark and light adaptation in a live moth. Environ Entomol 51:643–648. https://doi.org/10.1093/ee/nvac105
Bizheva KK, Unterhuber A, Hermann BM, Považay B, Sattmann H, Drexler W, Stingl A, Le TM, Mei M, Holzwarth R, Reitsammer H, Morgan JE, Cowey A (2004) Imaging ex vivo and in vitro brain morphology in animal models with ultrahigh resolution optical coherence tomography. J Biomed Opt 9:719–724. https://doi.org/10.1117/1.1756920
Brandenburg R, Bogaerts A, Bongers W, Fridman A, Fridman G, Locke BR, Miller V, Reuter S, Schiorlin M, Verreccken T, Ostrikov K (2019) White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process Polym 16:1700238. https://doi.org/10.1002/ppap.201700238
Carpen L, Chireceanu C, Teodorescu M, Chiriloaie A, Teodoru A, Dinescu G (2019) The effect of argon/oxygen and argon/nitrogen atmospheric plasma jet on stored products pests. Rom J Phys 64:1–11
Choma MA, Izatt SD, Wessells RJ, Bodmer R, Izatt JA (2006) Images in cardiovascular medicine: in vivo imaging of the adult Drosophila melanogaster heart with real-time optical coherence tomography. Circulation 114:35–36. https://doi.org/10.1161/circulationaha.105.593541
Decru E, Mul M, Nisbet AJ, Vargas Navarro AH, Chiron G, Walton J, Norton T, Roy L, Sleeckx N (2020) Possibilities for IPM strategies in European laying hen farms for improved control of the poultry red mite (Dermanyssus Gallinae): details and state of affairs. Front Vet Sci 7:565866. https://doi.org/10.3389/fvets.2020.565866
Donohue KV, Bures BL, Bourham MA, Roe RM (2006) Mode of action of a novel nonchemical method of insect control: atmospheric pressure plasma discharge. J Econ Entomol 99:38–47. https://doi.org/10.1093/jee/99.1.38
Donohue KV, Bures BL, Bourham MA, Roe RM (2008) Effects of temperature and molecular oxygen on the use of atmospheric pressure plasma as a novel method for insect control. J Econ Entomol 101:302–308. https://doi.org/10.1093/jee/101.2.302
Ebeling W (1971) Sorptive dusts for pest control. Annu Rev Pest Control 16:123–158. https://doi.org/10.1146/annurev.en.16.010171.001011
Faghihzadeh Gorji S, Faghihzadeh Gorji S, Rajabloo M (2014) The field efficacy of garlic extract against Dermanyssus gallinae in layer farms of Babol, Iran. Parasitol Res 113:1209–1213. https://doi.org/10.1007/s00436-014-3759-2
FAO (2020) Briefing note: Integrated pest management. https://www.fao.org/3/cb1199en/CB1199EN.pdf. Accessed 10 February 2024
Flochlay AS, Thomas E, Sparagano O (2017) Poultry red mite (Dermanyssus Gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasit Vectors 10:16. https://doi.org/10.1186/s13071-017-2292-4
Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, Steffes T, Nosenko T, Zimmermann JL, Karrer S (2010) Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol 25:1–11. https://doi.org/10.1111/j.1468-3083.2010.03702.x
Hwang ET (2023) Management of the poultry red mite Dermanyssus gallinae with physical control methods by inorganic material and future perspectives. Poult Sci 102:102772. https://doi.org/10.1016/j.psj.2023.102772
Jannasch A, Schnabel C, Galli R, Faak S, Büttner P, Dittfeld C, Tugtekin SM, Koch E, Matschke K (2021) Optical coherence tomography and multiphoton microscopy offer new options for the quantification of fibrotic aortic valve disease in ApoE–/– mice. Sci Rep 11:5834. https://doi.org/10.1038/s41598-021-85142-4
Ji WO, Lee MH, Kim GH, Kim EH (2019) Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-56160-0
Kilpinen O, Steenberg T (2009) Inert dusts and their effects on the poultry red mite (Dermanyssus Gallinae). Exp Appl Acarol 48:51–62
Kim SI, Yi JH, Tak JH, Ahn YJ (2004) Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 120:297–304. https://doi.org/10.1016/j.vetpar.2003.12.016
Kirkwood A (1963) Longevity of the mites Dermanyssus gallinae and Liponyssus Sylviarum. Exp Parasitol 14:358–366. https://doi.org/10.1016/j.vetpar.2003.12.016
Lesna I, Sabelis MW, Van Niekerk TGCM, Komdeur J (2012) Laboratory tests for controlling poultry red mites (Dermanyssus Gallinae) with predatory mites in small ‘laying hen’ cages. Exp Appl Acarol 58:371–383. https://doi.org/10.1007/s10493-012-9596-z
Liebisch A, Liebisch G (2003) Biologie, Schäden Und Bekämpfung Beim Befall Durch die rote vogelmilbe (Dermanyssus Gallinae). Lohmann Inform 4:1
Lima-Barbero JF, Villar M, Höfle U, de la Fuente J (2020) Challenges for the control of poultry red mite (Dermanyssus Gallinae). In: Pacheco GAB, Kamboh AA (eds) Parasitol Microbiol Res. IntechOpen, London, pp 233–253. https://doi.org/10.5772/intechopen.90439
Locher N (2010) Untersuchungen zur Wirksamkeit eines Neem-Präparates (Mite-Stop®) auf die Entwicklungsstadien der Roten Vogelmilbe Dermanyssus gallinae. Dissertation. Freie Universität Berlin
Lu Y, Shen Y, Xing X, Ye C, Meng MQ-H (2023) Boundary-enhanced semisupervised retinal layer segmentation in optical coherence tomography images using fewer labels. Comput Med Imaging Graph 105:102199. https://doi.org/10.1016/j.compmedimag.2023.102199
Marangi M, Cafiero MA, Capelli G, Camarda A, Sparagano O, Giangaspero A (2009) Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp Appl Acarol 48:11–18. https://doi.org/10.1007/s10493-008-9224-0
Matthes R, Koban I, Bender C, Masur K, Kindel E, Weltmann KD, Kocher T, Kramer A, Hübner NO (2013) Antimicrobial efficacy of an Atmospheric pressure plasma Jet Against biofilms of Pseudomonas aeruginosa and Staphylococcus epidermidis. Plasma Processes Polym 10:161–166. https://doi.org/10.1002/ppap.201100133
Maurer V, Perler E (2006) Silicas for control of the poultry red mite Dermanyssus gallinae Proceedings of the European Joint Organic Congress, Odense, Denmark. pp. 504–505
Maurer V, Perler E, Heckendorn F (2009) In vitro efficacies of oils, silicas and plant preparations against the poultry red mite Dermanyssus Gallinae. In: Sparagano O (ed) Control of Poultry mites (Dermanyssus). Springer, Dordrecht, pp 31–41. https://doi.org/10.1007/978-90-481-2731-3_5
Mul M (2013) Fact sheet Poultry Red Mite in Europe. https://www.researchgate.net/publication/258553789_Fact_sheet_Poultry_Red_Mite_in_Europe. Accessed 10 February 2024
Mul M (2017) Advancing integrated pest management for Dermanyssus gallinae in laying hen facilities. Doctoral Thesis. Wageningen University and Research. https://doi.org/10.18174/394911
Mul MF, Koenraadt C (2009) Preventing introduction and spread of Dermanyssus gallinae in poultry facilities using the HACCP method. Exp Appl Acarol 48:167–181. https://doi.org/10.1007/s10493-009-9250-6
Nasr M, Zinhoum RA, Lotfy K (2020) Efficacy of cold plasma against three of stored grain insects. Int J Entomol Res 5:113–117
Nawaz M, Uvaliyev A, Bibi K, Wei H, Abaxi SMD, Masood A, Shi P, Ho H-P, Yuan W (2023) Unravelling the complexity of Optical Coherence Tomography image segmentation using machine and deep learning techniques: a review. Comput Med Imaging Graph, 102269
Nordenfors H, Höglund J (2000) Long term dynamics of Dermanyssus gallinae in relation to mite control measures in aviary systems for layers. Bri Poult Sci 41:533–540. https://doi.org/10.1080/713654991
Nunn F, Baganz J, Bartley K, Hall S, Burgess S, Nisbet AJ (2020) An improved method for in vitro feeding of adult female Dermanyssus gallinae (poultry red mite) using Baudruche membrane (goldbeater’s skin). Parasit Vectors 13:1–6
Ohta T (2016) Chap. 8– Plasma in agriculture. In: Misra NN, Schlüter O, Cullen PJ (eds.) Cold Plasma in Food and Agriculture Academic Press, San Diego, pp. 205–221. https://doi.org/10.1016/B978-0-12-801365-6.00008-1
Ramanan KR, Sarumathi R, Mahendran R (2018) Influence of cold plasma on mortality rate of different life stages of Tribolium castaneum on refined wheat flour. J Stored Prod Res 77:126–134. https://doi.org/10.1016/j.jspr.2018.04.006
Roy L, Eladouzi M, Moraza ML, Chiron G, Villeneuvede Janti E, Lepeutrec G, Bonato O (2017) Arthropod communities of laying hen houses: an integrative pilot study toward conservation biocontrol of the poultry red mite Dermanyssus Gallinae. Biol Control 114:176–194. https://doi.org/10.1016/j.biocontrol.2017.08.006
Rüster V, Werner H, Wieneke S, Avramidis G, ten Bosch L, Krause ET, Strube C, Bartels T (2022) Short-time cold atmospheric pressure plasma exposure can kill all life stages of the poultry red mite Dermanyssus gallinae under laboratory conditions. Exp Appl Acarol 88:139–152. https://doi.org/10.1007/s10493-022-00751-6
Rüster V, Lückemann AK, Wittmann M, Strube C, Bartels T (2023) Successful long-term control of poultry red mite (Dermanyssus Gallinae) infestations in floor-kept laying hens via integrated pest management—a case report. Parasitol Res 122:2549–2555. https://doi.org/10.1007/s00436-023-07954-9
Sayed WAA, Hassan RS, Sileem TM, Rumpold BA (2021) Impact of plasma irradiation on Tribolium castaneum. J Pest Sci 94:1405–1414. https://doi.org/10.1007/s10340-021-01360-9
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinivez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Schlesinger WH, Bernhardt ES (2020) The Atmosphere. Biogeochemistry 2020:51–97. https://doi.org/10.1016/B978-0-12-814608-8.00003-7
Schnabel C, Gaertner M, Kirsten L, Meissner S, Koch E (2013) Total liquid ventilation: a new approach to improve 3D OCT image quality of alveolar structures in lung tissue. Opt Express 21:31782–31788. https://doi.org/10.1364/OE.21.031782
Schnabel C, Jannasch A, Faak S, Waldow T, Koch E (2014) Ex vivo 4D visualization of aortic valve dynamics in a murine model with optical coherence tomography. Biomed Opt Express 5:4201–4212. https://doi.org/10.1364/BOE.5.004201
Schulz J, Berk J, Suhl J, Schrader L, Kaufhold S, Mewis I, Hafez HM, Ulrichs C (2014) Characterization, mode of action, and efficacy of twelve silica-based acaricides against poultry red mite (Dermanyssus Gallinae) in vitro. Parasit Res 113:3167–3175. https://doi.org/10.1007/s00436-014-3978-6
Sommer D (2011) Die Rote Vogelmilbe, Dermanyssus gallinae de Geer, 1778, ein experimentell nachgewiesener mechanischer Vektor von Influenza a-virus und versuche zur Bekämpfung der roten Vogelmilbe mit einem Phenolderivat. Doctoral Thesis, Justus-Liebig-Universität Giessen
Soulié A, Sleeckx N, Roy L (2021) Repellent properties of natural substances against Dermanyssus gallinae: review of knowledge and prospects for Integrated Pest Management. Acarol 61:3–19. https://doi.org/10.24349/acarologia/20214412
Sparagano O, George D, Harrington D, Giangaspero A (2014) Significance and control of the poultry red mite, Dermanyssus gallinae. Ann Rev Entomol 59:447–466. https://doi.org/10.1146/annurev-ento-011613-162101
Stafford KA, Lewis PD, Coles GC (2006) Preliminary study of intermittent lighting regimes for red mite (Dermanyssus Gallinae) control in poultry houses. Vet Record 158:762–763. https://doi.org/10.1136/vr.158.22.762
Su Y, Wei L, Tan H, Li J, Li W, Fu L, Wang T, Kang L, Yao XS (2020) Optical coherence tomography as a noninvasive 3D real time imaging tool for the rapid evaluation of phenotypic variations in insect embryonic development. J Biophotonics 13:201960047. https://doi.org/10.1002/jbio.201960047
ten Bosch L, Habedank B, Siebert D, Mrotzek J, Viöl W (2019) Cold atmospheric pressure plasma comb—a physical approach for pediculosis treatment. Int J Environ Res Public Health 16:1–16. https://doi.org/10.3390/ijerph16010019
ten Bosch L, Habedank B, Candeo A, Bassi A, Valentini G, Gerhard C (2022) Light sheet fluorescence microscopy for the investigation of blood-sucking arthropods dyed via artificial membrane feeding. Parasit Vectors 15:1–8. https://doi.org/10.1186/s13071-022-05157-2
Ueno T, Mizobe Y, Ninomiya J, Inoue T, Furukawa T, Hatta T (2023) Studies on the control of Dermanyssus Gallinae via High-Voltage impulse. Electronics 12:1038. https://doi.org/10.3390/electronics12041038
Ulrichs C, Han YJ, Abdelhamid MT, Mewis I (2020) Management of the poultry red mite, Dermanyssus Gallinae, using silica-based acaricides. Exp Appl Acarol 82:243–254. https://doi.org/10.1007/s10493-020-00541-y
Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA (2006) Drosophila as a model for the identification of genes causing adult human heart disease. Proc Natl Acad Sci 103:1394–1399. https://doi.org/10.1073/pnas.0507359103
Zhang X, Liu D, Zhou R, Song Y, Sun Y, Zhang Q, Niu J, Fan H, Yang SZ (2014) Atmospheric cold plasma jet for plant disease treatment. Appl Phys Lett 104:043702. https://doi.org/10.1063/1.4863204
Zoons J (2004) The effect of light programs on red mite (Dermanyssus gallinae) in battery cage housing. In: Perry, GC (Ed.) Welfare of the Laying Hen, p. 416 (CABI)
Funding
The project was supported by funds of the German Government’s Special Purpose Fund held at Landwirtschaftliche Rentenbank (grant number 863 622).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: Vanessa Rüster, Henrik Werner, Christian Schnabel, Stephan Wieneke, Georg Avramidis, Christina Strube, Thomas Bartels; Methodology, formal analysis and investigation: Vanessa Rüster, Henrik Werner, Christian Schnabel, Thomas Bartels; Writing - original draft preparation: Vanessa Rüster; Writing - review and editing: Henrik Werner, Christian Schnabel, Stephan Wieneke, Georg Avramidis, Christina Strube, Thomas Bartels; Supervision: Christina Strube, Thomas Bartels. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Dermanyssus gallinae maintenance by experimental infestation of chickens was permitted by the ethics commission of the Animal Care and Use Committee of the German Lower Saxony State Office for Consumer Protection and Food Safety (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) under reference number 33.19-42502-04-22-00109.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rüster, V., Werner, H., Avramidis, G. et al. Morphological changes in plasma-exposed poultry red mites (Dermanyssus gallinae) using high-resolution video camera and optical coherence tomography (OCT). Exp Appl Acarol (2024). https://doi.org/10.1007/s10493-024-00934-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10493-024-00934-3