Abstract
Population density is one of the main socio-environmental factors that have critical impacts on reproduction of animals. Consequently, they need to adjust their reproductive strategies in response to changes of local population density. In this study we used a haplodiploid spider mite, Tetranychus ludeni Zacher (Acari: Tetranychidae), to test how population density dynamics during the reproductive period altered female reproductive performance. We demonstrate that females produced fewer eggs with a significantly higher female-biased sex ratio in dense populations. Reducing fecundity and increasing daughter production in a dense environment could be an advantageous strategy to minimise the intensity of local food competition. However, females also reduced their fecundity after arrival in a new site of larger area from a dense population, which may be associated with higher web production costs because females need to produce more webs to cover the larger area. There was no trade-off between egg number and size, and egg size had little impact on reproductive fitness. Therefore, T. ludeni females could adapt to the shift of population density during their reproductive period by manipulating the fecundity and offspring sex ratio but not the egg size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population density is one of the major components of social environments that can affect population dynamics. A local population density may vary over time due to aggregation (immigration), dispersal (emigration), or mortality (Roeder 1992; Roff 1992; Stearns 1992; Bowman et al. 2002; Schausberger et al. 2021). Animals may aggregate in a habitat to increase reproduction opportunities (e.g., Snead and Alcock 1985; Bengtsson 2008; Wheeler and Jr Welsh 2008; Le Goff et al. 2010; Pérez-González et al. 2010; Bonsignore and Jones 2014; DeVries et al. 2017. Dar et al. 2021) or to reduce predation risk (e.g., Spieler 2003; Morrell and James 2008; Yano 2012; Clotuche et al. 2014). However, aggregation may raise population density in the habitat, leading to intensive resource competition and reducing reproductive fitness (Li and Zhang 2021; Weerawansha et al. 2020, 2022). In this scenario, the reproductive females would disperse to seek new habitats for the next generation (Schaub and von Hirschheydt 2009; Azandémè-Hounmalon et al. 2014; Lutz et al. 2015; Kingma et al. 2017; Kusch et al. 2020; Manguette et al. 2020; Vaishali and Krushnamegh 2020; Schausberger et al. 2021; Zhou et al. 2021). With a few exceptions (e.g., Roeder 1992; Fox et al. 1997; Matsuura and Kobayashi 2010; Maenoa et al. 2020), studies on the effects of population density on reproductive plasticity have been carried out under constant population densities during female breeding time (e.g., Wrensch and Young 1978; Fischer et al. 2011; Weerawansha et al. 2020, 2022). To date, it is still unclear how females alter their reproductive strategies in response to the varying population density during their reproductive life.
After settling in new habitats, females are expected to adjust their reproductive strategies to optimize their fitness (Roff 1992; Stearns 1992; West et al. 2005; Fischer et al. 2011; Bowers et al. 2017; Maenoa et al. 2020; Weerawansha et al. 2022). For example, if the population is dense, females may lay fewer (van Noordwijk and de Jong 1986; Khan et al. 2018; Li and Zhang 2021) but larger eggs (Parker and Begon 1986; Sibly et al. 1988; Fischer et al. 2011), trading-offs the number with size of eggs to make best utilization of limited resources and maximise offspring fitness (Smith and Fretwell 1974; Parker and Begon 1986; Stearns 1992; Fox and Czesak 2000; Fischer et al. 2011; Macke et al. 2012; Walzer and Schausberger 2015; Maenoa et al. 2020). In species with sexual size dimorphism, resource-deficient females either reduce the egg size of the larger sex (Fox and Czesak 2000; Walzer and Schausberger 2013, 2015) or produce fewer eggs of the larger sex (Trivers and Willard 1973; Charnov 1982; Walzer and Schausberger 2015) to optimize their reproductive fitness returns. Moreover, if one sex is dispersive and the other is philopatric, females often skew investment towards philopatric offspring when local resources are abundant but allocate more resources to the dispersive sex when local resources are deficient (Clark 1978; Silk 1983, 1984; West et al. 2005; Hjernquist et al. 2009; West 2009).
Spider mites (Acari: Tetranychidae) are phytophagous invertebrates, often living as groups (Helle and Sabelis 1985; Le Goff et al. 2010; Schausberger et al. 2021) in discrete patches (Mitchell 1973; Nachappa et al. 2011; Sarwar 2013). Female adults are larger than male adults (Mitchell 1973) and thus more likely to compete for food with their mothers or siblings (Young et al. 1986). However, female adults, rather than male adults and immature nymphs, may disperse to found new colonies (Mitchell 1973; Brandenburg and Kennedy 1982) especially when the populations are crowded or when food is insufficient or poor in quality (Suski and Naegele 1968; McEnroe 1969). As spider mites are haplodiploid, mated females can manipulate offspring sex ratio by fertilizing relatively larger eggs that develop to daughters (Young et al. 1986; Roeder et al. 1996; Macke et al. 2011). It has been reported that females produce fewer eggs with more dispersing daughters in large and dense populations to reduce local competition for food (Weerawansha et al. 2022). Moreover, spider mites aggregate and cooperate in spinning silk webs for dispersal and protection against environmental hazards (Le Goff et al. 2010; Yano 2012), and group-living females produce more silk and lay more eggs per mite than single females (Le Goff et al. 2010). Therefore, spider mites should be able to adjust offspring sex ratio in response to the social environments.
Here, we used an invasive pest spider mite, Tetranychus ludeni Zacher (Zhang 2003), to examine how changes in population density during female reproductive life altered egg production and sex allocation. We simulated the aggregation by moving females from low to high population density and the dispersal by shifting females from high to low population density. We recorded the number and size of eggs laid and offspring sex ratio (i.e., proportion of daughters) before and after density changes. Based on the knowledge outlined above, we hypothesize that (1) females lay fewer but larger eggs and produce offspring with a more female-biased sex ratio in response to the aggregation scenario, and (2) the opposite case occurs in response to the dispersal scenario. This study provides insight into the mechanisms behind the adjustment of fecundity and sex ratio in response to the varying social environments.
Materials and methods
Mite colony
We maintained a colony of T. ludeni on kidney bean plants (Phaseolus vulgaris L.) in the laboratory—and carried out the experiment—at 25 ± 1 ºC, 40 ± 10% RH and L16:D8 h photoperiod. We used the first expanded leaves of 1- to 2-week-old plants for the experiment.
Experiment
To determine how females adjusted their fecundity and sex allocation in response to population density dynamics in T. ludeni, we set up two treatments, each with 32 leaf squares as replicates. Treatments 1 and 2 tested the effects of density changes from high to low (Fig. 1a) and from low to high (Fig. 1b), respectively. Briefly, we randomly selected the quiescent female deutonymphs just before emergence (silvery in colour) from the colony. We individually transferred them onto 1-cm2 leaf squares placed upside down on a water-saturated cotton pad in a Petri dish (9.5 cm diameter, 1 cm high) with a hole (1 cm diameter) in the middle of the lid covered by a fine metal mesh (aperture size 0.25 × 0.25 mm). We then introduced a newly emerged virgin male adult produced by virgin females onto each square. We monitored the pair until the end of copulation, after which time, we removed the male. For each replicate in Treatment 1, we introduced 16 newly mated females onto a 1-cm2 clean leaf square and allowed them to stay on the square for 1 day. We then transferred them to a new square daily for two consecutive days. On the 4th day, we randomly selected 16 of the 32 leaf squares and transferred mites from each leaf square onto a new 16-cm2 leaf square (from high to low density) and those from each of the remaining 16 leaf squares onto a new 1-cm2 leaf square (from hight to high density as control) daily for three consecutive days. The same procedure was carried out for Treatment 2 except that we transferred mites from low to high density and from low to low density (as control).
We checked each leaf square twice a day during the six oviposition days and replaced any dead females immediately with females of the same age and social experience. We recorded the number of eggs laid on each leaf square. To determine the egg size, we randomly selected 30 eggs from each leaf square and individually measured their diameter under a stereomicroscope (Leica MZ12, Germany) connected to a digital camera (Olympus SC30, Japan) and imaging software (CellSens GS-ST-v.1.7, Olympus, Japan). We calculated the egg radius (r = diameter/2) and egg size (volume = 4/3πr3). After eggs hatched, we transferred all live individuals onto a clean leaf square of the same size once every 5 days and recorded the sex of newly emerged adults.
Statistical analysis
We analysed all data using SAS v.9.4 with a rejection threshold set at α = 0.05. Data on the number of eggs laid and egg size were normally distributed (Shapiro-Wilk test; UNIVARIATE procedure). We analysed the data on egg number and size using a linear mixed model (GLM procedure) with treatment (i.e., density shift) as a main factor and replicate as a random factor, and a Tukey-Kramer test for multiple comparisons. The mean egg size and number for each female before and after density shift were calculated and used for analysis. The data on sex ratio (proportion of daughters) were analysed by a generalized linear model (GLIMMIX procedure) with a binomial distribution and a Logit link function after the model, and a Tukey-Kramer test was applied for multiple comparisons. A general linear regression model (GLM procedure) was applied to determine the relationships between egg size and number, between immature survival rate and egg size, and between sex ratio and egg size. The mean egg size and number, immature survival rate, and sex ratio for each female were used for regressions.
Results
Effect of population density shifts on fecundity, egg size and immature survival
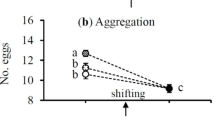
Our results show that females at low density laid significantly more eggs than at high density before density shift but laid significantly fewer eggs after the shift regardless of whether it was from high to low or from low to high (F5,107 = 28.79, P < 0.0001) (Fig. 2a). If the shift occurred at the same density levels, females produced similar numbers of eggs before and after shift (Fig. 2a). However, population density and its shift had no significant impact on egg size (F5,107 = 0.37, P = 0.87) (Fig. 2b). Moreover, increasing number of eggs laid did not significantly reduce the egg size (Fig. 3a) and egg size had no significant impact on immature survival rate (Fig. 3b).
Effects of female population shift between high (black dots) and low (white dots) densities on the mean (± SE) number of eggs laid (a) and egg size (b) in Tetranychus ludeni. Means within a panel with the same letters are not significantly different (Tukey-Kramer test: P > 0.05). Female population density shift occurred on the 4th day of oviposition period
Effect of population density shifts on sex allocation
We demonstrate that the sex ratio (proportion of daughters) was significantly higher at high density than at low density regardless of shifts; density shift from high to high, from low to low, or from low to high significantly increased the sex ratio, and density shift from high to low significantly reduced the sex ratio (F5,122 = 11.26, P < 0.0001) (Fig. 4). Egg size had no significant impact on sex ratio (F1,126 = 0.03, P = 0.86) (Fig. 5).
Effects of female population shift between high (black dots) and low (white dots) densities on the mean (± SE) sex ratio (proportion of females) in Tetranychus ludeni. Means with the same letters are not significantly different (Tukey-Kramer test: P > 0.05). Female population density shift occurred on the 4th day of oviposition period
Discussion
Our results indicate that T. ludeni females reduced their fecundity after the population density changed during their productive period (Fig. 2a). We suggest that when the density quickly increases, they lower their fecundity to prevent the collapse of the local population due to the increase of resource competition and overexploitation of the host plants (Krips et al. 1998) or hostile interference or aggression among offspring for resource access (Estevez et al. 2007; Wong et al. 2013; Li and Zhang 2021). Tetranychid mites construct silk webs (Saito 1983; Mori and Saito 2005; Clotuche et al. 2009; Le Goff et al. 2010) in the new habitats to protect themselves and their offspring from environmental hazards (Davis 1952; McMurtry et al. 1970; Hazan et al. 1975; Ashley 2003; Oku et al. 2003, 2004; Mori and Saito 2005; Le Goff et al. 2010) but the silk consists of mainly proteins (Hazan et al. 1975), the production of which incurs a considerable cost (Oku et al. 2009). Therefore, when they arrive in a new site of much larger area from a higher density population, they need to allocate more resources per female to produce enough silk to cover the area, leading to fecundity decline (Fig. 2a).
We did not observe a trade-off between the egg number and size in response to the density changes (Fig. 3a), challenging theoretical assumptions (Smith and Fretwell 1974; Parker and Begon 1986; Roff 1992, 2002; Stearns 1992; Fox and Czesak 2000; Fischer et al. 2011). The lack of such trade-offs has also been reported in some animal species (e.g., Doughty and Shine 1997; Zera and Harshman 2001; Jordan and Snell 2002; Bowden et al. 2004; Uller and Olsson 2005). Our results show that increasing egg size did not significantly increase the proportion of daughters (Fig. 5), contradictory to the previous assumption that sex allocation in spider mites is mediated by egg size (Macke et al. 2011). These findings suggest that T. ludeni females only adjust their fecundity but not egg size in response to density dynamics as reported in some birds (Christians 2002) because egg size has little impact on reproductive fitness, such as offspring survival (Fig. 3b) and sex allocation (Fig. 5). Therefore, egg size is not a reliable indicator of offspring fitness when future environmental conditions are uncertain or unpredictable (Wiklund and Persson 1983; Karlsson and Wiklund 1985; McEdward and Carson 1987; Lalonde 2005; Morrongiello et al. 2012).
We demonstrate that regardless of density changes, offspring produced by females in high population density was significantly more female-biased than in low density (Fig. 4). This could be due to sex-specific dispersal tendency in spider mites. Female spider mites usually disperse from dense conditions after mating (Suski and Naegele 1968; McEnroe 1969; Brandenburg and Kennedy 1982; Li and Margolies 1993) to establish new colonies (Mitchell 1973; Brandenburg and Kennedy 1982) and reduce future competition for food or space (Clark 1978; Silk 1983; Mari et al. 2008; Hjernquist et al. 2009; West 2009; Visser et al. 2014; Song et al. 2016; Weerawansha et al. 2022), resulting in production of more dispersing daughters in dense conditions. Compared to density shift from high to high or from low to low, that from low to high led to a faster increase in proportion of daughters produced (Fig. 4). This suggests that T. ludeni females can quickly adjust their sex allocation in response to the change of social environment for optimal fitness of their offspring.
In the present study, we demonstrate that T. ludeni females could adjust their reproductive strategies in response to dynamic social environments during their reproductive period. Females reduce fecundity and produce more dispersive female offspring in dense environments, which will reduce the local resource competition. However, females do not adjust the egg size in response to the shift of population density, as egg size imposes no significant effect on fecundity and offspring sex ratio and survival. Therefore, T. ludeni females adapt to the shift of population density by manipulating the fecundity and offspring sex ratio but not the egg size. Whether T. ludeni females could manipulate sex allocation via adjusting egg size in response to the shift of population size remains unclear and is warranted for future investigations.
References
Ashley JL (2003) Toxicity of selected acaricides on Tetranychus urticae Koch (Tetranychidae: Acari) and Orius insidiosus Say (Hemiptera: Anthocoridae) life stages and predation studies with Orius insidiosus. MSc thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, p 60
Azandémè-Hounmalon GY, Fellous S, Kreiter S, Fiaboe KKM, Subramanian S, Kungu M, Martin T (2014) Dispersal behavior of Tetranychus evansi and T. urticae on tomato at several spatial scales and densities: implications for integrated pest management. PLoS ONE 9(4):e95071. https://doi.org/10.1371/journal.pone.0095071
Bengtsson J (2008) Aggregation in non-social insects: an evolutionary analysis. https://www.pub.epsilon.slu.se/3437/1/Aggregation_17_v2.pdf. Accessed 05 Feb 2022
Bonsignore CP, Jones TM (2014) Aggregation and mating success of Capnodis tenebrionis (Coleoptera: Buprestidae). Insect Sci 21:203–212. https://doi.org/10.1111/1744-7917.12035
Bowden RM, Harms HK, Paitz RT, Janzen FJ (2004) Does optimal egg size vary with demographic stage because of a physiological constraint? Funct Ecol 18:522–529. https://doi.org/10.1111/j.0269-8463.2004.00861.x
Bowers EK, Thompson CF, Sakaluk SK (2017) Maternal natal environment and breeding territory predict the condition and sex ratio of offspring. Evol Biol 44:11–20. https://doi.org/10.1007/s11692-016-9380-9
Bowman J, Cappuccino N, Fahrig L (2002) Patch size and population density: the effect of immigration behavior. Conserv Ecol 6(1):9. https://doi.org/10.5751/ES-00354-060109
Brandenburg RL, Kennedy GG (1982) Intercrop relationships and spider mite dispersal in a corn/peanut agro-ecosystem. Entomol Exp Appl 32:269–276. https://doi.org/10.1111/j.1570-7458.1982.tb03217.x
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton, NY, p 355
Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biol Rev 77:1–26. https://doi.org/10.1017/S1464793101005784
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165. https://doi.org/10.1126/science.201.4351.163
Clotuche G, Le Goff G, Mailleux A-C, Deneubourg J-L, Detrain C, Hance T (2009) How to visualize the spider mite silk? Microsc Res Tech 72(9):659–664. https://doi.org/10.1002/jemt.20712
Clotuche G, Yano S, Akino T, Amano H (2014) Chemical investigation of aggregation behavior in the two-spotted spider mite Tetranychus urticae. Exp Appl Acarol 63:377–387. https://doi.org/10.1007/s10493-014-9779-x
Dar SA, Yaqoob M, Gajger IT (2021) The nesting and mating behavior of Andrena patella (Hymenoptera: Andrenidae). Vet Arh 91(2):207–219. https://doi.org/10.24099/vet.arhiv.1163
Davis DW (1952) Influence of population density on Tetranychus multisetis. J Econ Entomol 69:652–654. https://doi.org/10.1093/jee/45.4.652
DeVries Z, Mick R, Balvín O, Schal C (2017) Aggregation behavior and reproductive compatibility in the family Cimicidae. Sci Rep 7:13163. https://doi.org/10.1038/s41598-017-12735-3
Doughty P, Shine R (1997) Detecting life history trade-offs: measuring energy stores in “capital” breeders reveals costs of reproduction. Oecologia 110:508–513. https://doi.org/10.1007/s004420050187
Estevez I, Andersen I-L, Nævdal E (2007) Group size, density and social dynamics in farm animals. Appl Anim Behav Sci 103(3–4):185–204. https://doi.org/10.1016/j.applanim.2006.05.025
Fischer B, Taborsky B, Kokko H (2011) How to balance the offspring quality-quantity trade-off when environmental cues are unreliable. Oikos 120:258–270. https://doi.org/10.1111/j.1600-0706.2010.18642.x
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369. https://doi.org/10.1146/annurev.ento.45.1.341
Fox CW, Thakar MS, Mousseau TA (1997) Egg size in a seed beetle: an adaptive maternal effect. Am Nat 149:149–163. https://doi.org/10.1086/285983
Hazan A, Gertler A, Tahori AS, Gerson U (1975) Spider mite webbing–III. Solubilization and amino acid composition of the silk protein. Comp Biochem Physiol 51B:457–462. https://doi.org/10.1016/0305-0491(75)90038-3
Helle W, Sabelis MW (1985) Spider mites: Their biology, natural enemies and control. Elsevier, Amsterdam, p 4458
Hjernquist MB, Thuman Hjernquist KA, Forsman JT, Gustafsson L (2009) Sex allocation in response to local resource competition over breeding territories. Behav Ecol 20:335–339. https://doi.org/10.1093/beheco/arp002
Jordan MA, Snell HL (2002) Life history trade-offs and phenotypic plasticity in the reproduction of Galapagos lava lizards (Microlophus delanonis). Oecologia 130:44–52. https://doi.org/10.1007/s004420100776
Karlsson B, Wiklund C (1985) Egg weight variation in relation to egg mortality and starvation endurance of newly hatched larvae in some satyrid butterflies. Ecol Entomol 10:205–211. https://doi.org/10.1111/j.1365-2311.1985.tb00549.x
Khan I, Prakash A, Issar S, Umarani M, Sasidharan R, Masagalli JN, Lama P, Venkatesan R, Agashek D (2018) Female density-dependent chemical warfare underlies fitness effects of group sex ratio in flour beetles. Am Nat 191(3):306–317. https://doi.org/10.5061/dryad.p9v3q
Kingma SA, Komdeur J, Burke T, Richardson DS (2017) Differential dispersal costs and sex-biased dispersal distance in a cooperatively breeding bird. Behav Ecol 28(4):1113–1121. https://doi.org/10.1093/beheco/arx075
Krips OE, Witul A, Willems PEL, Dicke M (1998) Intrinsic rate of population increase of the spider mite Tetranychus urticae on the ornamental crop gerbera: intraspecific variation in host plant and herbivore. Entomol Exp Appl 89:159–168. https://doi.org/10.1046/j.1570-7458.1998.00395.x
Kusch JM, Crill Matzke C, Lane JE (2020) Reproductive failure predicts intracolony dispersal of female black-tailed prairie dogs (Cynomys ludovicianus) in a Northern population. West N Am Nat 80(2):157–164. https://doi.org/10.3398/064.080.0203
Lalonde RG (2005) Egg size variation does not affect offspring performance under intraspecific competition in Nasonia vitripennis, a gregarious parasitoid. J Anim Ecol 74(4):630–635. https://doi.org/10.1111/j.1365-2656.2005.00958.x
Le Goff GJ, Mailleux A-C, Detrain C, Deneubourg J-L, Clotuche G, Hance T (2010) Group effect on fertility, survival and silk production in the web spinner Tetranychus urticae (Acari: Tetranychidae) during colony foundation. Behaviour 147:1169–1184. https://doi.org/10.1163/000579510x510980
Li J, Margolies DC (1993) Effects of mite age and host quality on aerial dispersal behaviour in the twospotted spider mite. Entomol Exp Appl 68:79–86. https://doi.org/10.1111/j.1570-7458.1993.tb01691.x
Li G-Y, Zhang Z-Q (2021) The costs of social interaction on survival and reproduction of arrhenotokous spider mite Tetranychus urticae. Entomol Gen 41:49–57. https://doi.org/10.1127/entomologia/2020/0911
Lutz CL, Diefenbach DR, Rosenberry CS (2015) Population density influences dispersal in female white-tailed deer. J Mammal 96(3):494–501. https://doi.org/10.1093/jmammal/gyv054
Macke E, Magalhães S, Do-Thi Khan H, Luciano A, Frantz A, Facon B, Olivieri I (2011) Sex allocation in haplodiploids is mediated by egg size: evidence in the spider mite Tetranychus urticae Koch. Proc R Soc B Biol Sci 278:1054–1063. https://doi.org/10.1098/rspb.2010.1706
Macke E, Magalhães S, Do-Thi Khanh H, Frantz A, Facon B, Olivieri I (2012) Mating modifies female life history in a haplodiploid spider mite. Am Nat 179:147–162. https://doi.org/10.1086/665002
Maenoa KO, Piou C, Ghaout S (2020) The desert locust, Schistocerca gregaria, plastically manipulates egg size by regulating both egg numbers and production rate according to population density. J Insect Physiol 122:104020. https://doi.org/10.1016/j.jinsphys.2020.104020
Manguette ML, Robbins AM, Breuer T, Stokes EJ, Parnell RJ, Robbins MM (2020) Female dispersal patterns influenced by male tenure duration and group size in western lowland gorillas. Behav Ecol Sociobiol 74:81. https://doi.org/10.1007/s00265-020-02863-8
Mari L, Gatto M, Casagrandi R (2008) Local resource competition and the skewness of the sex ratio: a demographic model. Math Biosci Eng 5:813–830. https://doi.org/10.3934/mbe.2008.5.813
Matsuura K, Kobayashi N (2010) Termite queens adjust egg size according to colony development. Behav Ecol 21:1018–1023. https://doi.org/10.1093/beheco/arq101
McEdward LR, Carson SF (1987) Variation in egg organic content and its relationship with egg size in the starfish Solaster stimpsoni. Mar Ecol Prog Ser 37:159–169. https://doi.org/10.3354/MEPS037159
McEnroe WD (1969) Spreading and inbreeding in the spider mite. J Hered 60:343–345. https://doi.org/10.1093/oxfordjournals.jhered.a108011
McMurtry JA, Huffaker CB, van de Vrie M (1970) Ecology of Tetranychid mites and their natural enemies: a review. I. Tetranychidae enemies: their biological characters and the impact of spray practices. Hilgardia 40:331–390. https://doi.org/10.3733/hilg.v40n11p331
Mitchell R (1973) Growth and population dynamics of a spider mite (Tetranychus urticae K., Acarina: Tetranychidae). Ecology 54:1349–1355. https://doi.org/10.1038/hdy.1997.10
Mori K, Saito Y (2005) Variation in social behavior within a spider mite genus, Stigmaeopsis (Acari: Tetranychidae). Behav Ecol 16(1):232–238. https://doi.org/10.1093/beheco/arh157
Morrell LJ, James R (2008) Mechanisms for aggregation in animals: rule success depends on ecological variables. Behav Ecol 19:193–201. https://doi.org/10.1093/beheco/arm122
Morrongiello JR, Bond NR, Crook DA, Wong BBM (2012) Spatial variation in egg size and egg number reflects trade-offs and bet-hedging in a freshwater fish. J Anim Ecol 81(4):806–817. https://doi.org/10.1111/j.1365-2656.2012.01961.x
Nachappa P, Margolies DC, Nechols JR, Campbell JF (2011) Variation in predator foraging behaviour changes predator-prey spatio-temporal dynamics. Funct Ecol 25:1309–1317. https://doi.org/10.1111/j.1365-2435.2011.01892.x
Oku K, Yano S, Takafuji A (2003) Spider mites use of a refuge during the quiescent stage in the presence of a predator. Entomol Exp Appl 108:71–74. https://doi.org/10.1046/j.1570-7458.2003.00069.x
Oku K, Yano S, Takafuji A (2004) Nonlethal indirect effects of a native predatory mite, Amblyseius womersleyi Schicha (Acari: Phytoseiidae), on the phytophagous mite Tetranychus kanzawai Kishida (Acari: Tetranychidae). J Ethol 22:109–112. https://doi.org/10.1007/s10164-003-0102-2
Oku K, Magalhães S, Dicke M (2009) The presence of webbing affects the oviposition rate of two-spotted spider mites, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 49:167–172. https://doi.org/10.1007/s10493-009-9252-4
Parker GA, Begon M (1986) Optimal egg size and clutch size: effects of environment and maternal phenotype. Am Nat 128:573–592. https://doi.org/10.1086/284589
Pérez-González J, Carranza J, Polo V (2010) Measuring female aggregation in ungulate mating-system research: a red deer case study. Wildl Res 37(4):301–310. https://doi.org/10.1071/WR09033
Roeder CM (1992) Sex ratio response of the two-spotted spider mite (Tetranychus urticae Koch) to changes in density under local mate competition. Can J Zool 70:1965–1967. https://doi.org/10.1139/z92-266
Roeder C, Harmsen R, Mouldey S (1996) The effects of relatedness on progeny sex ratio in spider mites. J Evol Biol 9(2):143–151. https://doi.org/10.1046/j.1420-9101.1996.9020143.x
Roff DA (1992) The evolution of life histories: Theory and analysis. Chapman and Hall, New York, p 547
Roff DA (2002) Life history evolution. Sinauer Associates, Sunderland, MA, p 527
Saito Y (1983) The concept of life types in Tetranychinae: an attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24(4):377–391
Sarwar M (2013) Management of spider mite Tetranychus cinnabarinus (Boisduval) (Tetranychidae) infestation in cotton by releasing the predatory mite Neoseiulus pseudolongispinosus (Xin, Liang and Ke) (Phytoseiidae). Biol Control 65(1):37–42. https://doi.org/10.1016/j.biocontrol.2012.09.017
Schaub M, von Hirschheydt J (2009) Effect of current reproduction on apparent survival, breeding dispersal, and future reproduction in barn swallows assessed by multistate capture-recapture models. J Anim Ecol 78:625–635. https://doi.org/10.1111/j.1365-2656.2008.01508.x
Schausberger P, Yano S, Sato Y (2021) Cooperative behaviors in group-living spider mites. Front Ecol Evol 9:745036. https://doi.org/10.3389/fevo.2021.745036
Sibly R, Calow P, Smith RH (1988) Optimal size of seasonal breeders. J Theor Biol 133:13–21. https://doi.org/10.1016/S0022-5193(88)80021-3
Silk JB (1983) Local resource competition and facultative adjustment of sex ratios in relation to competitive abilities. Am Nat 121:56–66. https://doi.org/10.1086/284039
Silk JB (1984) Local resource competition and the evolution of male-biased sex ratios. J Theor Biol 108:203–213. https://doi.org/10.1016/s0022-5193(84)80066-1
Smith CC, Fretwell SD (1974) Optimal balance between size and number of offspring. Am Nat 108:499–506. https://doi.org/10.1086/282929
Snead JS, Alcock J (1985) Aggregation formation and assortative mating in two meloid beetles. Evolution 39(5):1123–1131. https://doi.org/10.1111/j.1558-5646.1985.tb00452.x
Song Z, Lou Y, Hu Y, Deng Q, Gao W, Zhang K (2016) Local resource competition affects sex allocation in a bird: experimental evidence. Anim Behav 121:157–162. https://doi.org/10.1016/j.anbehav.2016.08.023
Spieler M (2003) Risk of predation affects aggregation size: a study with tadpoles of Phrynomantis microps (Anura: Microhylidae). Anim Behav 65:179–184. https://doi.org/10.1006/anbe.2002.2030
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford, p 262
Suski ZW, Naegele JA (1968) Environmental determinants of white light response in the two-spotted spider mite. Tetranychus urticae K. I. Humidity and food reserve depletion. University of Massachusetts Press, Amherst, p 43
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92. https://doi.org/10.1126/science.179.4068.90
Uller T, Olsson M (2005) Trade-offs between offspring size and number in the lizard Lacerta vivipara: a comparison between field and laboratory conditions. J Zool 265:295–299. https://doi.org/10.1017/S0952836904006326
Vaishali B, Krushnamegh K (2020) Dispersal and migration have contrasting effects on butterfly flight morphology and reproduction. Biol Lett 16:20200393. https://doi.org/10.1098/rsbl.2020.0393
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142. https://doi.org/10.1086/284547
Visser B, Le Lann C, Snaas H, Hardy ICW, Harvey JA (2014) Consequences of resource competition for sex allocation and discriminative behaviors in a hyperparasitoid wasp. Behav Ecol Sociobiol 68:105–113. https://doi.org/10.1007/s00265-013-1627-1
Walzer A, Schausberger P (2013) Intra- and trans-generational costs of reduced female body size caused by food limitation early in life in mites. PLoS ONE 8:e79089. https://doi.org/10.1371/journal.pone.0079089
Walzer A, Schausberger P (2015) Food stress causes sex-specific maternal effects in mites. J Exp Biol 218:2603–2609. https://doi.org/10.1242/jeb.123752
Weerawansha N, Wang Q, He XZ (2020) Effect of foundress population density and size on reproduction and population growth of a haplodiploid mite. Syst Appl Acarol 25(11):2063–2076. https://doi.org/10.11158/saa.25.11.11
Weerawansha N, Wang Q, He XZ (2022) Adjustment of fecundity and sex ratio in response to social environments in a haplodiploid mite. Syst Appl Acarol 27(1):61–70. https://doi.org/10.11158/saa.27.1.7
West SA (2009) Sex allocation. Princeton University Press, Princeton, NJ, p 482
West SA, Shuker DM, Sheldon BC (2005) Sex-ratio adjustment when relatives interact: a test of constraints on adaptation. Evolution 59(6):1211–1228. https://doi.org/10.1554/04-158
Wheeler CA, Jr Welsh HH (2008) Mating strategy and breeding patterns of the foothill yellow-legged frog (Rana boylii). Herpetol Conserv Biol 3(2):128–142
Wiklund C, Persson A (1983) Fecundity, and the relation of egg weight variation to offspring fitness in the speckled wood butterfly Pararge aegeria or why don’t butterfly females lay more eggs? Oikos 40:53–63. https://doi.org/10.2307/3544198
Wong JWY, Meunier J, Kölliker M (2013) The evolution of parental care in insects: the roles of ecology, life history and the social environment. Ecol Entomol 38:123–137. https://doi.org/10.1111/een.12000
Wrensch DL, Young SSY (1978) Effects of density and host quality on rate of development, survivorship, and sex ratio in the carmine spider mites. Environ Entomol 7:499–502. https://doi.org/10.1093/ee/7.4.499
Yano S (2012) Cooperative web sharing against predators promotes group living in spider mites. Behav Ecol Sociobiol 66:845–853. https://doi.org/10.1007/s00265-012-1332-5
Young SSY, Wrensch DL, Kongchuensin M (1986) Control of sex ratio by female spider mites. Entomol Exp Appl 40:53–60. https://doi.org/10.1111/j.1570-7458.1986.tb02155.x
Zera A, Harshman L (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Evol Syst 32:95–126. https://doi.org/10.1146/annurev.ecolsys.32.081501.114006
Zhang Z-Q (2003) Mites of greenhouses: identification, biology and control. CABI Publishing, Cambridge, p 244
Zhou P, He XZ, Chen C, Wang Q (2021) Resource relocations in relation to dispersal in Tetranychus ludeni Zacher. Syst Appl Acarol 26(11):2018–2026. https://doi.org/10.11158/saa.26.11.3
Acknowledgements
We thank Professor Z.-Q. Zhang for identification of this spider mite to species, and K. Sinclair, P. Zhou and D. Ristyadi for technical assistance. We are very grateful to an anonymous reviewer for the constructive comments made in an earlier version, which have significantly improved the quality of the paper.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was jointly funded by the Accelerating Higher Education Expansion and Development Project (AHEAD) launched by the Sri Lankan Government under the funds of the World Bank and Massey University New Zealand.
Author information
Authors and Affiliations
Contributions
NW, XZH, and QW conceived and designed the study. NW collected the data. NW and XZH analysed the data. All authors contributed to manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weerawansha, N., Wang, Q. & He, X.Z. A haplodiploid mite adjusts fecundity and sex ratio in response to density changes during the reproductive period. Exp Appl Acarol 88, 277–288 (2022). https://doi.org/10.1007/s10493-022-00749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00749-0