Abstract
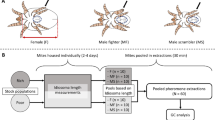
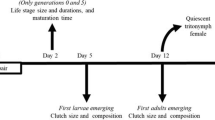
In male dimorphic species, growth influences morph expression and thereby the reproductive success of males. However, how variation in nutritional conditions affects male morph development and whether males can compensate for lost growth is poorly known. Here, we performed an experiment where males of the bulb mite (Rhizoglyphus robini)—which are fighters, able to kill other mites, or benign scramblers—were offered high quality food during the larval stage, but food of high or low quality during the protonymph and tritonymph (=final) stage. When food quality was low during the latter two stages, males matured smaller, later and were more likely to be a scrambler than when food quality was high. We found no evidence for compensatory growth: when males had low quality food only during the protonymph stage, they matured at the same age, but grew at a slower rate and matured at a smaller size than males that had high quality food throughout ontogeny. Furthermore, males that experienced this transient period of low food quality were less likely to mature as a fighter. Interestingly, scrambler increase in body size during the protonymph and tritonymph stages was always lower than that of fighters. Given the strong link between adult size and fitness, combined with the different development times and life histories of the male morphs, the lack of ability to compensate for a transient period of food deprivation during ontogeny is likely to have consequences for the dynamics of bulb mite populations.



Similar content being viewed by others
References
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190
Bates D, Sarkar D (2007) lme4: linear mixed-effects models using S4 classes. R package version 0.9975-13
Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P (2002) Population dynamic consequences of delayed life-history effects. Trends Ecol Evol 17:263–269
Benton TG, Plaistow SJ, Beckerman AP, Lapsley CT, Littlejohns S (2005) Changes in maternal investment in eggs can affect population dynamics. Proc R Soc Lond B 272:1351–1356
Benton TG, St Clair JJH, Plaistow SJ (2008) Maternal effects mediated by maternal age: from life histories to population dynamics. J Anim Ecol 77:1038–1046
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Buoro M, Gimenez O, Prévost E (2012) Assessing adaptive phenotypic plasticity by means of conditional strategies from empirical data: the latent environmental threshold model. Evolution 66:996–1009
Buzatto BA, Simmons LW, Tomkins JL (2012) Genetic variation underlying the expression of a polyphenism. J Evol Biol 25:748–758
Cohen J (1969) Statistical power analysis for the behavioral sciences. Academic Press, NY
Coulson T, MacNulty DR, Stahler DR, vonHoldt B, Wayne RK, Smith DW (2011) Modeling effects of environmental change on wolf population dynamics, trait evolution, and life history. Science 334:1275–1278
D’Amico L, Davidowitz G, Nijhout H (2001) The developmental and physiological basis of body size evolution in an insect. Proc R Soc Lond B 268:1589–1593
Day T, Rowe L (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat 159:338–350
Díaz A, Okabe K, Eckenrode CJ, Villani MG, Oconnor BM (2000) Biology, ecology, and management of the bulb mites of the genus Rhizoglyphus (Acari: Acaridae). Exp Appl Acarol 24:85–113
Emlen DJ (1994) Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera, Scarabaeidae). Proc R Soc Lond B 256:131–136
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Pearson Education Ltd, Harlow
Gerson U, Capua S, Thorens D (1983) Life history and life tables of Rhizoglyphus robini Claparède (Acari: Astigmata: Acaridae). Acarologia 24:439–448
Gross MR (1991) Salmon breeding behavior and life history evolution in changing environments. Ecology 72:1180–1186
Hazel W, Smock R, Lively CM (2004) The ecological genetics of conditional strategies. Am Nat 163:888–900
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Nicieza AG, Álvarez D (2009) Statistical analyses of structural compensatory growth: how can we reduce the rate of false detection? Oecologia 159:27–39
Oliveira RF, Taborsky M, Brockmann HJ (eds) (2008) Alternative reproductive tactics. Cambridge University Press, Cambridge
Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T (2010) Coupled dynamics of body mass and population growth in response to environmental change. Nature 466:482–487
Plummer M, Best N, Cowles K, Vines K (2006) Coda: output analysis and diagnostics for MCMC. R package version 0.10-7
Radwan J (1995) Male morph determination in two species of acarid mites. Heredity 74:669–673
Radwan J (2001) Male morph determination in Rhizoglyphus echinopus. Exp Appl Acarol 25:143–149
Radwan J, Bogacz I (2000) Comparison of life-history traits of the two male morphs of the bulb mite, Rhizoglyphus robini. Exp Appl Acarol 24:115–121
Radwan J, Czyż M, Konior M, Kolodziejcyk M (2000) Aggressiveness in two male morphs of the bulb mite Rhizoglyphus robini. Ethology 106:53–62
Radwan J, Unrug J, Tomkins JL (2002) Status-dependence and morphological trade-offs in the expression of a sexually selected character in the mite, Sancassania berlesei. J Evol Biol 15:744–752
Roff DA (2002) Life history evolution. Sinauer Associates, Sunderland
Scriber J, Slansky F (1981) Nutritional ecology of immature insects. Annu Rev Entomol 26:183–212
Smallegange IM (2011a) Complex environmental effects on the expression of alternative reproductive phenotypes in the bulb mite. Evol Ecol 25:857–873
Smallegange IM (2011b) Effects of paternal phenotype and environmental variability on age and size at maturity in a male dimorphic mite. Naturwissenschaften 98:339–346
Smallegange IM, Coulson T (2011) The stochastic demography of two coexisting male morphs. Ecology 92:755–764
Smallegange IM, Coulson T (2013) Towards a general, population-level understanding of eco-evolutionary change. Trends Ecol Evol 28:143–148
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Tomkins JL, Hazel W (2007) The status of the conditional evolutionarily stable strategy. Trends Ecol Evol 22:522–528
Tomkins JL, LeBas NR, Unrug J, Radwan J (2004) Testing the status-dependent ESS model: population variation in fighter expression in the mite Sancassania berlesei. J Evol Biol 17:1377–1388
Tomkins JL, Hazel WN, Penrose MA, Radwan J, LeBas NR (2011) Habitat complexity drives experimental evolution of a conditionally expressed secondary sexual trait. Curr Biol 21:569–573
Twombly S (1996) Timing of metamorphosis in a freshwater crustacean: comparison with anuran models. Ecology 77:1855–1866
Woodring JP (1969) Environmental regulation of andropolymorphism in Tyroglyphids (Acari). In: Evans GO (ed) Proceedings of the second international congress of acarology. Academiai Kiado, Budapest, pp 433–440
Acknowledgments
DL thanks Hedwig Ens and Jacques Deere for their help with identifying the different juvenile life stages. This work was sponsored by an ERC advanced grant awarded to Tim Coulson and conforms to the legal requirements of the UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leigh, D.M., Smallegange, I.M. Effects of variation in nutrition on male morph development in the bulb mite Rhizoglyphus robini . Exp Appl Acarol 64, 159–170 (2014). https://doi.org/10.1007/s10493-014-9822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9822-y