Abstract
Punctoribates is one of few genera in Poronota (Acari: Oribatida) containing species with porose areas and species with saccules, the two types of the octotaxic system. These porose organs are the main difference between two morphologically similar species, P. punctum with porose areas and P. zachvatkini with saccules. As the octotaxic system can vary within species, species separation solely based on this trait might be insufficient. To assess the species status of P. zachvatkini, we investigated additional differences from P. punctum by comparing habitat preferences of the two species regarding nature reserves and agricultural landscapes during a field study in the German Eifel region, and by examining Punctoribates material from four large German natural history museums. We also performed scanning electron microscopy (SEM) and a genetic analysis using the D3 marker of the nuclear 28S rDNA gene. In the field study, P. zachvatkini had higher densities in the nature reserves and P. punctum in the agricultural landscapes. Evaluation of the museum material revealed P. punctum occurred more regularly in disturbed sites such as urban, agricultural and post-mining areas compared to P. zachvatkini. Pairwise distances of the 28S D3 genetic marker as well as an additional base pair in P. zachvatkini further support the separation of the two species, and SEM investigations revealed new details regarding the punctulation of P. zachvatkini. The review of the museum material showed that P. zachvatkini already occurred in Germany in 1967 and has a wider distribution than previously known.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Punctoribates, a member of the family Punctoribatidae (junior synonym Mycobatidae; see Subías 2004 for discussion), is a globally distributed genus of Poronota (Acari: Oribatida) with currently 30 known species (Seniczak et al. 2020). It is one of few genera within the Poronota to contain species with porose areas as well as species with saccules, the two regular types of the octotaxic system. The octotaxic system is a set of usually four pairs of porose organs on the notogaster with secretory function (Alberti et al. 1996). In most Poronota, the octotaxic system is expressed as either porose areas (exposed pore fields on the cuticle) or saccules (invaginated pockets of pores beneath the cuticle).
Most species in Punctoribates express porose areas. Among them is Punctoribates punctum (Koch), the globally distributed type species of the genus, which is present throughout most of Germany (Weigmann et al. 2015). A species with saccules is Punctoribates zachvatkini (Shaldybina), which has been mostly recorded in Eastern Europe and Russia (Shaldybina 1969; Ivan and Călugăr 2004; Shevchenko and Kolodochka 2014). The first findings of P. zachvatkini in the German federal states of Saxony and Bavaria (Weigmann et al. 2015) represent the most western record of the species currently known.
For Punctoribates species with saccules, the genus Semipunctoribates has been proposed (Mahunka 1987; Bayartogtokh et al. 2000). However, there is discussion as to whether the octotaxic system suffices to split up a genus (Weigmann 2010; Weigmann and Ermilov 2016; Seniczak et al. 2020). Although the occurrence of both types of the octotaxic system in the same genus is rare, it can vary between genera of a family (Alberti and Norton 1997; Schäffer et al. 2010). This suggests a plasticity of the trait, possibly linked to functional selection (Schäffer et al. 2010; Klimov and Ermilov 2017), although the advantage of either an exposed or invaginated secretory surface is not clear (Alberti and Norton 1997). In a few cases, variability is known to extend to the level of populations or even individuals: multiple specimens in a population of Protoribates paracapucinus (Mahunka) (Haplozetidae) expressed saccules instead of porose areas (Weigmann and Ermilov 2016), and a specimen of Peloptulus phaeonotus (Koch) (Phenopelopidae) had one porose area replaced by a saccule (Weigmann 2010). This raises the question of how reliable the type of octotaxic system is for species separation.
Morphologically, P. zachvatkini is very similar to P. punctum, being smaller on average, but their size ranges overlap. In addition, there are slight differences in the shape of bothridial and length of notogastral setae (Seniczak et al. 2020). However, the most notable distinction is their octotaxic system. The slight morphological differences and possible plasticity of the octotaxic system lead to the question of whether P. zachvatkini and P. punctum are indeed two separate species or just represent intraspecific variation within P. punctum. Due to their morphological similarity and as P. zachvatkini is not included in the most recent identification key for Central European Oribatida, published by Weigmann (2006), P. zachvatkini may have been confused with P. punctum in the past. The actual distribution of the two species and their ecological requirements are therefore not known, nor is whether they are truly two species.
A combined approach including genetics and ecology is known to reliably separate even cryptic species (Rissler and Apodaca 2007; Padial et al. 2010; Fišer et al. 2018). Closely related, sympatric species often differ in habitat use (Reinert 1984; Friberg et al. 2008), meaning that information about habitat preferences can provide arguments regarding species status. Furthermore, genetic markers have become a standard method to test separation of species. For Oribatida, the nuclear 28S D3 region is a reliable marker for this purpose (Maraun et al. 2004; Lehmitz and Decker 2017).
The aim of the present study is to clarify the morphologically ambiguous separation of P. zachvatkini and P. punctum. Therefore, we investigated habitat preferences in a field study in the Eifel region in Germany, where both species occurred sympatrically, and we reviewed Punctoribates-collection material from four large German natural history museums, in order to gather all available information on the distribution of the possibly overlooked P. zachvatkini in Germany. We hypothesized (1) that P. punctum and P. zachvatkini occur in different habitats, (2) the nuclear 28S D3 marker separates P. zachvatkini from other members of its genus, and (3) morphological traits [detected by scanning electron microscopy (SEM) pictures] separate the two species P. punctum and P. zachvatkini.
Material and methods
Sampling
As part of a soil zoological survey within the project INPEDIV (‘Integrative analysis of the influence of pesticides and land use on biodiversity in Germany’), we took soil samples from the Eifel region in Germany in the federal states of North Rhine-Westphalia and Rhineland-Palatinate. Sampling took place at five study sites in May 2019 (RL1-RL5) and five different sites in May 2020 (RL6-RL10) (Table 1).
At every site, we established a linear transect of four plots reaching from an agricultural field into an adjacent dry meadow located in a nature reserve. The first sampling plot was located 25 m within the agricultural field (plot ‘field’). The second sampling plot was located on the border between the agricultural field and the nature reserve (‘border’). The third and fourth sampling plot lay 25 m (‘nr25’) and 50 m (‘nr50’) apart from the border in the nature reserve (Fig. 1). At a distance of 4 m from the center of each plot, we took three soil samples with a metal cylinder (2.5 cm radius, 5 cm high), closed with lids on the top and bottom and cooled until extraction 2 or 3 days after field sampling. The samples were extracted over a 10-day period by a high-temperature gradient (from 20 °C on the first to 55 °C on the last day) using a MacFadyen apparatus (MacFadyen 1961), and preserved in 96% ethanol. We identified specimens of P. punctum and P. zachvatkini under the light microscope (Leica DM 2500) using the keys of Weigmann (2006), Shaldybina (1969), Bayartogtokh et al. (2000) and Seniczak et al. (2020). All specimens were deposited in the collection of the Senckenberg Museum of Natural History Görlitz (SMNG).
To test for correlations with soil parameters, we measured the water content, pH, nitrogen content, carbon content and particle size distribution separately for every soil sample. We calculated water content as weight loss per g of dry soil by weighing the soil samples before and after drying on the MacFadyen apparatus. We measured pH with a pH-meter model HI2210 (Hanna instruments, Vöhringen, Germany) in 1-M potassium chloride according to DIN ISO 10390 (DIN 2005). We performed measurements of carbon and nitrogen contents with 5–10 µg of dried and sieved sample soil in a vario PYRO cube elemental analyzer (Elementar Analysesysteme, Hanau, Germany). We measured particle size distribution as the proportion of clay (particle size < 2 µm), silt (2–200 µm) and sand (200–2000 µm) in each sample using a LS 13 320 particle size analyzer (Beckman Coulter, Miami, FL, USA).
For the genetic analyses, further specimens were provided within ‘MetaInvert’, a Senckenberg project in association with the ‘Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg’. The MetaInvert specimens of P. punctum were sampled in 2018 at Olba Lake near Wartha (51°16′18.5″N, 14°36′36.8″E) and in Görlitz (51°07′56.6″N, 14°58′20.2″E), both in Saxony, Germany. Furthermore, we added one specimen of Minunthozethes semirufus (C.L. Koch) sampled in 2018 from the Sernitz bog (53°04′58.8″N, 13°55′08.4″E), Brandenburg, Germany, to the dataset as an outgroup, and downloaded additional sequences from GenBank (Table 2).
Habitat preferences
We calculated densities (number of individuals per m2) of both species for each sample from the field study and pooled samples according to plot (field, border, nr25, nr50). We found no specimens of P. punctum at three sites in 2020 (RL7, RL8, RL10) and excluded them from the analyses of P. punctum, leaving 84 samples from seven sites (RL1-RL6, RL9). Punctoribates zachvatkini was present in all 10 sites, so we used all 120 samples for analyses of the species. We created boxplot diagrams of the average densities of the two species along the gradient from agricultural site into nature reserve using R v 4.0.4 (R Core Team 2021).
To extract ecological information from the museum material, we grouped sampling sites (if possible) into six categories depending on habitat type, namely: ‘grasslands’ for any kind of meadows including pastures; ‘agricultural sites’ for crop fields, plantations, intense pastures and vineyards; ‘coniferous forests’ and ‘deciduous/mixed forests’ for the respective forest types; ‘lake and river banks’ for floodplains, floating material at river banks and two peatland sites; and ‘anthropogenic’ for urban areas and former coal mining sites.
Genetic distances
We extracted DNA non-destructively from 71 single whole individuals sampled in the Eifel region (see Sampling, above) using the Qiagen DNAeasy Blood&Tissue kit following the standard kit protocol. Specimens were incubated for 2–3 days in Qiagen Incubation lysis buffer (Lehmitz and Decker 2017) and the remaining body was transferred to 70% ethanol and stored in the voucher collection of the SMNG. Each specimen received an individual code consisting of the site where it was collected, the abbreviation of the plot, the number of the sample from that plot (1–3) and an individual letter for each individual of a sample (e.g., RL1-acr-3a = individual a from the third sample of the agricultural plot at RL1).
We used DNA extracts from 24 specimens (12 of each species) for DNA sequencing in both directions. We amplified the non-coding D3-D5 fragment of the 28S rDNA (and its flanking regions) of approximately 400–600 base pairs (bp) length using the forward primer D3A (5’-GACCCGTCTTGAAACACGGA-3’; Litvaitis et al. 1994) and the reverse primer 28Sbout (5’-CCCACAGCGCCAGTTCTGCTTACC-3’; Tully et al. 2006). The PCR reaction mix had a total volume of 10 µl, containing 0.12 µM of each Primer, 0.2 U Maximo Taq 1xl Taq buffer, 0.12 µM of each dNTP, and additive magnesium to a total of 2.0 mM (all components: GeneON, Ludwigshafen, Germany), 1 µl template DNA and 7.6 µl double-distilled water. PCR program ran with one pre-heating cycle at 95 °C for 60 s, followed by 33 amplification cycles (denaturation: 94 °C for 20 s, 49 °C for 20 s, and 68 °C for 45 s) and one post-annealing cycle at 72 °C for 10 min.
We inspected PCR products on a 1% agarose gel, purified single band amplifications with ExoSap-IT (Applied Biosystems) and sequenced them at the BiK-F Bio-diversity and Climate Research Centre, Frankfurt am Main, Germany. We checked all obtained sequences with BLAST in GenBank for contamination and manually aligned them in ClustalX v.1.83 (Chenna et al. 2003).
Scanning electron microscopy (SEM)
We took SEM images of four specimens of P. punctum and five of P. zachvatkini from the field study (see Sampling, above). After dehydration of specimens in 96% alcohol, we critical-point-dried them in CO2 and mounted them on aluminum stubs before sputter-coating them with gold. We took images with a JEOL JSM-6510 LV microscope, which we edited using Adobe Photoshop CS4 for better contrast and discernment of fine structures.
Distribution in Germany
To evaluate the distribution of P. zachvatkini and P. punctum in Germany, we re-identified all material of P. punctum and P. zachvatkini under the light microscope (Leica DM 2500) available from museum collections of SMNG (1841 specimens earlier identified as P. punctum and 4925 specimens earlier identified as P. zachvatkini), the Senckenberg Research Institute and Museum in Frankfurt (49 specimens earlier identified as P. punctum), the State Museum of Natural History in Karlsruhe (655 specimens earlier identified as P. punctum) and the Museum for Natural History in Berlin (486 specimens earlier identified as P. punctum). The material from the museum collections originated from Germany, Austria, Czech Republic and Slovakia.
We created a distribution map of P. zachvatkini in Germany with Edaphobase (www.portal.edaphobase.org), an online database for soil fauna (Burkhardt et al. 2014) and added further findings of P. zachvatkini from Weigmann et al. (2015), the field study in the Eifel region, and the museum material not included in Edaphobase using Adobe Photoshop CS4.
Statistical analysis
Habitat preferences
We performed a two-way ANOVA in Past v.4.07b (Hammer et al. 2001) with the factors ‘plot’ and ‘site’ on each species independently to see whether the factors had a significant effect on density. For significant results, we performed Tukey’s post-hoc test to identify which sites/plots differed significantly (α = 0.05) from each other.
Furthermore, we tested for correlations between species abundances and the soil parameters water content, pH, nitrogen content, carbon content, and particle size distribution. As clay content was low in all samples (< 12%), the particle size distribution mostly differed in relation of silt to sand. We chose sand content as the parameter to represent particle size distribution. For each species and soil parameter, we calculated the Pearson correlation coefficients R2 in Microsoft Excel. We used all samples in these analyses.
To see if combinations of parameters affected dominance, we created contour diagrams of both species and each of the 10 possible parameter combinations in R using R packages ggplot2 3.3.5 (Wickham 2016) and contourPlot v0.2.0 (Murphy 2020). A species was considered dominant when it was more abundant in a sample compared to the other species. We excluded samples with equal densities in both species from the analyses, leaving 64 samples. We considered species to differ in dominance when the contour plots of the relevant parameters did not overlap.
Genetic distances
There was no variation within the D5 fragment of Punctoribates in our dataset, so we only used the D3 fragment (315 bp) for analyses as most GenBank sequences only cover this region. We selected eight high-quality sequences of P. punctum and six of P. zachvatkini for analyses, and added additional sequences from GenBank of P. punctum as well as Punctoribates sellnicki (Willmann), a species with porose areas, to the dataset. Furthermore, we added sequences of P. punctum and Minunthozetes semirufus (C.L. Koch) obtained during the MetaInvert project (see Sampling, above). In total, the dataset consisted of 30 sequences from four species of the family Punctoribatidae (Table 2).
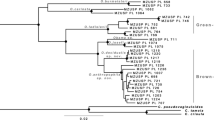
Sequences were aligned with MUSCLE and modified in MEGA6. To test for species separation, we calculated pairwise distances (p-distances) as the number of differences per base position between sequences, and created a Neighbor-Joining tree (Saitou and Nei 1987) to see if species formed separate clades. The optimal tree with the sum of branch length = 0.10 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distances method (Nei and Kumar 2000) and are in the units of the number of base differences per site. There were in total 316 positions in the final dataset. For p-distances and the tree, all ambiguous positions were removed for each sequence pair. All evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013).
Results
Habitat preferences
Overall, we found 99 specimens of P. punctum and 209 specimens of P. zachvatkini in the Eifel region. Densities differed between sites (2-way ANOVA, P. punctum: F6, 56 = 3.07, P = 0.011; P. zachvatkini: F9, 80 = 2.14, P = 0.036) and along the gradient from the agricultural fields into the nature reserves (P. punctum: F3, 56 = 10.35; P. zachvatkini: F3, 80 = 10.84, both P < 0.001). There was no significant interaction between site and plot. Densities of P. punctum were higher in the field compared to other plots (Tukey’s test: field vs. border: P = 0.001; field vs. nr25/nr50: P < 0.001), whereas densities of P. zachvatkini were higher in nr50 plots (nr50 vs. field/border: P < 0.001; nr50 vs. nr25: P < 0.05) (Fig. 2).
Densities (no. individuals m−2) of a Punctoribates punctum (n = 21) and b P. zachvatkini (n = 30) along the gradient from agricultural sites into nature reserves. Boxes represent 50% of variation, thick lines inside the boxes indicate the median, and whiskers 1.5 × the interquartile range, with dots marking outliers. Plots significantly different from others according to Tukey’s post-hoc test are marked with an asterisk. nr25 = plot 25 m inside the nature reserve, nr50 = plot 50 m inside the nature reserve
No single abiotic parameter explained the distribution of either species (Pearson correlation, R2 < 0.2). Contour plots showed that P. zachvatkini was dominant in nutrient-rich and P. punctum in nutrient-poor soils (Fig. 3a). Punctoribates zachvatkini also dominated in moist and sandy soils (Fig. 3b).
Regarding the material from museum collections, we found more sampling sites were colonized by P. punctum than by P. zachvatkini or both species together. However, P. zachvatkini also occurred in almost all habitat types, including lake and river banks, forests and grasslands. Punctoribates punctum was the more common species in highly disturbed anthropogenic habitats (cities, post-mining sites) and on agricultural sites, whereas P. zachvatkini was rare (Fig. 4).
Genetic distances
Of the 316 base positions, 28 showed differences between the four species. Between P. punctum and P. zachvatkini p-distances of the D3 fragment of 28S rDNA ranged from 1.9 to 2.9%. Specifically, P. zachvatkini contained 5–8 substitutions compared to P. punctum. Among sequences of P. punctum from the Eifel region p-distances varied by 0–0.3%, whereas there was no variation within the sequences of P. zachvatkini. Comparing sequences of P. punctum among regions, those from the Eifel region differed by 0–1.6% from those of other regions: by up to 0.3% from specimens from Schleswig–Holstein and by up to 1.6% from specimens from Saxony. P. sellnicki and P. punctum differed by 2.9–3.8%, P. zachvatkini and P. sellnicki by 3.2%. To Minunthuzetes semirufus, the Punctoribates species differed by 4.8–7.6% (Table 3). All species formed separate clades in the Neighbor-Joining tree (Fig. 5).
Neighbor-Joining tree based on sequences of the D3 fragment of 28 s rDNA. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Names of Eifel specimens consist of site, plot, sample number of the plot and a letter individual to each specimen of a sample (see text for details). Sn specimen from Saxony, SH specimen from Schleswig–Holstein
Alongside the differences in p-distances, all six individuals of P. zachvatkini from the various study sites had an additional base pair at position 101 in the D3 region of 28S compared to the other Punctoribatidae species analyzed in this dataset.
Scanning electron microscopy
The SEM images of the four specimens of P. punctum and five specimens of P. zachvatkini showed the different expressions of the octotaxic system of both species in fine detail (Figs. 6, 7): four pairs of porose areas or saccules, respectively, one pair in the anterior half of the notogaster at the height of the posterior end of the pteromorphs, and three pairs near the posterior-lateral margin of the notogaster. The porose areas of P. punctum (Fig. 6a, 7c) were largest in the anterior pair (Aa) while smaller in the posterior ones (A1-3). The saccules of P. zachvatkini (Figs. 6b, 7a,b) were visible as small openings, all of similar size, in the notogaster.
Scanning electron microscopy images of the octotaxic system of (a, b) Punctoribates zachvatkini and (c) P. punctum, dorsal view. a Lateral posterior part of notogaster of P. zachvatkini; b close-up on saccule of P. zachvatkini; c close-up on porose area of P. punctum. White arrows point at saccules, black arrowhead points at region of large cuticular protrusions
The investigated specimens showed another fine structure that is different between the two species: they both had an uneven surface with small cuticular protrusions on the notogaster, but in P. zachvatkini these protrusions were noticeably larger near the central-lateral areas of the notogaster (Figs. 6b, 7a, arrow heads). The cuticle of P. punctum on the other hand was similar in this region to the rest of the body (Fig. 6a).
Distribution in Germany
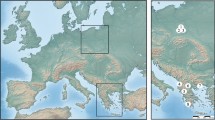
We identified 5259 specimens of P. zachvatkini and 2687 specimens of P. punctum from the museum collections. We re-identified 351 specimens of P. punctum as P. zachvatkini, and seven specimens of P. zachvatkini as P. punctum. The earliest record of P. zachvatkini in Germany stemmed from 1967 from a chalk slope near Mücheln, Thuringia. The 41 individuals of this sample were collected by Manfred Moritz and are stored in the collection of Jason Dunlop at the Museum of Natural History in Berlin. The most western distribution of P. zachvatkini in Europe stemmed from the field study in the Eifel region, at site RL10 near Gönnersdorf (Fig. 8).
Findings of Punctoribates zachvatkini in Germany from museum collections after review, the field study in the Eifel region and Weigmann et al. (2015). Map created in Edaphobase (www.portal.edaphobase.org) and edited with Adobe Photoshop CS4
Discussion
Punctoribates punctum and P. zachvatkini have mainly been distinguished by the type of their octotaxic system, a trait that may be variable within other species of Poronota (Weigmann 2010; Weigmann and Ermilov 2016). The present study shows that these two species also differ in regard to their habitat requirements, in regard to the sequence of the genetic species marker 28S D3, and in regard to the fine protrusions on their notogaster. These findings support their status as two separate species.
Intraspecific variation of the D3 fragment of 28S rDNA in Oribatida usually neither exceeds 0.5% nor includes indels (Lehmitz and Decker 2017). We found both to be the case when comparing sequences of P. punctum and P. zachvatkini, with all specimens of P. zachvatkini having at least five substitutions and an additional base pair. In accordance with the p-distances, the neighbor joining tree splits the species into different clades. Variation within P. punctum from different geographical regions exceeded 0.5%. Specifically, some sequences from Saxony differ by 1% or more from individuals of other regions. Examination of the corresponding vouchers confirmed this variation is neither due to contamination nor misidentification, and the sequences were not ambiguous. Further studies are needed to clarify whether P. punctum is possibly a complex of cryptic species and whether the genetic variation is also reflected morphologically. As this is the first genetic analysis conducted on P. zachvatkini, so far no information on genetic variance between populations is available for comparison.
There was no correlation of the abundance of P. zachvatkini or P. punctum with single soil parameters. According to Acarofauna Germanica published by Weigmann et al. (2015), P. zachvatkini is associated with wet or moist habitats such as fresh meadows or alluvial forests (in Germany). Punctoribates zachvatkini from the field study and museum material was present in other habitats as well, such as dry meadows and coniferous forests. Punctoribates punctum avoids low pH (Wissuwa et al. 2013). As the pH values of the study sites ranged from 5.5 to 8.1, they may have not been acidic enough to verify this effect. Instead, we found that combinations of soil parameters affected distribution of the two species, with P. zachvatkini being more dominant in nutrient-rich, wet and sandy soils. The observed niche ranges match the generalist ecology of P. punctum, which is able to colonize habitats outside the range of P. zachvatkini.
Parameters relating to agricultural practices such as pesticide application or ploughing may also play a role. In the field study in the German Eifel region, P. zachvatkini had higher densities in the plots furthest away from the field in the protected dry meadows of the nature reserves, whereas densities of P. punctum were highest in the agricultural plots. This is in line with earlier research which found P. punctum to be an early colonizer of disturbed habitats, including agricultural sites (Sheals 1956; Murvanidze et al. 2013; Wissuwa et al. 2013), and usually more often present at sites of anthropogenic influence compared to P. zachvatkini (Ivan and Călugăr 2004; Kolodochka and Shevchenko 2013; Shevchenko and Kolodochka 2014). In the museum material, P. punctum was also more common in highly disturbed habitats such as urban areas or post mining sites than P. zachvatkini. Their opposing distributions might make these species good indicators for anthropogenic disturbances of soil fauna.
The observed difference in habitat preference can be the result of a competitor-colonizer trade-off, where one species is a better competitor in regard to a resource while the other is better at colonizing habitats (Kneitel and Chase 2004). Punctoribates punctum can be transported by wind and water (Schuppenhauer et al. 2019) and accordingly, might be able to recolonize a habitat more quickly after disturbance than can P. zachvatkini. In contrast, P. zachvatkini may have a competitive advantage over P. punctum in less disturbed areas. Taken together this would explain our present findings.
Differences in the habitat preferences and type of octotaxic system of the two species might be linked. Little is known about how the type and function of the octotaxic system are related to the ecological demands of the animals. Alberti and Norton (1997) suggested invagination of open pore fields, meaning expression of saccules, might minimize water loss across the cuticle, but found no correlation with desiccating environments. In contrast, they found that secretory porose areas are often enlarged in species associated with dry environments. This is in line with the findings of the present field study where P. punctum with open porose areas was more abundant in the generally dryer (agricultural) sites.
In addition to having different octotaxic systems, P. zachvatkini is seen in SEM images to have larger cuticular protrusions in certain regions of the notogaster compared to P. punctum. In the original description, Shaldybina (1969) described P. zachvatkini with dark punctulation on the integument. The protrusions seen in the SEM images are probably identical to this punctulation. Though Shaldybina describes the punctulation to be dark and noticeable, the protrusions seem rather inconspicuous under the light microscope and share the same color as the notogaster. They only appear dark and striking in individuals that have been intensively cleared in lactic acid or in voucher specimens after DNA extraction. Ultimately, the octotaxic system is a better trait to morphologically distinguish P. zachvatkini from P. punctum.
A comprehensive review of collection material from natural history museums has shown that due to the morphological similarity, P. punctum and P. zachvatkini were insufficiently separated in the past, at least in Central and Western Europe. Before this study, the only German findings of P. zachvatkini stemmed from Saxony and Bavaria (Weigmann et al. 2015), and were considered its most western distribution. The present study extends its distribution to 10 of the 16 German federal states, covering the majority of the country from east to west. Its distribution might well expand further to the west into the neighbouring countries such as France, Belgium and Luxembourg. The earliest known record of P. zachvatkini in Germany now dates back to 1967, 2 years before Shaldybina (1969) described the species. This highlights the value of museum collections, which allow earlier findings to be reviewed in the light of the latest knowledge.
Conclusion
The present investigation supports the status of P. punctum and P. zachvatkini as separate species. They are morphologically distinct beyond the expression of their octotaxic system and show clear differences in their habitat preferences as well as with respect to the nuclear 28S rDNA genetic marker. Although the octotaxic system may be labile in families and genera and can in few cases vary within populations of the same species, it appears to be reliable in separating species of Punctoribates. In addition, the present examination of museum material revealed an insufficient distinction between the two species in the past. With increasing knowledge about species and their distribution, re-evaluation of old findings and the storage of individuals in museum collections for future research is an important aspect of scientific work.
Data availability
Data on the distribution of Punctoribates punctum and P. zachvatkini in the Eifel region will be uploaded to Edaphobase, an open-access data warehouse on soil biodiversity (www.edaphobase.org), alongside the data from the review of the museum material. DNA sequences are available at GenBank (www.ncbi.nlm.nih.gov/genbank/, see Table 2 for access numbers).
References
Alberti G, Norton RA, Adis J, Fernandez NA, Franklin EN, Kratzmann M, Moreno AI, Ribeiro EF, Weigmann G, Woas S (1996). Porose areas and related organs in oribatid mites (Oribatida). In: IX International Congress of Acarology (Ohio Biological Survey), pp. 277–283
Alberti G, Norton RA (1997) Porose integumental organs of oribatid mites (Acari, Oribatida): 3. Evolut Ecol Asp Zool 146:115–143
Bayartogtokh B, Grobler L, Cobanoglu S (2000) A new species of Punctoribates (Acari: Oribatida: Mycobatidae) collected from mushrooms in Turkey with remarks on the taxonomy of the genus. Navorsinge Van Die Nasionale Museum: Res Natl Mus 16:29–30
Burkhardt U, Russell DJ, Decker P, Döhler M, Höfer H, Lesch S, Rick S, Römbke J, Trog C, Vorwald J (2014) The Edaphobase project of GBIF-Germany—A new online soil-zoological data warehouse. Appl Soil Ecol 83:3–12. https://doi.org/10.1016/j.apsoil.2014.03.021
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500. https://doi.org/10.1093/nar/gkg500
DIN ISO 103901:2005–12, Soil quality - Determination of pH (ISO 10390:2005)
Fišer C, Robinson CT, Malard F (2018) Cryptic species as a window into the paradigm shift of the species concept. Mol Ecol 27:613–635.
Friberg M, Bergman M, Kullberg J, Wahlberg N, Wiklund C (2008) Niche separation in space and time between two sympatric sister species—a case of ecological pleiotropy. Evol Ecol 22:1–18. https://doi.org/10.1007/s10682-007-9155-y
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Ivan O, Călugăr A (2004) On the diversity and distribution of edaphic mites (Acari: Gamasida, Oribatida) in some saxicolous, low-altitude habitats in the North-Eastern Romania. Anuarul Complexului Muzeal Al Bucovinei 16–17:151–168
Klimov PB, Ermilov SG (2017) Phylogeny of the large-winged mites (Galumnoidea): investigating comparative evolutionary dynamics of a complex and possibly polygenic trait using nearly complete taxonomic sampling. Biol J Lin Soc 121:600–612. https://doi.org/10.1093/biolinnean/blx016
Kneitel JM, Chase JM (2004) Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol Lett 7:69–80. https://doi.org/10.1046/j.1461-0248.2003.00551.x
Kolodochka LA, Shevchenko OS (2013) Bидoвыe кoмплeкcы opибaтид (Sarcoptiformes, Oribatei) зeлeныx зoн гopoдa Киeвa. Hayкoвi зaпиcки Дepжaвнoгo пpиpoдoзнaвчoгo мyзeю: 95–103
Lehmitz R, Decker P (2017) The nuclear 28S gene fragment D3 as species marker in oribatid mites (Acari, Oribatida) from German peatlands. Exp Appl Acarol 71:259–276.
Litvaitis MK, Nunn G, Thomas WK, Kocher TD (1994) A molecular approach for the identification of meiofaunal turbellarians (Platyhelminthes, Turbellaria). Mar Biol 120:437–442. https://doi.org/10.1007/BF00680218
Macfadyen A (1961) Improved funnel-type extractors for soil arthropods. J Anim Ecol. https://doi.org/10.2307/2120
Mahunka S (1987) A survey of the oribatids of the Kiskunság National Park (Acari: Oribatida). Fauna Kiskuns Natl Park 2:346–397
Maraun M, Heethoff M, Schneider K, Scheu S, Weigmann G, Cianciolo J, Thomas RH, Norton RA (2004) Molecular phylogeny of oribatid mites (Oribatida, Acari): evidence for multiple radiations of parthenogenetic lineages. Exp Appl Acarol 33:183–201. https://doi.org/10.1023/B:APPA.0000032956.60108.6d
Murphy T (2020) contourPlot: Plots x,y,z Co-Ordinates in a Contour Map. R package version 0.2.0. Retrieved January 21, 2022 from https://CRAN.R-project.org/package=contourPlot
Murvanidze M, Mumladze L, Arabuli T, Kvavadze E (2013) Oribatid mite colonization of sand and manganese tailing sites. Acarologia 53:203–215. https://doi.org/10.1051/acarologia/20132089
Nei M, Kumar S (2000) Molecular Evolution and Phylogenetics. Oxford University Press, New York
Padial JM, Miralles A, De la Riva I, Vences M (2010) The integrative future of taxonomy. Front Zool 7:1–14. https://doi.org/10.1186/1742-9994-7-16
R Core Team (2021) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved February 12th, 2021 from https://www.R-project.org/
Reinert HK (1984) Habitat variation within sympatric snake populations. Ecology 65:1673–1682. https://doi.org/10.2307/1939146
Rissler LJ, Apodaca JJ (2007) Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Syst Biol 56:924–942. https://doi.org/10.1080/10635150701703063
Saitou M, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schäffer S, Koblmüller S, Pfingstl T, Sturmbauer C, Krisper G (2010) Ancestral state reconstruction reveals multiple independent evolution of diagnostic morphological characters in the “Higher Oribatida”(Acari), conflicting with current classification schemes. BMC Evol Biol 10:1–17. https://doi.org/10.1186/1471-2148-10-246
Schuppenhauer MM, Lehmitz R, Xylander WE (2019) Slow-moving soil organisms on a water highway: aquatic dispersal and survival potential of Oribatida and Collembola in running water. Mov Ecol 7:1–14. https://doi.org/10.1186/s40462-019-0165-5
Seniczak S, Ivan O, Marquardt T, Seniczak A (2020) Morphological ontogeny of Punctoribates astrachanicus (Acari: Oribatida: Punctoribatidae), and comments on Punctoribates Berlese. Zootaxa 4900:43–61. https://doi.org/10.11646/zootaxa.4900.1.6
Shaldybina YS (1969) New Species of beetle mites of the genus Punctoribates Berlese, 1908, of the Gorky region. Scientific Notes of the Gorky State Pedagogical Institute, Biological Series 99:53–68
Sheals JG (1956) Soil population studies. I.—The effects of cultivation and treatment with insecticides. Bull Entomol Res 47:803–822. https://doi.org/10.1017/S0007485300047039
Shevchenko OS, Kolodochka LA (2014) Species composition and distribution of oribatids (Acari, Oribatei) in urbanized biotopes of Kyiv. Becтник Зooлoгии. https://doi.org/10.2478/vzoo-2014-0018
Subías LS (2004) Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758–2002). Graellsia 60:3–305. https://doi.org/10.3989/graellsia.2004.v60.iextra.218
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Tully T, D’Haese CA, Richard M, Ferriere R (2006) Two major evolutionary lineages revealed by molecular phylogeny in the parthenogenetic collembola species Folsomia candida. Pedobiologia 50:95–104. https://doi.org/10.1016/j.pedobi.2005.11.003
Weigmann G (2010) Anomalies of notogastral structures in poronotic oribatid mites (Oribatida: Poronota) interpreted as cryptic ancestral characters modulated by regulatory genes. Trends in Acarology. https://doi.org/10.1007/978-90-481-9837-5_3
Weigmann G, Ermilov SG (2016) Intraspecific variation of the octotaxic system in Protoribates paracapucinus (Acari, Oribatida, Haplozetidae), with systematic and taxonomic considerations. Zootaxa 4067:473–478. https://doi.org/10.11646/zootaxa.4067.4.6
Weigmann G, Horak F, Franke K, Christian A (2015) Verbreitung und Ökologie der Hornmilben (Oribatida) in Deutschland. Peckiana 10:1–171
Weigmann G (2006) Hornmilben (Oribatida). In: Dahl (ed) Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, Volume 76. Goecke & Evers, Keltern
Wickham H (2016) Ggplot2: Elegant graphics for data analysis, 2nd edn. Springer International Publishing
Wissuwa J, Salamon JA, Frank T (2013) Oribatida (Acari) in grassy arable fallows are more affected by soil properties than habitat age and plant species. Eur J Soil Biol 59:8–14. https://doi.org/10.1016/j.ejsobi.2013.08.002
Acknowledgements
We thank Hubert Höfer from the State Museum in Karlsruhe, Peter Jäger from the Senckenberg Museum in Frankfurt and Jason Dunlop from the Museum for Natural History in Berlin, for giving us the opportunity to study their collections of P. punctum and P. zachvatkini. The first author personally thanks Franz Horak for advice on Punctoribates, Ilya Noskov for the translation of Russian and Ukrainian papers, and all colleagues from the department of soil zoology from SMNG, especially Ulrich Burkhardt for his expertise with PCR, Birgit Balkenhol for guidance with the SEM, and Gabriel Salako for advice on creating the contour plots. We also thank the two anonymous reviewers as well as Jan Bruin, the editor of Experimental and Applied Acarology, for their suggestions to improve the quality of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is part of the project INPEDIV (‘Integrative analysis of the influence of pesticides and land use on biodiversity in Germany’), led by the Zoological Research Museum Alexander König, Bonn, Germany and funded by the Leibniz Association (funding code K120/2018). Sequencing of some specimens was funded by ‘Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg’ as part of the collaboration with Senckenberg in the MetaInvert project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection and analyses. The first draft of the manuscript was written by JE and all authors commented on subsequent versions of the manuscript. All figures except for Fig. 4 were created by JE. Figure 4 was created by RL and edited by JE. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Escher, J., Hohberg, K., Decker, P. et al. Ecology, genetics and distribution of Punctoribates zachvatkini, an oribatid mite so far overlooked in Germany. Exp Appl Acarol 87, 289–307 (2022). https://doi.org/10.1007/s10493-022-00738-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00738-3