Abstract
Stigmaeopsis nanjingensis (Ma and Yuan) (Acari: Tetranychidae) is an important pest of bamboo—feeding behavior and silk production by the female adult mites is seriously harmful to bamboo leaves. Due to its small size, silking and cocooning, its management is difficult. This study discusses a fast and easy method for management of the pest by disturbing the spinning behavior. Stigmaeopsis nanjingensis is host specific and feeds only on bamboo leaves. Leaf margins of bamboo are highly hydrophobic, which makes dsRNA difficult to immerse. Hence, it is a challenge to apply the commonly used feeding method to inhibit gene expression in mites. In this study, we deliver dsRNA to interfere with the expression of fibroin by body wall permeation with a nanocarrier-based delivery system. The dsRNA/nanocarrier formulation droplets could enter the body cavity within 2 min after falling on the mite. The fibroin silencing efficiency was 75.4%, and the results of electron microscopy showed that dsRNA/nanocarrier damage the morphological structure of the silk thread. This study demonstrated the effectiveness of a nanocarrier-based percutaneous dsRNA delivery system in S. nanjingensis and its effect on the fibroin gene that influences the spinning behavior of S. nanjingensis. These findings may provide a new delivery system for RNAi-based control of spider mites that utilize protective webbing in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silk is produced by a wide variety of arthropods (Hoffman et al. 1996) including Arachnida and Myriapoda (Craig et al. 1997) and more than 20 insect orders, such as Hymenoptera, Lepidoptera and Neuroptera. Silk plays an important role in the life history, growth, foraging, nesting, breeding, and metamorphosis of arthropods (Sutherland et al. 2010). Insect silk is composed of fibroin, sericin, external wax and various impurities. Fibroin is secreted and synthesized by posterior silk glands of insects and is the main component of arthropod silk. Fibroin is a natural biological macromolecule with no physiological activity and is insoluble in water. It is mainly composed of low-complexity dipeptide repeating units composed of Gly-Ala or Gly-Ser (Zhou et al. 2001). According to the conformation of the fibroin molecular chain, the structures of silk are mainly divided into three types: α-helices, β-folds, and random coils.
At present, research on fibroin focuses mostly on silkworm (Song 2002). In 1972, Suzulki isolated the fibroin mRNA of the posterior silk gland of Bombyx mori, and identified part of its sequence (Zhou et al. 2000). Subsequently, the full sequences of 13 genes containing silk fibroin were obtained from the posterior silk gland, and some of these genes were cloned (Ohshima and Suzuki 1977). During physical chromatogram analysis, it was found that the 5′ end of the silk fibroin gene was rich in AT (Manning et al. 1978). Then, its function and regulation were extensively studied. Research showed that the transcription factor trx (Li 2017), E2F and Ras1 gene play a regulatory role in the synthesis of silk proteins, the tyrosine kinase gene Abl has a certain effect on silk protein secretion (Liu 2018), and miR-2805 significantly downregulate the expression of B. mori fibroin light-chain gene (Chen 2019). Studies have also been conducted on Sylepta derogata, a main pest for cotton and another cash crop, the larvae of which cause damage by spinning silk and leaf rolling. The fibroin of S. derogata was cloned in 2016, RNAi was used to interfere with the expression of fibroin and affect the silking behavior (Chen 2018).
In Acari, Tetranychus spp. are typical species that spin silk for dispersal and movement between plants (Clotuche et al. 2011). However, at present, analysis of spider mite silk is limited. Seventeen genes of Tetranychus urticae Koch were annotated by very weak sequence similarity with fibroin genes of other arthropods, encoding fibroin with an abnormally high serine content (27–39%) (Grbić et al. 2011). In 2020, Tetranychus lintearius was shown to produce a large amount of nanosized silk, and fluorescence labelling showed that this silk was a new cytocompatible material and a potential source of natural nanoparticles with various potential applications (Lozano-Perez et al. 2020). Through comparative genomics and multigroup analysis, two kinds of fibroin were identified and showed a high Young's modulus (Arakawa et al. 2021), even exceeding that of spider silk, showing that spider mite silk has considerable application prospects.
Stigmaeopsis nanjingensis belongs to the family Tetranychidae and can produce a large amount of silk; the function of its silk is different from that of the other spider mites. Stigmaeopsis nanjingensis is a main pest of bamboo, and uses dense silk threads to make nests (Pellizzari and Duso 2009) (Fig. 1a). Both male and female mites have silk spinneret (Fig. 1c, d), but only female adult mites can weave nests. In the nests, female mites weave sticky silk and stick waste residue to the top, so as to clean the nest; Male mites cooperate to maintain their nests. In addition, S. nanjingensis weaves a web mat around the eggs to fix their position to prevent them from being disturbed during mite activities (Kanazawa et al. 2011) (Fig. 1b). These are strong indicators that silk is essential for S. nanjingensis growth and development. Stigmaeopsis nanjingensis caused severe damage to bamboo leaves during high-temperatures season from July to August that led to plant dieback due to falling of leaves. Under these circumstances, it is difficult to provide relief via biological and chemical control. The design of the nest can reduce the risk of predation (Mori et al. 1999), and being located on the underside of the leaves makes the control of S. nanjingensis more complicated. Therefore, we provide a control measure from the perspective of interfering with the silking behavior of S. nanjingensis. However, a systematic study of the fibroin genes expressed in S. nanjingensis has not been reported yet. Here, we report the transcriptome of S. nanjingensis and verify one of the fibroin genes.
Electron micrographs of female Stigmaeopsis nanjingensis and her spinnerets. a Colony of S. nanjingensis, after manual removal of the woven net top. (b) Web mat woven for fixing eggs. c and d Spinnerets of S. nanjingensis at c 13,000× and d 5000×. suζ: terminal eupathidium; ul′ζ and ul″ζ: two lateral eupathidia; ω: solenidion; a, c: two tactile setae
RNAi is a post-transcriptional gene-silencing mechanism (Hutvangner and Zanmore 2002). RNAi with double-stranded RNA (dsRNA) has been used for analysis of gene functions in arthropods (Wilkins et al. 2005). Although the RNAi system was first established for insects, its application in mite research is common now. Several studies mainly focused on basic gene function analysis and screening of lethal target genes (Niu et al. 2018). Several methods have been developed for the delivery of dsRNA, including external contact, injection and feeding (Suzuki et al. 2017a, b; Guang et al. 2019). However, no research has been carried out on dsRNA transmission in S. nanjingensis to date. Due to the small size of the mites and the risks of physical damage or infection, injection is relatively complex. The soaking method consumes a large amount of dsRNA. Feeding is the most commonly used method for spider mites, such as Tetranychus urticae (= cinnabarinus) (Shen et al. 2017; Yoon et al. 2018) and Amphitetranychus viennensis (Zacher) (Zhao 2020). Stigmaeopsis nanjingensis is known to occur only on Gramineae plants (Sakagami et al. 2009), and bamboo is its main host. Leaf structure of bamboo makes dsRNA transmission difficult to enter the leaves for the feeding of mites. As the leaf develops, it forms two different regions of wettability which persists as the leaf bends down into its natural position (Wigzell et al. 2016). Leaf margins are highly hydrophobic whereas the middle of the leaf is hydrophilic in nature. Liquid landing on the leaf margins forms beads which when large enough roll towards the middle. There the liquid collects and coalesces to form a stream like film of water that is funneled to the tip and falls to the ground (Wigzell et al. 2016). There are difficulties in determining the transmission of intended target through food intake. Based on this, we used a nanomaterial-based delivery system to deliver dsRNA via body wall permeation in mites (Zheng et al. 2019).
Gene vectors rationale is that polycations pack large gene coils into nanoparticles by charge neutralizing, which prevents gene degradation and facilitates its cellular uptake through endocytosis pathways (Ponnuswamy et al. 2017). The nanocarrier used is a star polycation (SPc) (Yan et al. 2019). Its star-shaped structure has a high density of functional groups and unique chemical properties of dendrimers (Fig. 2a), which are considered good for mass production of functional polymers. The chemical source of SPc, the monomer 2-N-(dimethyl aminoethyl) methacrylate (DMAEMA), is commercially available and an inexpensive chemical raw material (Li et al. 2019). In this study, for the first time we evaluate the gene-silencing effect and the lethal effect of dsRNA/nanocarrier on S. nanjingensis. Nanocarrier-based delivery systems not only have a simple operation procedure and high gene-silencing efficiency but also have low dsRNA consumption. This system was applied to S. nanjingensis for the first time, providing a new management method for mites. SPc is a kind of nanomaterial with high efficiency and low cost that can be applied in practical production (Yan et al. 2019).
a Chemical structure of the nanocarrier SPc (from Li et al. 2019). b The dsRNA/nanocarrier transportation system
Materials and methods
Stigmaeopsis nanjingensis rearing and experimental conditions
The original strain of S. nanjingensis was collected from the bamboo forest near the library of Guizhou University in Guiyang City, China, and kept in a climate control chamber, Institute of Entomology, Guizhou University. During the experiments, the bamboo leaves covered with wet cotton wool were placed in the culture dish for the feeding of S. nanjingensis. The culture conditions were 26 ± 0.5 °C, 70% RH, and L16:D8 photoperiod.
Gene cloning and analysis of biological information
The fibroin gene was obtained by transcriptome sequencing of S. nanjingensis. Total RNA was extracted with MiniBEST Universal RNA Extraction Kit, following the manufacturer’s instructions (Takara Biomedical Technology, Beijing, China). First-strand cDNA was synthesized using StarScript II First-strand cDNA Synthesis Mix with gDNA Remover (Gene Star, Beijing, China), and RNA and cDNA concentrations were measured by a NanoDrop 2000c spectrophotometer. After quality checking, samples were stored at −80 and –20 °C for subsequent experiments.
All primers (Table S1) were designed by Primer v.5.0 software and synthesized by Sangon Biotech (Shanghai, China). The fibroin gene was amplified by using 2 × SanTaq PCR Master Mix (with Blue Dye) (Sangon Biotech, Shanghai, China). The PCR procedure was as follows: pre-denaturation at 94 °C for 4 min; 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min; and a final cycle of 72 °C for 10 min. The recovered purified product was ligated to the pGEM-T Easy vector (Promega, Beijing, China) and transformed into the Escherichia coli DH5α strain (Sangon Biotech). Positive colonies were selected and sent to Sangon Biotech for sequencing.
The cloned sequence was uploaded to the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov). Amino acid composition of the proteins was calculated using PredictProtein (https://predictprotein.org/). The transmembrane structure was calculated using the TMHMM Server (http://www.cbs.dtu.dk/services/TMHMM/). Hydrophobicity was calculated using ExPASy-ProtScale (https://web.expasy.org/protscale/). Signal peptide was calculated using SignalP v.3.0 (http://www.cbs.dtu.dk/services/SignalP/). Phosphorylation site prediction was carried out by using the NetPhos3.1 Server (http://www.cbs.dtu.dk/services/NetPhos/). Prediction of glycosylation sites was performed using the online software YinOYang v.1.2 (http://www.cbs.dtu.dk/services/Yin OYang/). The physicochemical properties were computed using the ExPASy-ProtParam tool (https://web.expasy.org/protparam/). Using the SOPMA tool, protein secondary structures were visualized (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automa t.pl?page = npsa_sopma.html).
Relative expression of fibroin genes in different mite developmental stages
To investigate the expression profiles of fibroin, different developmental stages (egg, larva, nymph and adult) were collected with three biological replicates. In total 120 eggs, 100 larvae, 80 nymphs and 50 adults were collected for this experiment. The nine housekeeping genes (Actin, EF1A, RPL13, v-ATPase Tubulin, UBC, 28S rRNA, TBP, and 18S rRNA) were selected to design primers (Table S1) for screening the most stable housekeeping genes in different development stages, and their expression abilities in four developmental stages were evaluated by four algorithms (geNorm (Vandesompele et al. 2002), NormFinder (Andersen et al. 2004), BestKeeper (Pfaffl et al. 2004) and the ΔCt (Silver et al. 2006). All RNA was extracted by an RNA extraction kit (Takara Biomedical Technology) according to the manufacturer’s protocol. Integrity of RNA was determined by 1% agarose electrophoresis. The total RNA was reverse transcribed as described above.
The enzyme used in the qPCR was 2 × RealStar Green Fast Master Mix (Gene Star). The reaction conditions for the qPCR were as follows: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. A melting curve was generated to ensure that no reaction produced nonspecific amplification. Relative standard curves for each step were generated from a fivefold cDNA dilution series. Using the 2−∆∆Ct method, the gene expression levels were normalized to the abundance of the reference. Three technical repetitions were performed for each sample to determine the average value.
Preparation of the dsRNA/nanocarrier formulation and the RNAi effect detection
Off target effects were detected using si-Fi tools (https://bio.tools/si-Fi). Synthesis of dsRNA was performed using the Transcript T7 High Yield Transcription Kit (Thermo Scientific, Shanghai, China). After synthesis, dsRNA was purified using the Gene JET RNA Purification Kit (Thermo Scientific). Nanomaterials and dsRNA were mixed gently at the recommended mass ratio of 1:1 (the final concentration of both nanomaterials and dsRNA was 500 ng/µl) to form a complex (Yan et al. 2019). The green fluorescent protein gene was used as a control (Thairu et al. 2017). Twenty female adult mites were placed onto a new leaf disc, and the dsFib/nanocarrier is dropped on the mite body wall by a microinjector (Narishige IM-31, Tokyo, Japan), at pressure 35 kPA. Amount of each injection was 20–50 nl (Fig. 2b). Treated leaf disc was placed in a climate chamber at 25 ± 1 °C with 70 ± 5% RH and L16:D8 photoperiod. As shown in Fig. 2b, female adult mites were treated with dsFib/nanocarrier, dsFib, dsGFP and nanocarrier, respectively. Each treatment consisted of 20 mites, three repetitions were used to determine the interference efficiency, and six repetitions were used to detect lethal effects. Time was recorded after the complete absorption of droplets, then three groups of droplets with the same volume were dropped on the blade, and their volatilization time was recorded (nine drops in each group).
After 24 h of treatment, RNA sample was extracted and reverse transcribed into cDNA. Gene interference efficiency was determined by quantitative PCR (qPCR) as described above. Morphological changes of silk were observed by electron microscopy.
Statistical analysis
The 2−∆∆Ct method was used for relative quantitation. The experimental data were analyzed by t-test in IBM-SPSS v.21.0 (IBM, Armonk, NY USA) to compare the difference between the treatment group and the control group. The expression pattern of the target gene was analyzed by the ANOVA in GraphPad Prism v.8.0 software. The software geNorm, NormFinder, BestKeeper and RefFinder were used to screen housekeeping genes. All column charts are made with GraphPad Prism v.8.0.
Results
Transcriptome sequencing
We separately completed the transcriptome sequencing of S. nanjingensis. In total, 6.41 Gb data were measured using the BGISEQ-500 platform (Fig. S1). 26,896 Unigenes were assembled and de-redundant. With BUSCO completeness scores (c) of 94% for S. nanjingensis, the Q20 base percentage exceeded 97.0%, the Q30 base percentage exceeded 92.4% (Table S2). Assembly of clean reads followed by clustering deredundant transcripts resulted in unigenes; 26,896 unigenes with an N50 of 2453 bp were generated (Fig. S2a). The transcriptome sequence data have been deposited in the Sequence Read Archive under BioProject ID PRJNA768242. UniGene functions were annotated to seven databases (Table 1).
The highest two percentages of unigenes annotated were found in the NR and KEGG databases and were almost equivalent, with 67 and 55%, respectively. Moreover, the NT database had the lowest number of annotated unigenes with 6728 unigenes (25%). Based on the NR annotation information, a total of 17,480 unigenes have been annotated in the S. nanjingensis transcriptome, of which the most similar genes to T. urticae accounted for 81.8%, followed in order by Limulus polyphemus (0.91%), Centruroides sculpturatus (0.62%), Dermatophagoides pteronyssinus (0.55%), and Tetranychus truncatus (0.49%) (Fig. S2b).
Based on the GO database, functional classification statistics were performed on the transcriptome of S. nanjingensis, in which there were 7332 unigenes in the biological process, 9348 unigenes in the cellular component, 12,155 unigenes in total by molecular function (Fig. S2c). According to the annotation information of the KEGG database, a total of 14,765 unigenes were annotated onto six categories of metabolic pathways: cellular processes, environmental information processing, genetic information processing, human diseases, metabolism, and organismal systems (Fig. S2d).
Cloning, characterization and expression patterns in different developmental stages of fibroin
In this study, 17 fibroins were identified in the transcriptome. Eight of the fibroins presented a complete ORF. Of these fibroins, the Unigene15321-Z1 and CL1353.contig8-Z1 have neither found the blast query nor structural features of fibroin. Both, CL1308.contig2-Z1 and CL2865.contig1-Zl found the blast query for the insect fibroin gene, the identity was between 67 and 74%. The CL1703.contig2-Z1 and Unigene15298_Z1 found the blast query for T. urticae fibroin gene, the identity was between 67 and 74%. The CL479.contig1-Zl has typical fibroin gene motif and a general motif of spider silk protein. The multiple alignment results are shown in Fig. 3. We found the composition similar to other fibroins, all with relatively conserved glycines, and comprising dipeptide repeating units composed of glycine-alanine or glycine-serine elements of low complexity. The ORF of target fibroin (CL479.contig1-Zl) was obtained from transcriptome data and verified by PCR. The sequence can be obtained in GenBank under accession number MZ436653. The complete ORF of the fibroin gene was 2493 bp long, encoding a total of 831 amino acids (Fig. S3), considered a heavy-chain component. The fibroin protein sequence contains 20 amino acids, among which glycine, serine and alanine account for > 50% of the total composition (Fig. S4a). The protein has no obvious transmembrane structure, so it was preliminarily identified as a non-transmembrane protein (Fig. S4b). Hydrophobic and hydrophilic residues are distributed throughout the polypeptide chain of fibroin, but the proportion of hydrophobic amino acids is lower than that of hydrophilic amino acids, so fibroin is considered a hydrophilic protein (Fig. S4c), and there are also signal peptide sites between amino acids 18 and 19 (Fig. S4d).
The secondary structure of fibroin consisted of an alternating pattern of random coils and α-helix and β-chain motifs (Fig. S4e), fitting the known fibroin secondary structure motifs. And the domain composition of S. nanjingensis fibroin protein has a typical fibroin motif. Two internal domains (domain 1: 330–485 and domain 2: 541–670), with distinct repetitive motifs (forming β-sheets), are separated by spacer domain (485–541). And there is a motif GGX (X: Ala, Ser), which is a general motif in spider silk protein (Table S3; Jelinski 1998). The unique toughness of these biopolymers is due to the secondary structure forming alternating crystalline (rigid) and amorphous (flexible) blocks (Sarkar et al. 2019). Fibroin has multiple phosphorylation and glycosylation sites. The calculated theoretical isoelectric point is 5.78, and the molecular weight is estimated to be 80.6 kDa.
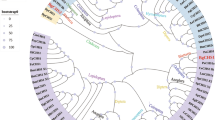
The sequence obtained in this study was compared with those of some silking arthropods recorded in the NCBI database, the results showed that the homology of fibroin gene in the arthropods was not high—even in the same family (Tetranychidae) it is not conservative (Fig. 4). By NCBI blast analysis, it was found that its sequence did not show strong homogeneity with the silk fibroin gene sequences of other arthropods. It is possible that the conservation of the silk protein gene in arthropods is not strong or that the fibroin of mites has a unique specificity.
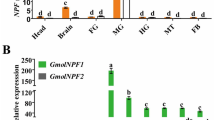
Standard curves were generated for Fib and the nine housekeeping genes, using fivefold serial dilution of pooled cDNA. The correlation coefficient (R2) and PCR efficiency (E%) for each standard curve are shown in Table S4. R2 is 0.98–1.0, and efficiency of RT-qPCR is 90–110%. Using geNorm analysis, overall ranking of reference genes from most to least stable was: UBC > EF-1α > 18 s RNA > TBP > α-Tubulin > RPL13 > β-actin > 28 s RNA > v-ATPase (Fig. S5). The genetic stability ranking of BestKeeper analysis was significantly different from the other three methods. 18 s RNA, UBC, and EF-1α were all ranked in the top three in the stability analysis of ΔCt, geNorm, and NormFinder, whereas TBP was ranked first in the BestKeeper calculations. Comprehensive ranking calculated by RefFinder is: UBC > 18 s RNA > EF-1α > β-actin > 28 s RNA > Tubulin > RPL13 > v-ATPase > TBP (Table S5). It turned out that the most stable housekeeping gene in different developmental stages of S. nanjingensis was UBC. Therefore, UBC was selected as the housekeeping gene in this study. Relative expression of the fibroin gene was measured by qPCR after sampling each state of S. nanjingensis (Fig. 5). Results showed that expression level of fibroin varied greatly among mite stages. Expression in larvae was relatively high, followed by nymphs, and expression in eggs and adults was relatively low.
Effects of dsFib/nanocarrier complexes on mite survival rate
To verify the safety of nanocarriers for S. nanjingensis, we explored the lethal effects of nanocarriers and dsFib/nanocarrier composites on S. nanjingensis. Survival rate was 98.4% after 24 h of dsGFP treatment, 95.9% after nanocarrier treatment, 96% after dsFib treatment, and 92.8% after dsFib/nanocarrier complex treatment (Fig. 6). There was no significant difference among the three groups, which indicated that the nanocarrier was safe for S. nanjingensis.
The effect of introducing nanocarrier on the survival rate of Stigmaeopsis nanjingensis can be neglected. Under the interference concentration of 500 ng/μl, the mean (± SEM) survival rates of S. nanjingensis treat with dsGFP, nanocarrier and dsFib/nanocarrier were > 92%. Means did not differ significantly (t-test: P > 0.05)
Detection of silence effect of dsFib/nanocarrier
The dsFib/nanocarrier droplets naturally adhered to the dorsa of S. nanjingensis and absorbed within 2 min, nanocarriers also absorbed in mites within 2 min, and average permeation time of dsGFP and dsFib is 2 min 14 s, whereas the average evaporation time of droplets with the same volume is 7 min 10 s. Our results indicate that all treatment groups penetrate into the body (Fig. S6). To determine the RNAi delivery system efficiency, fibroin gene levels were determined by RT-qPCR after 24 h of treatments. There was no significant difference between the nanocarrier and dsGFP control groups (Fig. 7). In dsFib/nanocarrier treatment, fibroin gene expression decreased by 75.4%, and interference efficiency of dsFib treatment group was 59.8%. synergized nanocarriers 15.6%. These results indicated that dsRNA and dsFib/nanocarrier complex effectively inhibit the expression of target genes, but interference efficiency of the dsFib/nanocarrier treatment group was significantly higher than that of the dsRNA treatment group. This shows that addition of nanocarriers improves the interference efficiency. The findings provide a new RNAi method for Tetranychidae and a pest control delivery system that could be applied in practical fields.
Effect of gene interference on silk morphology of Stigmaeopsis nanjingensis
Comparison of scanning electron microscopy showed that morphological structure of the silk was not different between the two control treatments (Fig. 8). Silk in the dsGFP and nanocarrier treatment groups was clearly smoother and tighter than dsFib/nanocarrier treatment group, and net structure is neat and orderly. In dsFib and dsFib/nanocarrier treatment group, silk structure has uneven thickness, crimp and irregularity. The diameter of silk was generally smaller in the treated compared to the control group, and network structure was disorganized. The morphological structure of S. nanjingensis silk infiltrated with dsFib was damaged up to some extent after interference. This shows that fibroin may be related to the silk spinning behavior of S. nanjingensis.
Actual electron micrographs of female adult silk of Stigmaeopsis nanjingensis after RNAi by body wall permeation based on nanocarriers. (a–d, a'–d') Silk produced by female adult S. nanjingensis under the treatments of dsGFP, nanocarrier, dsFib and dsFib/nanocarrier groups, at (a–d) 500× and (a'–d') 5000×
Discussion
Fibroin has traditionally been used to make textiles. Increasing research has shown that fibroin has important value in daily life and scientific research. Fibroin has good biocompatibility, material universality, mechanical resistance and controllable degradability; it can be used in artificial skin, blood vessels, bones materials as a suture in surgery and in biomedical materials (Barrett et al. 2013). At present, research on silk-producing arthropods is mainly focused on economic insects and spiders for the purpose of economic perspectives and national defense. However, many insects caused great loss to cash crops and ornamental plants by silk production. For example, S. derogata curle and eat cotton leaves, consequently the cotton plants cannot blossom and normally bear bolls, which seriously affects cotton production (Tang 2016). Tetranychus urticae has a strong reproductive capacity, and a large number of individuals spread by spinning silk and relying on wind, causing a sustained increase in the population and a wide range of hazards (Fleschner et al. 1956). Stigmaeopsis nanjingensis also has a strong silk spinning ability, which also increases the difficulty of biological and chemical control. Therefore, a new method with low environmental pollution risks has become an urgent requirement for the control of S. nanjingensis.
Most research on S. nanjingensis has focused on classification and biological habits, and less attention has been paid on molecular biology. Based on integral role play by silk in the life cycle of S. nanjingensis, we screened a fibroin gene through transcriptome information to explore the impact on silk production and functionality. In this study, we used silking performance to curb silking and reduce harm to target crops. The findings are expected to provide a new way to control silk-producing pests. Our results obtained from transcriptome data showed that fibroin genes are not conservative with each other in S. nanjingensis, that are similar to T. urticae. An evolutionary tree of other arthropods with fibroin genes, showed that different forms of fibroin lack homology (Numata 2020), even compared to T. urticae. However, it has characteristics that are similar to other arthropod fibroins, such as repeat motifs and amino acid composition. Secondary structure is a repeating pattern of alternating random coils and α-helix and β-chain motifs. In amino acid composition, serine, glycine and alanine account for the highest proportion (> 50%) of the amino acid composition, similar amino acid compositional bias is observable in silkworm fibroin. Subsequently, we explore its function through RNAi technology.
When using RNAi technology to control pests, effective introduction of dsRNA synthesized in vitro into insects is one of the strongest limiting factors. Different arthropods require appropriate dsRNA delivery systems to ensure efficient gene silencing and ease of manipulation (Zhang et al. 2013; Vogel et al. 2019). In spider mites, methods of dsRNA delivering include feeding method, soaking method and transgenic plants (Suzuki et al. 2017b). In this research, dsRNA was transmitted by the body wall permeation method using nanomaterials as the carrier. The nanocarrier used in this study is SPC, and its toxicity to S. nanjingensis is negligible. The SPc is a cationic dendrimer that condense random nucleic acids into complexes that are taken up through endocytosis (Win and Feng 2005). High-density amine functional groups at the periphery of nanocarrier enhance the affinity of complex for barrier components e.g., epidermis layer of insect body wall and cell membrane, body wall of Tetranychidae mainly composed of upper epidermis, outer epidermis, inner epidermis and dermatocyte layer (Hong et al. 1994), that are easily penetrated into the body compared to insects. In addition, SPc rapidly internalized into living cells with high efficacy of gene delivery and low cytotoxicity (Li et al. 2019). In our study, dsRNA/nanocarrier dose was placed on the dorsa of S. nanjingensis by a microinjector, the interference efficiency of dsFib/nanocarrier treatment was 75.4%, whereas dsFib treatment was 59.8%—addition of nanocarrier improved the interference efficiency (Fig. 6). As compared to common feeding methods, the amount of dsRNA required for a nanocarrier-based transdermal dsRNA delivery system is particularly low. In this study, 20–50 nl dsFib per S. nanjingensis and 2–4 uL dsRNA was sufficient for one treatment. During feeding method, a large amount of dsRNA was consumed without successful interference. Nanocarrier-based transdermal dsRNA delivery system reduces the consumption of dsRNA and saves the experimental cost. Electron microscopy results showed that silk in the control group was smooth and tight, with good fullness. However, after inhibition of the fibroin gene, the silk threads showed varying thickness, messy network structure, and the network gap became larger. Our results showed that fibroin gene played an important role in the silk formation process, and dsRNA targeting S. nanjingensis fibroin effectively affect the surface morphology of the net and flatness of the silk. How it affects the silk morphology remains to be further studied and analyzed. Moreover, web-spinning behavior is a complex trait controlled by multiple genes, in order to better explain the mechanism of silk spinning harm for S. nanjingensis and to curb the harm of silk spinning in application, there is still need much work to be carried out.
At present, pesticides are mainly used to control pests, but excessive use of chemical pesticides has caused various environmental problems, and long-term use has also made pests resistant (Gavrilescu et al. 2015). Nanocarriers carry dsRNA and pesticides, the delivery system reduces the use of pesticides and relieves the pressure of ecosystems on organisms (Xu et al. 2014a, b). However, RNAi is mainly used for the management of small arthropod pests, which have difficulties in determining the feed, are small in size, have weak body walls and are equipped with piercing-sucking mouthparts (Zheng 2018). The outermost wax layer on the body wall of arthropods is relatively thick—e.g., as in aphids—and it is necessary to add detergent to dsRNA/nanocarrier complexes to form dsRNA/nanocarrier/detergent complexes (Shen et al. 2014). The surfactant molecule in the detergent is amphiphilic, with hydrophilic groups forming a protective layer around the surface of the dsRNA/nanocarrier complex and lipophilic groups facing outward for subsequent adsorption to the insect body wall. The whole process only needs a simple operation by dropping, and high mortality and systematic errors caused by mechanical damage can be avoided, which provides an efficient and convenient operation scheme for scientific researchers and a new RNAi technology for mites. Thus, the nanocarrier-based transdermal dsRNA delivery system provides a new approach for the management of mite pests.
Conclusion
This work explored a new control method from the perspective of biological silk production, mining fibroin protein genes of S. nanjingensis and using a body wall infiltration method based on nanomaterials for gene interference. Results showed that dsRNA/nanocarrier enters the mite body within 2 min after being dripped on the surface, and silencing efficiency is 75.4%. Interference efficiency is increased by 15.6% compared with direct dropping dsRNA. After interference, morphological structure of silk is obviously damaged. Our experimental results showed that interfering with the expression of the female adult fibroin protein gene by using nanomaterials may represent a new method for the prevention and control of S. nanjingensis. This study provides a research basis for elucidating the mechanisms of silk laying, cocoon formation and transfer damage in many agricultural and forest pests.
References
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496
Arakawa K, Mori M, Kono N, Suzuki T, Gotoh T, Shimano S (2021) Proteomic evidence for the silk fibroin genes of spider mites (Order Trombidiformes: Family Tetranychidae). J Proteomics 239:104195. https://doi.org/10.1016/j.jprot.2021.104195
Barrett DG, Fullenkamp DE, He L, Holten-Andersen N, Lee KYC, Messersmith PB (2013) pH-based regulation of hydrogel mechanical properties through mussel- inspired chemistry and processing. Adv Funct Mater 23:1111–1119. https://doi.org/10.1002/adfm.201201922
Chen CX (2018) Cloning and functional verification on of silk fibroin light chain in Sylepta derogata fabricius, Dissertation, Yangzhou University of China
Chen YH, Jiang T, Tan ZC, Xue P, Xu J, Tang SM, Yi YZ, Shen XJ (2019) Bom-miR-2805 upregulates the expression of Bombyx mori fibroin light chain gene in vivo. J Cell Biochem 120:14326–14335. https://doi.org/10.1002/jcb.28538
Clotuche G, Mailleux AC, Fernandez AA, Deneubourg JL, Detrain C, Hance T (2011) The formation of collective silk balls in the spider mite tetranychus urticae Koch. PLoS ONE 6:e18854. https://doi.org/10.1371/journal.pone.0018854
Craig CL (1997) Evolution of arthropod silks. Annu Rev Entomol 42:231–267. https://doi.org/10.1146/annurev.ento.42.1.231
Fleschner CA, Badgley ME, Ricker DW, Hall JC (1956) Air drift of spider mites. Econ Entomol 49:624–627
Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N Biotechnol 32:147–156. https://doi.org/10.1016/j.nbt.2014.01.001
Grbić M, Van Leeuwen T, Clark RM, Rombauts S et al (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:478–492. https://doi.org/10.1038/nature10640
Guang MS, Wen C, Chuan ZL, Shi YO, Lin H (2019) RNAi targeting ecdysone receptor blocks the larva to adult development of Tetranychus cinnabarinus. Pestic Biochem Physiol 159:85–90. https://doi.org/10.1016/j.pestbp.2019.05.020
Hoffman RT, Schmidt ER, Case ST (1996) A cell-specific glycosylated silk protein from Chironomus thummi salivary glands. Cloning, chromosomal localization, and characterization of cDNA. J Biol Chem 271:9809–9815. https://doi.org/10.1074/jbc.271.16.9809
Hong XY, Wang MC, You ZP (1994) Cuticle structure and moulting process of Tetranychus cinnabarinus abarinus (boisduval). J Nanjing Agric Univ 4:42–48 (in Chinese)
Hutvagner G, Zamore PD (2002) RNAi: nature abhors a double-strand. Curr Opin Genet Dev 12:225–232. https://doi.org/10.1016/S0959-437X(02)00290-3
Jelinski LW (1998) Establishing the relationship between structure and mechanical function in silks. Curr Opin Solid State Mater 3:237–245. https://doi.org/10.1016/S1359-0286(98)80097-1
Kanazawa M, Sahara K, Saito Y (2011) Silk threads function as an ‘adhesive cleaner’ for nest space in a social spider mite. Proc R Soc B 1278:1653–1660. https://doi.org/10.1098/rspb.2010.1761
Li J (2017) Screening and analysis of genes related with the characters of cocoon and silk production in silkworm. Dissertation, Jiangsu University of Science and Technology
Li J, Qian J, Xu Y, Yan S, Shen J, Yin M (2019) A facile-synthesized star polycation constructed as a highlyeicient gene vector in pest management. ACS Sustain Chem Eng 7:6316–6322. https://doi.org/10.1021/acssuschemeng.9b00004
Liu N (2018) Research on the correlation of cocoon silk trait and the part of genes related with silk production in silkworm. Dissertation, Jiangsu University of Science and Technology
Lozano-Perez AA, Pagan A, Zhurov V, Hudson SD, Hutter JL, Pruneri V, Perez-Moreno I, Grbić V, Cenis JL, Grbić M, Aznar-Cervantes S (2020) The silk of gorse spider mite Tetranychus lintearius represents a novel natural source of nanoparticles and biomaterials. Sci Rep 10:18471. https://doi.org/10.1038/s41598-020-74766-7
Manning RF, Gage LP (1978) Physical map of the Bombyx mori DNA containing the gene for silk fibroin. J Biol Chem 253(6):2044–2052
Mori K, Saito Y, Sakagami T (1999) Effects of the nest web and female attendance on survival of young in a subsocial spider mite, Schizotetranychus longus (Acari: Tetranychidae). Exp Appl Acarol 23:411–418
Niu JZ, Shen GM, Christiaens O, Smagghe G, He L, Wang JJ (2018) Beyond insects: current status, achievements and future perspectives of RNAi in mite pests. Pest Manage Sci 74:2680–2687. https://doi.org/10.1002/ps.5071
Numata K (2020) How to define and study structural proteins as biopolymer materials. Polym J 52:1043–1056. https://doi.org/10.1038/s41428-020-0362-5
Ohshima Y, Suzuki Y (1977) Cloning of the silk fibroin gene and its flanking sequences. PNAS 74:5363–5367. https://doi.org/10.1073/pnas.74.12.5363
Pellizzari G, Duso C (2009) Occurrence of Stigmaeopsis nanjingensis in Europe. B Insecto 62:149–151
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable reference genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. https://doi.org/10.1023/B:BILE.0000019559.84305.47
Ponnuswamy N, Bastings MMC, Nathwani B, Ryu JH, Chou LYT, Vinther M, Li WA, Anastassacos FM, Mooney DJ, Shih WM (2017) Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat Commun 8:15654. https://doi.org/10.1038/ncomms15654
Sakagami T, Saito Y, Kongchuensin M, Sahara K (2009) Molecular phylogeny of Stigmaeopsis, with special reference to speciation through host plant shift. Ann Entomol Soc Am 3:360–366. https://doi.org/10.1603/008.102.0303
Sarkar A, Connor AJ, Koffas M, Zha RH (2019) Chemical synthesis of silk-mimetic polymers. Materials 12:4086. https://doi.org/10.3390/ma12244086
Shen D, Zhou F, Xu Z, He B, Li M, Shen J, Yin MZ, An CJ (2014) Systemically interfering with immune response by a fluorescent cationic dendrimer delivered gene suppression. Mater Chem B 2:4653–4659. https://doi.org/10.1039/c4tb00411f
Shen GM, Song CG, Ao YQY, Xiao YH, Zhang YJ, Pan Y, He L (2017) Transgenic cotton expressing CYP392A4 double-stranded RNA decreases the reproductive ability of Tetranychus cinnabarinus. Insect Sci 24:559–568. https://doi.org/10.1111/1744-7917.12346
Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Bio 7:33. https://doi.org/10.1186/1471-2199-7-33
Song FZ (2002) Study on fibroin heavy chain of the silkworm Bombyx mori by fluorescence in situ hybridization (FISH). Sci China Ser 45:663–668. https://doi.org/10.1007/BF02879755
Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ (2010) Insect silk: one name, many materials. Annu Rev Entomol 55:171–188. https://doi.org/10.1146/annurev-ento-112408-085401
Suzuki T, España MU, Nunes MA, Zhurov V, Dermauw W, Osakabe M, Van Leeuwen T, Grbić M, Grbić V (2017a) Protocols for the delivery of small molecules to the two-spotted spider mite, Tetranychus urticae. PLoS ONE 12:e0190025. https://doi.org/10.1371/journal.pone.0180658
Suzuki T, Nunes MA, Espana MU, Namin HH, Jin PY, Bensoussan N, Zhurov V, Rahman T, De Clercq R, Hilson P, Grbić V, Grbić M (2017b) RNAi-based reverse genetics in the chelicerate model Tetranychus urticae: A comparative analysis of five methods for gene silencing. PLoS ONE 12:e0180654. https://doi.org/10.1371/journal.pone.0180654
Tang RY (2016) Cloing, functional verification and application in production of fibroin heavy chain gene in Sylepta derogata fabricius. Dissertation, Yangzhou University of China
Thairu MW, Skidmore IH, Bansal R, Novakova E, Hansen TE, Li-Byarlay H, Wickline SA, Hansen AK (2017) Efficacy of RNA interference knockdown using aerosolized short interfering RNAs bound to nanoparticles in three diverse aphid species. Insect Mol Biol 26:356–368. https://doi.org/10.1111/imb.12301
Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1
Vogel E, Santos D, Mingels L, Verdonckt TW (2019) RNA interference in insects: protecting beneficials and controlling pests. Front Physiol 9:1912. https://doi.org/10.3389/fphys.2018.01912
Wigzell JM, Racovita RC, Stentiford BG, Wilson M, Harris MT, Fletcher IW, Mosquin DPK, Justice D, Beaumont SK, Jetter R, Badyal JPS (2016) Smart water channelling through dual wettability by leaves of the bamboo Phyllostachys aurea. Colloid Surf A 506:344–355. https://doi.org/10.1016/j.colsurfa.2016.06.058
Wilkins C, Dishongh R, Moore SC, Whitt MA, Chow M, Machaca K (2005) RNA interference is an antiviral defense mechanism in Caenorhabditis elegans. Nature 436:1044–1047. https://doi.org/10.1038/nature03957
Win KY, Feng SS (2005) Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 26:2713–2722
Xu L, Duan X, Lv Y, Zhang X, Nie Z, Xie C, Ni ZF, Liang RQ (2014a) Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobion avenae tolerance of Phoxim insecticides. Transgenic Res 23:389–396. https://doi.org/10.1007/s11248-013-9765-9
Xu ZJ, He BC, Wei W, Liu KL, Yin MZ, Yang WT, Shen J (2014b) Highly water-soluble perylenediimide-cored poly (amido amine) vector for efficient gene transfection. J Mater Chem B 2:356–368. https://doi.org/10.1039/c4tb00195h
Yan S, Qian J, Cai C, Ma Z, Li J, Yin M, Ren B, Shen J (2019) Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. Pest Sci 93:449–459. https://doi.org/10.1007/s10340-019-01157-x
Yoon JS, Sahoo DK, Maiti IB, Palli SR (2018) Identification of target genes for RNAi-mediated control of the twospotted spider mite. Sci Rep 8:14687. https://doi.org/10.1038/s41598-018-32742-2
Zhang H, Li HC, Miao XX (2013) Feasibility limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci 20:15–30. https://doi.org/10.1111/j.1744-7917.2012.01513.x
Zhao J (2020) Study on gene silencing and RNAi efficiency by dsRNA feeding in Amphitetranychus viennensis. Dissertation, Shanxi University
Zheng Y (2018) The nanocarrier-based novel strategy for pest control. Dissertation, China Agricultural University
Zheng Y, Hu YS, Yan S, Zhou H, Song DL, Yin MZ, Shen J (2019) A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manage Sci 75:1993–1999. https://doi.org/10.1002/ps.5313
Zhou CZ, Confalonierif F, Medina N, Zivanovic Y, Esnault C, Yang T, Jacquet M, Janin J, Duguet M, Perasso R, Li ZG (2000) Fine organization of Bombyx fibroin heavy chain gene. Nucleic Acids Res 28:2413–2419. https://doi.org/10.1093/nar/28.12.2413
Zhou CZ, Confalonieri F, Jacquet M, Perasso R, Li ZG, Janin J (2001) Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 44:119–122. https://doi.org/10.1002/prot.1078
Acknowledgements
We thank Professor Shen Jie (China Agricultural University) for providing nanomaterials and Dr. Gary R. Bauchan (Electron and Confocal Microscopy Unit, ARS, USDA) for taking SEM micrographs. The research was supported by Ministry of Agriculture and Rural Affairs (15216014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Liu, R., Li, G. et al. Identification of the fibroin of Stigmaeopsis nanjingensis by a nanocarrier-based transdermal dsRNA delivery system. Exp Appl Acarol 87, 31–47 (2022). https://doi.org/10.1007/s10493-022-00718-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00718-7