Abstract
Amblyomma species are non-endemic ticks in Egypt, which have been recorded from imported animals. This study was carried out in 2022 to monitor Amblyomma spp. from dromedary camels, cattle, and snakes in Egypt. During this study, 400 camels, 200 cattle, and two snakes (Pythonidae) were inspected for tick infestation. Collected specimens were identified based on morphological characters and confirmed by phylogenetic analysis of the 12S rRNA gene. Camels were infested by adult specimens of Amblyomma variegatum and Amblyomma lepidum, but no Amblyomma spp. were collected from cattle. Amblyomma variegatum showed high genetic similarity to other A. variegatum from Guinea-Bissau and São Tomé (> 99.99%), and A. lepidum showed high genetic similarity to other A. lepidum from Israel and Sudan (99.99%). Amblyomma latum is recorded in Egypt from the ball python snake for the first time and showed high genetic similarity with South African A. latum (99.87%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Amblyomma is represented by approximately 138 species worldwide, ca. 20% of all world tick species (Guglielmone et al. 2015; Okely et al. 2022). Species of this genus are distributed in Neotropical, Afrotropical, and Australasian regions, with the highest diversity in the Neotropical region (Barker and Burger 2018; Horak et al. 2018). A wide range of pathogens has been reported in ticks from this genus, such as Borrelia spp., Ehrlichia spp., Rickettsia spp., and Anaplasma spp. (Ogrzewalska et al. 2019; Jiang et al. 2021; Saijuntha et al. 2021; Qiu et al. 2021; Vieira et al. 2022).
In Egypt, Amblyomma ticks are represented by only six non-endemic species (Okely et al. 2022). In neighboring regions, Amblyomma spp. have been collected from domestic animals such as dromedary camels and cattle in countries such as Sudan, Ethiopia, Nigeria, and Somalia (Okely et al. 2021). These species are Amblyomma eburneum, Amblyomma gemma, Amblyomma hebraeum, Amblyomma lepidum, Amblyomma marmoreum, and Amblyomma variegatum (Robinson 1926; Hoogstraal 1952, 1956; El Kammah et al. 2001, 2007; Ghoneim et al. 2017, 2020; Elhelw et al. 2021; Okely et al. 2021). Amblyomma eburneum was reported in Egypt from imported cattle to Cairo only once (Robinson 1926). Ghoneim et al. (2017, 2020) recorded A. hebraeum from imported dromedary camels to Basatin abattoir, Cairo. Amblyomma marmoreum was recorded by El Kammah et al. (2001, 2007), but without data about its host. Amblyomma gemma, A. lepidum, and A. variegatum have been reported in Egypt in several studies (Hoogstraal 1952, 1956; Youssef et al. 2015; Ghoneim et al. 2017, 2020; Elhelw et al. 2021; Okely et al. 2021).
The snake tick, Amblyomma latum, is a hard tick species with an Afrotropical distribution, especially prevalent in sub-Saharan African countries (Guglielmone and Robbins 2018). Several families of squamates have been recorded as the principal hosts for all parasitic stages, with immature stages also reported from rodents (Muridae), and adults occasionally collected from anurans, tortoises, rodents, and soricomorphs (Guglielmone et al. 2014; Horak et al. 2018).
In the present study, we confirm the presence of Amblyomma species in Egypt using morphological and molecular identification and estimate phylogenetic relationships between the recorded species in Egypt and other Amblyomma spp. from GenBank records. Additionally, we report the first record of A. latum infesting a snake in Egypt.
Materials and methods
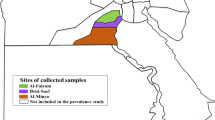
In 2022, several collection trips were conducted to monitor Amblyomma tick species from domestic and wild animals in Egypt. In total 600 domestic animals (400 dromedary camels and 200 cattle), and two snakes belonging to family Pythonidae were inspected for Amblyomma species infestation. Camels, cattle, and snakes were examined monthly, twice, and only once, respectively. Camels were examined in the Birqash camel market, cattle were examined in cattle farms, and snakes were examined in markets for live snake trade (Figure S1). The collection sites of each animal were georeferenced using ArcGIS v.10.3 mapping software (Esri, Redlands, CA, USA) (Table 1).
All Amblyomma spp. specimens were removed from animals using fine forceps and then stored in vials containing 70% ethyl alcohol for preservation. Drops of 20% glycerol were added for morphological identification in the Parasitology Laboratory, Department of Entomology, Ain Shams University. Specimens were identified to the species level based on morphological characters using diagnostic characters and identification keys (Hoogstraal 1956; Voltzit and Keirans 2003; Walker et al. 2003; Okely et al. 2021). Morphological characters were observed using a CZM4 Stereo Microscope (Labomed, Fremont, CA, USA) with an Am Scope LED-144 W-ZK white adjustable luminance. Species were photographed using an Am Scope MU1000 10MP Microscope camera (Am Scope, Irvine, CA, USA). Specimens preserved in 70% ethanol were processed for extraction of DNA. DNA was extracted utilizing QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. DNA was stored at -20 °C until use. PCR targeted amplification of the 12S rRNA gene using primers (5´-GAGGAATTTGCTCTGTAATGG-3´ and 5´-AAGAGTGACGGGCGATATGT-3´) according to Norris et al. (1999).
Amplified PCR products were excised from agarose gel, purified, and sent for sequencing. Sanger sequencing was performed by Solgent (Daejeon, South Korea). Sequences were then analyzed using BLAST (Johnson et al. 2008) to confirm they belonged to Amblyomma. Sequence data (GenBank acc. nrs. OP775457, OP785088, OP785089, and OP783984) were compiled with GenBank reference data to represent Afrotropical and related Amblyomma (32 sequences). Misidentified tick species in GenBank sequence data is a growing problem that can only be addressed with relatively complete taxon sampling of the underlying evolutionary tree. Phylogenetic tree estimation of the collected Amblyomma tick species from Egypt and other Amblyomma species available at the GenBank database used the hard tick Ixodes spp. (L43903, MF095802, and MF095798) as outgroup taxa. Sequence data were aligned using MAFFT (Q-INS-i, 200PAM/k = 2; gap opening penalty, 1.53) (Katoh et al. 2002). The optimal nucleotide substitution model was selected using BIC calculations in MEGA v.7.0.14 (Kumar et al. 2016) and was determined as TN93 + G with 5 gamma rate categories. Maximum likelihood analysis was performed in MEGA using 1000 bootstrap replications.
Results
Morphological identification
In total 66 individual Amblyomma ticks were collected (Table 2). Camels were infested by adult A. lepidum and A. variegatum. One of the two snakes was infested by only one A. latum nymph. The key diagnostic morphological characters that differentiate A. lepidum from A. variegatum are: mesial area of enamel ornamentation with dense, coarse punctations in A. lepidum, but smooth in A. variegatum; lateral median areas of enamel ornamentation large in A. lepidum, while absent in A. variegatum; festoons with enamel on 6–8 festoons of 11 festoons in A. lepidum, whereas the festoons are without enamel in A. variegatum; medial and posterior margins of the spiracular plate slightly straight, dorsal prolongation relatively broad, nearly perpendicular to axis of spiracular plate in A. lepidum, whereas medial and posterior margin of spiracular plate straight, dorsal prolongation triangular, very narrow, forming an obtuse angle with spiracular plate axis in A. variegatum (Figs. 1 and 2).
Phylogenetic analysis
Tick species identifications were confirmed by phylogenetic analysis of the 12S rRNA gene. Lengths of amplified 12S rRNA sequences in all examined Amblyomma species in Egypt were found to be similar (approx. 300 bp); the length of the alignment was ca. 250 bp after trimming the low-quality ends of each sequence. The 12S rRNA sequences of A. lepidum collected from imported camels showed 99.99% similarity and were shown as a monophyletic group with specimens of A. lepidum from Israel (KPU987776) and Sudan (LC612437 and LC612433) (bootstrap support: 96%) and formed an independent, but weakly supported, sister clade with A. neumanii (KU284862 and KU284861) from Argentina with a bootstrap value of 36. Sample of A. variegatum from Giza governorate collected from imported camels showed > 99.99% similarity and formed a monophyletic group to other A. variegatum from Guinea-Bissau (KU568495) and São Tomé (MH781752) (bootstrap support: 77), in a clade sister to both A. variegatum (KY688458) and A. sparsum (AF150047) (indicating potential misidentified GenBank data). Amblyomma latum 12S rRNA sequences from Faiyum governorate and collected from imported ball python snake showed 99.87% similarity. They formed a monophyletic group to other A. latum (OP677567 and OP677566) reported from South Africa, albeit with some distinct phylogenetic distance (bootstrap support: 99) (Fig. 3).
Discussion
Correct species identification of tick specimens is a crucial step to better understanding tick ecology, host-use patterns, life cycles, and epidemiology (Estrada-Peña et al. 2017; Okely et al. 2021; Perveen et al. 2021). Identifying ticks to species level may be based on morphological characters and molecular markers including 16S rRNA, 12S rRNA, cox1, 18S rRNA, 28S rRNA, ITS1 rDNA, and ITS2 rDNA (Cruickshank 2002; Nava et al. 2009; Araya-Anchetta et al. 2015; Estrada-Peña et al. 2017). In this study, we identified Amblyomma species recorded in Egypt using morphological characters and sequence data for the 12S rRNA gene. Although A. latum could not be identified using morphological features due to specimen damage, it was identified using molecular data in a phylogenetic context. As such, molecular tick identification helps overcome some difficulties associated with morphological identification (Abouelhassan et al. 2019).
In the present study, A. lepidum and A. variegatum infested imported camels; the highest number of specimens collected were A. lepidum. Our findings are in agreement with previous studies (Abdel-Shafy and Allam 2013; Hassan et al. 2017; Okely et al. 2021), which reported these species from imported camels in camel markets in Egypt. Phylogenetic analysis showed relatedness between the study sample sequences and the same species from other African countries such as Sudan, Guinea-Bissau, and Uganda. No Amblyomma species were collected from cattle because the cattle examined during this study were native to Egypt and not imported from other countries. As in the previous studies, Amblyomma species were not recorded from native cattle in Egypt. However, there are studies reporting A. lepidum and A. variegatum from imported cattle to Egypt (Robinson 1926; Hoogstraal 1956; Okely et al. 2022). Hence our results and previous studies suggest that native cattle were not infected by Amblyomma spp. in Egypt.
Illegal wildlife trade is a practice bringing several ecological and public health concerns, such as the spreading of zoonotic pathogens and their diseases continue to emerge and the introduction of exotic animals into new geographical areas (Brianti et al. 2010; Bezerra-Santos et al. 2021). There is growing concern that international trade without existing legislation and regulations will contribute to a loss in biological diversity and the extinction of several animal species worldwide by emerging novel infectious diseases (Smith et al. 2009). The trade in live reptiles has increased in recent years worldwide, which threatens wildlife conservation and public health (Franke and Telecky 2001). International trade in exotic reptiles increases the chance of ectoparasites and their pathogens to be introduced into new geographical locations (Burridge 2001). Tick species are the most reported parasites on internationally traded exotic reptiles (Mihalca 2015) and investigated infectious agents in imported reptiles and ectoparasitic ticks are a public health concern (Takano et al. 2010).
The number of ticks introduced to new exotic locations has increased and caused problems even in developed countries (Burridge 2001). The importation of exotic ticks into a new geographic area via reptiles has been reported in several countries worldwide. For example, In Italy, tortoises imported from North Africa were infested by ticks (Brianti et al. 2010); Mihalca (2015) reviewed the ticks imported to Europe with exotic reptiles, and the tick species belonged to the genera Hyalomma and Amblyomma. Several Amblyomma species have been reported in Poland for the first time by the international trade in reptiles (Nowak 2010). In the USA, Amblyomma dissimile was identified for the first time from imported reptiles (Pietzsch et al. 2006). Recently, there has been extensive trade of exotic reptiles, especially snakes as pets in Egypt (Satour and Dewir 2018; Jensen et al. 2019). Herein, the first record is reported for the A. latum from an African Python imported to Egypt.
Phylogenetic analysis showed that the collected A. latum sequence is closely related to specimens from South Africa, despite some population-level sequence divergence. Distinct branch length but the high similarity between Egyptian and South African A. latum (Fig. 3) suggest some minor population-level divergence effects over geographic distance, possibly due to the limited dispersal ability of reptile hosts. Historically, A. latum was detected outside the Afrotropical zoogeographic region and introduced to different parts of the world probably due to the international trade in live reptiles (Guglielmone et al. 2014). Amblyomma latum was collected from imported pythons in Florida, USA (Nearctic region) (Burridge et al. 2006; Corn et al. 2014) and Python regius imported into Argentina and Chile (Neotropical region) (González-Acuña et al. 2005). In Japan (Palaearctic region), this species infested P. regius imported from Ghana (Goka et al. 2013; Andoh et al. 2015) and Phelsuma dubia imported from Madagascar (Qiu et al. 2021). Amblyomma latum was collected from Varanus exanthematicus imported from Ghana to Poland (Palaearctic region) (Nowak 2010).
Finally, this study recommends the monitoring of tick species and their pathogens on imported wild and domestic animals from different ecological zones in Egypt.
References
Abdel-Shafy S, Allam NA (2013) Quantitative real-time RT-PCR detection of flaviviruses associated with camel ticks in Egypt. Glob Vet 10:394–402
Abouelhassan EM, El-Gawady HM, Abdel-Aal AA, El-Gayar AK, Esteve-Gassent MD (2019) Comparison of some molecular markers for tick species identification. J Arthropod Borne Dis 13(2):153–164
Andoh M, Sakata A, Takano A, Kawabata H, Fujita H, Une Y, Goka K, Kishimoto T, Ando S (2015) Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PLoS ONE 10(7):e0133700
Araya-Anchetta A, Busch JD, Scoles GA, Wagner DM (2015) Thirty years of tick population genetics: a comprehensive review. Infect Genet Evol 29:164–179
Barker SC, Burger TD (2018) Two new genera of hard ticks, Robertsicus n. gen. and Archaeocroton n. gen., and the solution to the mystery of Hoogstraal’s and Kaufman’s “primitive” tick from the Carpathian Mountains. Zootaxa 4500(4):543–552
Bezerra-Santos MA, Mendoza-Roldan JA, Thompson RCA, Dantas-Torres F, Otranto D (2021) Illegal wildlife trade: a gateway to zoonotic infectious diseases. Trends Parasitol 37:181–184. https://doi.org/10.1016/j.pt.2020.12.005
Brianti E, Dantas-Torres F, Giannetto S et al (2010) Risk for the introduction of exotic ticks and pathogens into Italy through the illegal importation of tortoises, Testudo graeca. Med Vet Entomol 24:336–339. https://doi.org/10.1111/j.1365-2915.2010.00874.x
Burridge MJ (2001) Ticks (Acari: Ixodidae) spread by the international trade in reptiles and their potential roles in dissemination of diseases. Bull Entomol Res 91(1):3–23
Burridge MJ, Berube LR, Holt TJ (2006) Invasive ticks: introduction of Amblyomma kraneveldi (Anastos) and other exotic ticks (Acari: Ixodidae) into Florida on imported reptiles. Int J Acarol 32(3):315–321
Corn JL, Mertins JW, Hanson B, Snow S (2014) First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J Med Entomol 48(1):94–100
Cruickshank RH (2002) Molecular markers for the phylogenetics of mites and ticks. Syst Appl Acarol 7(1):3–14
El Kammah K, Oyoun L, El Kady G, Shafy S (2001) Investigation of blood parasites in livestock infested with argasid and ixodid ticks in Egypt. J Egypt Soc Parasitol 31(2):365–371
El Kammah K, Oyoun L, Shafy S (2007) Detection of microorganisms in the saliva and midgut smears of different tick species (Acari: Ixodoidea) in Egypt. J Egypt Soc Parasitol 37:533–539
Elhelw R, Elhariri M, Hamza D, Abuowarda M, Ismael E, Farag H (2021) Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet Res 17(1):1–9
Estrada-Peña A, D’Amico G, Palomar AM, Dupraz M, Fonville M, Heylen D, Habela MA, Hornok S, Lempereur L, Madder M, Nuncio MS (2017) A comparative test of ixodid tick identification by a network of european researchers. Ticks Tick Borne Dis 8(4):540–546
Franke MS, Telecky TM (2001) Reptiles as pets: an examination of the trade in live reptiles in the United States. The Humane Society of the United States
Ghoneim NH, Abdel-Moein KA, Zaher HM (2017) Molecular detection of Francisella spp. among ticks attached to camels in Egypt. Vector Borne Zoonotic Dis 17:384–387. https://doi.org/10.1089/vbz.2016.2100
Ghoneim NH, Abdel-Moein KA, Zaher HM, Abuowarda MM (2020) Investigation of Ixodidae ticks infesting camels at slaughterhouse and its potential role in transmitting Coxiella burnetii in Egypt. Small Rumin Res 191:106173. https://doi.org/10.1016/j.smallrumres.2020.106173
Goka K, Okabe K, Takano A (2013) Recent cases of invasive alien mites and ticks in Japan: why is a regulatory framework needed? Exp Appl Acarol 59(1):245–261
González-Acuña D, Beldomenico PM, Venzal JM, Fabry M, Keirans JE, Guglielmone AA (2005) Reptile trade and the risk of exotic tick introductions into southern south american countries. Exp Appl Acarol 35(4):335–339
Guglielmone AA, Robbins RG (2018) Hard ticks (Acari: Ixodida: Ixodoidea) parasitizing humans. Springer, A global overview, p 313
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG (2014) The hard ticks of the world: (Acari: Ixodida: Ixodidae). Springer Dordrecht, Heidelberg, p 738
Guglielmone AA, Sanchez M, Franco L, Nava S, Rueda L, Robbins R (2015) Hard ticks (Acari: Ixodida: Ixodidae): a non-profit open-access web portal for original descriptions of tick species (valid and invalid), dubious and uncertain names, and selected nominanuda. Available from http://rafaela.inta.gob.ar/nombresgarrapatas/ (Accessed 11 November 2017)
Hassan MI, Gabr HS, Abdel-Shafy S, Hammad KM, Mokhtar MM (2017) Prevalence of tick-vectors of Theileria annulata infesting the one-humped camels in Giza, Egypt. J Egypt Soc Parasitol 47:425–432
Hoogstraal H (1952) Notes on Egyptian ticks (Ixodoidea). I. The genus Argas (Argasidae) in the Cairo area. Proc Egypt Acad Sci 7:114–127
Hoogstraal H (1956) African Ixodoidea. I. ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Department of the Navy, Bureau of Medicine and Surgery, US Naval Medical Research Unit 3, Cairo, Egypt. 1100 pp
Horak IG, Heyne H, Williams R, Gallivan GJ, Spickett AM, Bezuidenhout JD, Estrada-Peña A (2018) The ixodid ticks (Acari: Ixodidae) of southern Africa. Springer Cham, Switzerland, p 676
Jensen TJ, Auliya M, Burgess ND, Aust PW, Pertoldi C, Strand J (2019) Exploring the international trade in african snakes not listed on CITES: highlighting the role of the internet and social media. Biodivers Conserv 28(1):1–19
Jiang BG, Wu AQ, Jiang JF, Yuan TT, Xu Q, Lv CL, Chen JJ, Sun Y, Fang LQ, Ruan XD, Que TC (2021) Molecular detection of Novel Borrelia Species, CandidatusBorrelia javanense, in Amblyomma javanense ticks from pangolins. Pathogens 10(6):728
Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL (2008) NCBI BLAST: a better web interface. Nucl Acids Res 36:W5–W9. 101093/nar/gkn201
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Mihalca AD (2015) Ticks imported to Europe with exotic reptiles. Vet Parasitol 213(1–2):67–71
Nava S, Guglielmone A, Mangold A (2009) An overview of systematics and evolution of ticks. Front Biosc 14:2857–2877
Norris DE, Klompen JSH, Black WC (1999) Comparison of the mitochondrial 12S and 16S ribosomal DNA genes in resolving phylogenetic relationships among hard ticks (Acari: Ixodidae). Ann Entomol Soc Am 92(1):117–129
Nowak M (2010) The international trade in reptiles (Reptilia)—the cause of the transfer of exotic ticks (Acari: Ixodida) to Poland. Vet Parasitol 169(3–4):373–381
Ogrzewalska M, Machado C, Rozental T, Forneas D, Cunha LE, De Lemos ERS (2019) Microorganisms in the ticks Amblyomma dissimile Koch 1844 and Amblyomma rotundatum Koch 1844 collected from snakes in Brazil. Med Vet Entomol 33(1):154–161
Okely M, Anan R, Gad-Allah S, Samy AM (2021) Hard ticks (Acari: Ixodidae) infesting domestic animals in Egypt: diagnostic characters and a taxonomic key to the collected species. Med Vet Entomol 35(3):333–351
Okely M, Chen Z, Anan R, Gad-Allah S (2022) Updated checklist of the hard ticks (Acari: Ixodidae) of Egypt, with notes of livestock host and tick-borne pathogens. Syst Appl Acarol 27(5):811–838
Perveen N, Muzaffar SB, Al-Deeb MA (2021) Prevalence, distribution, and Molecular Record of Four Hard ticks from Livestock in the United Arab Emirates. Insects 12(11):1016
Pietzsch M, Quest R, Hillyard PD, Medlock JM, Leach S (2006) Importation of exotic ticks into the United Kingdom via the international trade in reptiles. Exp Appl Acarol 38:59–65
Qiu Y, Kidera N, Hayashi M, Fujishima K, Tamura H (2021) Rickettsia spp. and Ehrlichia spp. in Amblyomma ticks parasitizing wild amphibious sea kraits and yellow-margined box turtles in Okinawa, Japan. Ticks Tick Borne Dis 12(2):101636
Robinson LE (1926) The genus Amblyomma-ticks: a monograph of the Ixodoidea. Part IV
Saijuntha W, Petney TN, Andrews RH, Robbins RG (2021) Ticks: a largely unexplored factor in disease transmission. In: Mehlhorn H, Saijuntha W, Petney TN (eds) Biodiversity of Southeast Asian parasites and vectors causing human disease. Parasitology research monographs, vol 14. Springer, Cham, pp 165–182
Satour NS, Dewir AW (2018) Some internal parasites of reptiles in Alexandria Province, Egypt. EVMSPJ 14(1):98–114
Smith KF, Acevedo-Whitehouse K, Pedersen AB (2009) The role of infectious diseases in biological conservation. Anim Conserv 12(1):1–12. https://doi.org/10.1111/j.1469-1795.2008.00228.x
Takano A, Goka K, Une Y, Shimada Y, Fujita H, Shiino T et al (2010) Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ Microbiol 12:134–146
Vieira TS, Collere FC, Ferrari LD, Baggio RA, Lange RR, Ferrari MV, Duque JC, Sanches GS, Pereira NA, Aguiar DM, Labruna MB (2022) Novel Anaplasmataceae agents Candidatus Ehrlichia hydrochoerus and Anaplasma spp. Infecting Capybaras, Brazil. Emerg Infect Dis 28(2):480–482
Voltzit OV, Keirans JE (2003) A REVIEW OF AFRICAN AMBL YOMMA SPECIES (ACARI, IXODIDA, IXODIDAE). Acarina 11(2):135–214
Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, Pegram RG, Preston PM (2003) Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh, Scotland, UK, Bioscience Reports, p 221
Youssef SY, Yasien S, Mousa WMA, Nasr SM, El-Kelesh EAM, Mahran KM, Abd-El-Rahman AH (2015) Vector identification and clinical, hematological, biochemical, and parasitological characteristics of camel (Camelus dromedarius) theileriosis in Egypt. Trop Anim Health Prod 47:649–656. https://doi.org/10.1007/s11250-015-0771-1
Acknowledgements
We thank the Department of Entomology Ain Shams University for their support of this work. The authors gratefully thank the owners of the animals for their help in tick sampling. We greatly appreciated the help of Dr. Mohamed G E-D Nasser (Department of Entomology, Ain Shams University, Egypt) for his kind help in identifying the snake.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MO collected and provided the ticks. MO and LCD did morphological identification. EMA and MSK did DNA extraction and molecular tests. MO prepared Figs. 1 and 2. EMA did sequence submission. DKB did the genetic analysis and prepared Fig. 3. EMA, MSK, and MO wrote the first draft of the manuscript. LCD and DKB read, edited, and reviewed the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abouelhassan, E.M., Kamel, M.S., Chitimia‑Dobler, L. et al. Molecular screening of Amblyomma species (Acari: Ixodidae) imported from African countries to Egypt, with the first report of Amblyomma latum from the ball python, Python regius (Squamata: Pythonidae). Exp Appl Acarol 91, 123–132 (2023). https://doi.org/10.1007/s10493-023-00829-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00829-9