Abstract
A bacterial strain was isolated from a brackish water sample of Tagus river, Alcochete, Portugal and was designated TO1_6T. It forms light pink colonies on M13 medium supplemented with N-acetylglucosamine. Cells are pear-shaped to spherical, form rosettes and divide by budding. Strain TO1_6T presents a mesophilic and neutrophilic profile, with optimum growth at 20 to 25 °C and pH 7.0 to 7.5, and vitamin supplementation is not required to promote its growth. The genome of the novel isolate is 7.77 Mbp in size and has a DNA G + C content of 56.3%. Based on its 16S rRNA gene sequence, this strain is affiliated with the phylum Planctomycetota. Further taxonomic characterization using additional phylogenetic markers, namely rpoB gene sequence (encoding the β-subunit of the DNA-dependent RNA polymerase), as well as Percentage of conserved proteins, average nucleotide identity and average amino acid identity, suggest the affiliation of strain TO1_6T to the genus Stieleria, a recently described taxon in the family Pirellulaceae, order Pirellulales and class Planctomycetia. Based on the genotypic, phylogenetic and physiological characterization, we here describe a new species represented by the type strain TO1_6T (= CECT 30432T, = LMG 32465T), for which the name Stieleria tagensis sp. nov. is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Planctomycetota is a phylum of Gram-negative bacteria that have received particular attention over the last decades, e.g. due to an increasing evidence of their bioactive potential, i.e. the production of antimicrobial and anticancer compounds (Calisto et al. 2019; Gimranov et al. 2022; Graca et al. 2016; Jeske et al. 2013, 2016; Sandargo et al. 2020) and of their potential as supplementary food source for microcrustaceans (Marinho et al. 2018, 2019). Additionally, their peculiar characteristics such as macromolecule uptake (Boedeker et al. 2017; Lonhienne et al. 2010), complex cellular ultrastructure (Boedeker et al. 2017; Santarella-Mellwig et al. 2010, 2013), broad-range antibiotic resistance (Cayrou et al. 2010; Godinho et al. 2019; Ivanova et al. 2021) and the unusual ftsZ-independent cell division (Jogler et al. 2012; Rivas-Marin et al. 2016; Rivas-Marin et al. 2020) also reinforces the interest in the study of fundamental biology within this phylum. Members of this phylum have been described across many environments, from freshwater (Kohn et al. 2020) to deep-sea hydrothermal deposits (Storesund et al. 2018; Storesund and Ovreas 2013) or boreal and subarctic wetlands (Dedysh and Ivanova 2019), associated with macroalgae (Bondoso et al. 2014, 2017; Lage and Bondoso 2011, 2014) and sponges (Izumi et al. 2013; Kallscheuer et al. 2020b), as well as in environments contaminated with metals (Akob et al. 2007; Halter et al. 2011) or hydrocarbons (Abed et al. 2011). Although widely distributed in the environment and abundant in some habitats, the number of isolated species is still moderate due to the difficulty of obtaining axenic cultures, mainly because they usually are slow growing bacteria with doubling times reported from around 5 h to up to 140 h (Vitorino et al. 2021; Vitorino and Lage 2022; Wiegand et al. 2020b). Recent efforts to expand the current collection of planctomycetal axenic cultures have led to the rapid increase of the description of new genera and species (Vitorino and Lage 2022; Wiegand et al. 2020b). An example is the newly described genus Stieleria (Kallscheuer et al. 2020a; Sandargo et al. 2020; Surup et al. 2020), which currently comprises four described species, to which the production of secondary metabolites with antimicrobial activity as well as potential quorum-sensing mechanisms have been linked (Kallscheuer et al. 2020a; Sandargo et al. 2020; Vitorino et al. 2022). Stieleria maiorica Mal15T, the first described strain within the genus, was isolated from a seawater sediment sample from Mallorca island, Spain (Kallscheuer et al. 2020a). Stieleria neptunia Enr13T was isolated from leaves of Posidonia sp. collected close to Panarea island, Italy (Sandargo et al. 2020), and Stieleria varia (type strain Pla52nT), was isolated from the biofilms on wood particles incubated in the Baltic Sea (Surup et al. 2020). More recently, Stieleria sedimenti ICT_E10.1T was isolated from sediments retrieved from the Tagus river estuary in Portugal (Vitorino et al. 2022). A taxonomic conflict has been previously detected between the genus Stieleria and the genus “Roseiconus”, which has two described species “Roseiconus lacunae” and “Roseiconus nitratireducens” (Kumar et al. 2021; Vitorino and Lage 2022). Although these two species do not have validly published names, their descriptions have been published and as such are included in this analysis. Here, we describe the strain TO1_6T (= CECT 30432T, = LMG 32465T), which was isolated from a river water sample from Tagus river, Portugal, for which the name Stieleria tagensis sp. nov. is proposed.
Materials and methods
Isolation and cultivation
Isolate TO1_6T was retrieved from a water sample from Tagus river, Alcochete (38° 45′ 20″ N 8° 57′ 55″ W) in May 2021. At the time of sampling, the water temperature and salinity (% (w/v) NaCl) were 20 °C and 1.54%, respectively. In brief, 250 mL of river water were filtered through a 0.22 µm pore size Whatman sterile membrane filter, which was then placed on M13 + NAG medium (0.25 g/L peptone, 0.25 g/L yeast extract, 50 mL/L 0.1 mM Tris–HCl buffer (pH 7.5), 10 mL/L 2.5% (w/v) glucose solution, 10 mL/L 5% (w/v) N-acetylglucosamine (NAG) solution, 10 mL vitamin solution (Lage and Bondoso 2011), 20 mL Hutner’s Basal Salts solution (Cohen-Bazire et al. 1957), 90% (v/v) of natural sea water, 1.6 g/L of agar) supplemented with streptomycin (1000 µg/mL), vancomycin (40 µg/mL) and cycloheximide (20 µg/mL). The membrane was incubated at 26 °C and checked routinely for colonies for almost a month, when a small pink colony was retrieved and labeled as strain TO1_6. Strain TO1_6 was maintained on M13 + NAG medium at 26 °C and preserved in medium M13 + NAG supplemented with 20% (v/v) glycerol at − 80 °C.
Phylogenetic inference and genome analysis
DNA extraction of an axenic culture of strain TO1_6T was performed using the E.Z.N.A. Bacterial DNA Isolation Kit (Omega BioTek) according to the manufacturer’s instructions. Extracted genomic DNA was used for PCR amplification of the 16S rRNA gene using the universal primers 27F and 1492R (Lane 1991) and for genome sequencing. The PCR mixture of 25 µL was prepared with 12.5 µL NZYTaq 2 × Green Master Mix (NZYTech), 0.25 µL of primer 27F (10 mM), 0.25 µL of primer 1492R (10 mM), 10 µL of H2O and 2 µL of DNA. The PCR was performed in a MyCycler™ Thermo Cycler (Bio-Rad) according to the following steps: initial denaturation at 95 °C for 5 min; 30 cycles of 95 °C for 1 min, 56 °C for 1 min, and 72 °C for 1:30 min; and last step of final extension at 72 °C for 10 min. The PCR products were then visualized after electrophoresis on a 0.8% (w/v) agarose gel in 1 × Tris–Acetate-EDTA (TAE) buffer stained with GreenSafe Premium (NZYTech). All amplicons were then purified with a GFX PCR DNA and Gel Band Purification Kit (Cytiva) and sent for sequencing at Eurofins Genomics. The obtained sequences were trimmed and analyzed using Geneious Prime 2021, and the consensus sequence was compared with the National Center for Biotechnology Information (NCBI) Genbank database (Benson et al. 2013) using NCBI’s Standard Nucleotide BLAST search (Altschul et al. 1990; Johnson et al. 2008) and with the 16S rRNA-based ID tool from the EzBioCloud platform (Yoon et al. 2017a) for phylogenetic affiliation.
Evolutionary analyses were carried out using MEGA7 software (Kumar et al. 2016) using 16S rRNA gene sequences retrieved from GenBank (Benson et al. 2013) from closely related strains and the type strain of Phycisphaera mikurensis, which was used as the outgroup, and aligned using ClustalW (Thompson et al. 1994). The dendrogram was constructed with the maximum likelihood method based on the General Time Reversible model and gamma distribution with invariant sites (G + I) (Tamura and Nei 1993) and phylogeny was tested with the bootstrap method with 1000 replications.
For genome sequencing, the library preparation was performed using the DNA Prep kit (Illumina), followed by sequencing on a MiSeq system (Illumina). The de novo genome assembly was performed using CLC Genomics Workbench (QIAGEN) version 21.0.1 and the completeness and contamination of the assembled genome was analyzed by checkM version 1.20 (Parks et al. 2015). Open reading frame calling was performed using Prodigal version 2.6.3 (Hyatt et al. 2010) and the coding sequences were annotated with Prokka version 1.14.6 (Seemann 2014). Comparative genomic analyses between strain TO1_6T and its current closely related species were performed. Genomes of closely related type strains were obtained from the NCBI Genbank database (Benson et al. 2013) and annotated simultaneously with the TO1_6T genome. The ANI values were calculated using CJ Bioscience's online ANI calculator at the EzBioCloud platform (Yoon et al. 2017a, b). The AAI values were calculated using the enveomics collection online ANI/AAI-Matrix: All-vs-all ANI/AAI matrix calculator (Rodriguez-R and Konstantinidis 2014, 2016). The rpoB and 16S rRNA genes identities were calculated with NCBI’s Standard Nucleotide BLAST search (Altschul et al. 1990; Johnson et al. 2008). The full rpoB gene enconding sequences were retrieved from genome annotation. The POCP was determined as previously described (Qin et al. 2014).
For the multi-locus sequence analysis (MLSA)-based phylogenetic tree, autoMLST was used. The analysis was performed with the Denovo method in fast alignment mode (MAFFT FFT-NS-2) with the default set of MLST genes and with filtering of inconsistent MLST genes and IQ-TREE Ultrafast Bootstrap analysis with 1000 replicates (Alanjary et al. 2019). Visualization of the tree was performed on the iTOL platform (Letunic and Bork 2019).
The presence of putative biosynthetic gene clusters was analyzed using antiSMASH 6.0 with relaxed detection strictness and with all extra features activated (Blin et al. 2021). The presence of antibiotic resistance genes was assessed with the CARD-RGI platform, using the genomic DNA sequence as input, considering only perfect and strict hits and excluding the nudge (Alcock et al. 2020).
Morphological and physiological characterization
The morphological characterization of strain TO1_6T was performed using optical and transmission electron microscopy (OM, TEM). Cell preparation for TEM followed a previously described protocol (Godinho et al. 2021) with slight modifications. Briefly, cells were harvested after 4 days of cultivation and were fixed in 2.5% (v/v) glutaraldehyde in marine buffer (Watson et al. 1986) for 2 h. Next, the cells were post-fixed in 1% (w/v) osmium tetroxide for 4 h in marine buffer and subsequently with 1% (w/v) uranyl acetate for 1 h. Dehydration was carried out with a graded ethanol series, followed by incubation in propylene oxide and embedding in Epon resin. Ultrathin sections of the embedded material were stained first with uranyl acetate, and then with Reynolds lead citrate, for 10 min each. Samples were visualized in a JEOL 100CXII transmission electron microscope.
All physiological tests were carried out in triplicates in a volume of 10 mL of M13 + NAG medium at room temperature for 4 days, after which the final OD600nm was measured (Thermo Scientific™ GENESYS™ 10UV Spectrophotometer), unless otherwise stated. The range and optimal temperatures were determined on M13 + NAG agar medium plates incubated from 5 to 40 °C, with 5 °C increments, by placing 3 droplets of 10 µL of exponential phase culture on each plate and incubating for 7 days at the respective testing temperature. Growth was observed visually. Salinity was tested in M13 + NAG broth prepared with artificial seawater (ASW) (Harrison et al. 1980) without NaCl and subsequently supplemented with different concentrations of NaCl from 0 to 12% (w/v) in 1% increments. For the determination of optimum and growth range regarding pH, M13 + NAG broth with pH ranging from 5.0 to 10.0, at 0.5 units intervals, was prepared using the following buffer systems: citrate buffer 0.1 M for pH 5.0, MES 0.1 M for pH 5.5–6.5, Tris–HCl 1 M for pH 7.0–8.5 and CHES 1 M for pH 9.0–10.0. To assess vitamin requirements, M13 + NAG broth without vitamins solution no. 6 (Lage and Bondoso 2011) was used, and for each experiment one of the following vitamins was individually added: biotin (20 µg/L), folic acid (20 µg/L), riboflavin (50 µg/L), thiamine-HCl (50 µg/L), nicotinamide (100 µg/L), calcium d-pantothenate (50 µg/L) and vitamin B12 (1 µg/L). For nitrogen utilization assays, the base of M13 + NAG broth without peptone, yeast extract and NAG was used and later individually supplemented with a total of 15 different nitrogen sources at 0.1% (w/v), namely l-arginine, l-tyrosine, l-threonine, l-glutamine, l-cysteine, l-methionine, l-isoleucine, l-serine, l-aspartic acid, peptone, yeast extract, NAG, sodium nitrate, sodium nitrite and urea. A negative control without any nitrogen source was included, as well as a positive control using standard M13 + NAG broth. These cultures were incubated for 4 days before measuring the results by absorbance reading at 600 nm. For carbon utilization assays, M13 + NAG broth without peptone and NAG, but with 1 g/L of yeast extract was used. A total of 16 different carbon sources were then added individually at 0.1% (w/v), namely d-arabinose, cellobiose, dulcitol, d-galactose, glycerol, myo-inositol, lactose, maltose, d-mannitol, d-sorbitol, saccharose, trehalose, d-xylose, d-glucose, raffinose and dextran. A negative control without any carbon source was included and M13 + NAG broth was applied as positive control. These cultures were incubated for 7 days before measuring the results by absorbance reading at 600 nm.
The growth rate of strain TO1_6 was inferred in medium M13 + NAG at 25 °C and 1000 rpm in a BioSan™ RTS-1C Personal bioreactor. Anaerobic and microaerophilic growth were tested using the GENbox system (bioMérieux S.A., France) containing a generator sachet, either Genbox anaer or Genbox microaer (bioMérieux S.A.), in which plates of M13 + NAG agar medium inoculated with strain TO1_6T were incubated at 26 °C for 1 month. Antibiotic susceptibility was evaluated by the modified Kirby-Bauer method as previously described (Godinho et al. 2019) in M13 medium. The tested antibiotics (amount per disc in brackets) were amikacin (30 µg), gentamicin (10 µg), tobramycin (10 µg), kanamycin (30 µg), chloramphenicol (30 µg), amoxicillin (10 µg), amoxicillin-clavulanic acid (30 µg), aztreonam (30 µg), cefotaxime (30 µg), cefoxitin (30 µg), ceftazidime (30 µg), imipenem (10 µg), meropenem (10 µg), piperacillin (100 µg), piperacillin-tazobactam (110 µg), fosfomycin (50 µg), teicoplanin (30 µg), vancomycin (30 µg), clindamycin (2 µg), erythromycin (15 µg), nitrofurantoin (300 µg), colistin sulphate (10 µg), polymyxin B (300 IU), ciprofloxacin (5 µg), nalidixic acid (30 µg), doxycycline (30 µg) and tetracycline (30 µg).
The fatty acid content of Stieleria tagensis T01_6T and Stieleria sedimenti ICT_E10.1T was evaluated by gas chromatography (GC) using the MIDI’s Sherlock™ Microbial ID System. Strains were first cultured for 4 days at 25 °C in modified R2A medium plates prepared as followed (per liter of Milli-Q water): 18.2 g of Difco™ R2A Agar powder and 30 g of Instant Ocean® Sea Salt. After autoclaving, the following supplements were added by filtration (0.22 µm pore filter): 10 mL vitamin solution (Lage and Bondoso 2011), 20 mL Hutner’s basal salts solution (Cohen-Bazire et al. 1957) and 40 mL glucose solution (stock at 2.5% w/v). Biomass was collected and the fatty acids obtained by saponification, methylation and extraction following the manufacturer’s instructions (MIDI 2017; Sasser 1990). Finally, the fatty acid content was analyzed in an Agilent 6890N Network Gas Chromatograph equipment.
Results and discussion
16S rRNA gene sequencing and phylogenetic analysis
A nucleotide BLAST search was conducted using the partial (1292 bp) 16S rRNA gene sequence of strain TO1_6T as query (in August 2022), and all the hits with more than 98.65% identity (the proposed threshold for species delineation) were analyzed. A total of five distinct hits were obtained from which three are from isolates and two from metagenomic samples. The closest hits are from metagenomic samples from the macroalga Chondrus crispus collected in Foz, Porto, North of Portugal in summer, and from seawater next to dolphin I in San Diego, California, USA, with 98.75 and 98.73% identities, respectively. The three isolates are strains TBK1, Enr13T and JC639, all with 98.68% identity. TBK1 was isolated from iron hydroxide deposits in Valu Fa ridge, Pacific Ocean (Storesund et al. 2018), while strain Enr13T was isolated from leaves of Posidonia sp. collected close to the Panarea island, Italy. Strain JC639 was isolated in Tamil Nadu, India, but no information regarding sample type was provided. This indicates a disperse geographic distribution of members of the genus Stieleria.
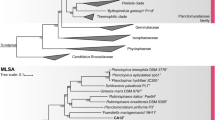
According to the nucleotide BLAST and the EzBioCloud analysis of the 16S rRNA gene of strain TO1_6T the closest sequence belongs to the type strain of the species Stieleria neptunia, strain Enr13T, with 98.68% identity. Strain TO1_6T clusters with the described species of the genus Stieleria in maximum likelihood phylogenetic trees based on 16S rRNA gene sequences (Fig. 1). The 16S rRNA gene similarity is presented in Table 1 taking into consideration described species of the genera Stieleria and “Roseiconus” and strain TO1_6T. The above-mentioned 16S rRNA gene similarity of strain TO1_6T to S. neptunia strain Enr13T is slightly above the 98.65% threshold for species delimitation (Kim et al. 2014). However, the 16S rRNA gene similarity alone has often been reported as insufficient to delineate species within the phylum Planctomycetota, as for example in case of strains that share 99.01% similarity, like S. neptunia strain Enr13T and S. maiorica Mal15T. Even higher 16S rRNA gene sequence similarities have been observed for strains within the phylum that turned out to belong to separate species based on whole-genome-based phylogenetic analyses (Kallscheuer et al. 2020a; Kohn et al. 2020; Sandargo et al. 2020; Wiegand et al. 2020a). Additional phylogenetic markers such as the sequence of the rpoB gene (encoding the β-subunit of the RNA polymerase), ANI, AAI and POCP are commonly used for the taxonomic delimitation within this phylum.
Phylogenetic 16S rRNA gene sequence-based dendrogram demonstrating the relationship between the type species of the different genera within the families Lacipirellulaceae, Thermoguttaceae and Pirellulaceae and the described species of the genus Stieleria, including the new strain TO1_6T. Phycisphaera mikurensis was used as outgroup. The numbers on the branches refer to percentage of trees in which the associated taxa clustered together from the total of bootstrap replications. GenBank accession numbers are presented. The scale bar refers to 0.02 substitutions per nucleotide position
Genome analysis
The main features of the genome of strain TO1_6T are presented in Table 2. Genome sequencing revealed a genome size of 7.77 Mbp and DNA G + C content of 56.3%. The assembly resulted in 196 contigs and the annotation resulted in 5752 protein coding sequences, 3803 of which are annotated as hypothetical proteins, representing a total of 66% of hypothetical proteins. The genome also encodes 11 giant genes (i.e. genes > 10 kb), most of which code for proteins with unknown function, with the exception of one that was automatically annotated as putative 6-phosphogluconolactonase. When compared to the other species of the genus Stieleria, the genome of strain TO1_6T has a DNA G + C content closer to S. varia than to the other two species, but it has a smaller size when compared to the genomes of the other three described Stieleria species and has a higher coding density. Remarkably, even though the genome is smaller, it harbors a higher percentage of hypothetical proteins (66%), which exceeds the range of 40–50% hypothetical proteins typically found in the genomes of other members of the phylum Planctomycetota (Wiegand et al. 2020b). The complete 16S rRNA gene (1529 bp) was retrieved from the annotation of the genome and it had 100% similarity with the partial 16S rRNA gene obtained from amplicon sequencing.
Given that members of the genus Stieleria have been linked to the production of bioactive compounds (Kallscheuer et al. 2020a; Sandargo et al. 2020), prediction of biosynthetic gene clusters potentially associated to secondary metabolite production was performed with antiSMASH. The analysis yielded a total of seven clusters, two of which are putatively related to the production of terpenes, while the remaining are related to the biosynthesis of polyketides (one type I and one type III polyketide synthases), non-ribosomal peptides (one cluster), hybrid polyketides/non-ribosomal peptides (one cluster) and N-acyl amino acids (one cluster) (Supplementary Table 1). The predicted terpene biosynthetic enzymes seem to be related to the production of carotenoids and the production of antimicrobial compounds. Antibiotic activity is also predicted regarding the clusters of both polyketide synthases classes and of the non-ribosomal peptide. The PKS/NRPS hybrid cluster might be related to the production of antioxidants. The type strains of two of the three characterized Stieleria species have been shown to produce stieleriacines. The key reactions of their biosynthesis were proposed to be catalyzed by enzymes encoded in an N-acyl amino acid cluster. However, when comparing the putative N-Acyl amino acid cluster identified in strain TO1_6T with the one described in S. neptunia Enr13T, only 35% of the genes from TO1_6T cluster show similarity to the ones in Enr13T, suggesting that the two clusters, although predicted to belong to the same class, may differ. This is not unexpected when taking into account that S. maiorica Mal15T and S. neptunia Enr13T produce compounds that belong to the same class (namely stieleriacines), but produce compounds that chemically differ in fatty acid chain length and aromatic ring substitutions (Kallscheuer et al. 2020a; Sandargo et al. 2020). Apart from this, the cluster in S. maiorica includes several additional genes coding for putative transporters and cell wall biosynthesis proteins, that are not necessarily related to stieleriacine biosynthesis.
Additional phylogenetic markers such as similarity of rpoB gene sequence, ANI, AAI and POCP are commonly used for the purpose of species and genus delineation within this phylum. The results of these different markers are reported in Table 1. POCP values show that TO1_6T belongs to the genus Stieleria (values above 50% with the three described species (Qin et al. 2014)), while ANI, AAI and rpoB similarity reinforce that strain TO1_6T represents a new species within this genus. The obtained values of 70.98–73.82% are below the 95% threshold for ANI (Goris et al. 2007), 61.28–69.16% are below the 95–98% threshold for AAI (Konstantinidis and Tiedje 2005), and rpoB identities are between 82.34% and 86.55% which are also below the established 96.3% threshold (Bondoso et al. 2013). Strain TO1_6T clusters with the described species of the genus Stieleria in maximum likelihood phylogenetic trees based on multi-locus sequence analysis but on a separate branch (Fig. 2).
Multi-locus sequence analysis-based phylogenetic tree highlighting the position of the novel strain TO1_6T. The web-based tool autoMLST was used to construct the phylogenetic tree, with 1000 bootstrap (values shown in % at the nodes). RefSeq accession numbers are presented. Genomes from Verrucomicrobium spinosum DSM 4136T and Phycisphaera mikurensis NBRC 102666T were used as outgroups. Scale bar represents 0.10 substitutions per nucleotide position
Morphology and physiology
When cultivated on M13 + NAG agar, isolate TO1_6T forms small, circular and white/beige colonies in the initial phases of incubation, but eventually these become light pink after a couple of days. When grown in M13 + NAG broth, cells of strain TO1_6T show the same pattern of pigmentation but grow aggregated in flakes, with fresh cultures having a clear medium with flakes. Turbidity is only observed in old cultures. Cells in the aggregates can be separated by vortexing. Under the OM it was possible to visualize the presence of rosettes, as well as single cells, and the occurrence of budding. The cells of strain TO1_6T are typically pear-shaped, but larger and more round-shaped cells can also occur. TEM micrographs showed the typical characteristics of the ultrastructure of members of the phylum Planctomycetota (Fig. 3). In detail, we observed complex membrane invaginations, condensed DNA, ribosomes as well as inclusion storage bodies. Thick polar fimbriae are present.
Strain TO1_6T was able to grow between 15 and 30 °C, with optimal growth at 20–25 °C, and in the presence of 0–11% (w/v) NaCl, with optimum growth at 1%. Growth was observed over a pH ranging from 5.0 to 9.5, with optimal growth at pH 7.0–7.5. Strain TO1_6T did not show an altered growth behavior in the absence of any of the tested vitamins, except for vitamin B12 and thiamine-HCl which promoted its growth when used to supplement M13 + NAG medium. Regarding nitrogen source utilization, strain TO1_6T was able to use l-arginine, l-tyrosine, l-threonine, l-glutamine, l-isoleucine, l-aspartic acid, peptone, yeast extract, NAG and nitrate. Optimal growth was obtained with yeast extract. For carbon source utilization, strain TO1_6T was able to use d-arabinose, cellobiose, glycerol, myo-inositol, lactose, maltose, d-mannitol, d-sorbitol, saccharose, trehalose, d-xylose, d-glucose, raffinose and dextran. Optimal growth was obtained with dextran as carbon source. A doubling time of 17.2 h was observed in M13 + NAG medium. Strain TO1_6T was able to grow under microaerophilic conditions but not under anaerobic conditions. The fatty acid content of S. tagensis TO1_6T and S. sedimenti ICT_E10.1T are presented in Supplementary Table 2. Major fatty acids for both strains are C16:0 and C18:1ω9c.
A comparison between the morphological and physiological characteristics between isolate TO1_6T and other described species of the genus Stieleria is presented in Table 3. Isolate TO1_6T differs from other members of the genus by its initial white to beige pigmentation, longer generation time, and lower optimal temperature.
The results from the antibiotic susceptibility testing are presented in Table 4. Strain TO1_6T showed no susceptibility to any of the tested antibiotics that target cell wall biosynthesis, including the two tested β-lactam/β-lactamase inhibitor combinations. Resistance to glycopeptides such as vancomycin and teicoplanin was expected since these antibiotic molecules are big and usually unable to cross the outer membrane of Gram-negative bacteria (Blair et al. 2015). The resistance to β-lactams, β-lactams/β-lactamase inhibitor combinations and fosfomycin, is in agreement with previous reports for this phylum (Cayrou et al. 2010; Godinho et al. 2019; König et al. 1984). Although some exceptions have been previously reported (Hu et al. 2013; Ivanova et al. 2021; Zaicnikova et al. 2011), the vast majority of the phylum members, for which antibiotic susceptibility has been tested, showed high resistance to various antibiotics that target cell wall biosynthesis (Cayrou et al. 2010; Godinho et al. 2019; Ivanova et al. 2021; König et al. 1984; Vitorino and Lage 2022). Regarding the remaining groups of antibiotics, strain TO1_6T showed mixed results. For compounds that target protein biosynthesis, the following observations were made: the strain was resistant to the four tested aminoglycosides, but was susceptible to chloramphenicol, clindamycin and erythromycin, and showed small inhibition zone diameters for doxycycline and tetracycline. Susceptibility to erythromycin has been reported for this phylum (Cayrou et al. 2010; Godinho et al. 2019; Ivanova et al. 2021). For those that target DNA replication, strain TO1_6T showed resistance to nalidixic acid, but was susceptible to ciprofloxacin. It was also susceptible to nitrofurantoin. And finally, among the ones that target the structure and integrity of the cell membrane, it showed resistance to colistin and susceptibility to polymyxin B. Given the broad range of resistance to antibiotics that target the cell wall biosynthesis, as well as to aminoglycosides, it was hypothesized that antibiotic resistance genes should be present in the genome of strain TO1_6T. However, prediction of antibiotic resistance genes with CARD-RGI online platform yielded only 3 hits, all of which are for adeF. According to the CARD, adeF codes for the membrane fusion protein of the multidrug efflux complex AdeFGH which has been linked to the resistance to fluoroquinolones, tetracycline, tigecycline, chloramphenicol, clindamycin, trimethoprim, and sulfamethoxazole (Alcock et al. 2020; Coyne et al. 2010). Even though this efflux pump might be responsible for resistance to nalidixic acid or tetracycline, no gene hits for the rest of the components of this pump were retrieved and other mechanisms could be at play. Surprisingly, no currently known genetic determinants of resistance to either β-lactams, fosfomycin or aminoglycosides were found.
Conclusion
The polyphasic analysis including morphological, physiological and genomic features supports the results of the phylogenetic inference, which together delineate strain TO1_6T from the known species of the genus Stieleria. Hence, we conclude that strain TO1_6T represents the type strain of a new species of the genus, for which we propose the name Stieleria tagensis sp. nov.
Description of Stieleria tagensis sp. nov.
Stieleria tagensis sp. nov. (ta.gen’sis. L. fem. adj. tagensis, pertaining to the Tagus River)
Cells are round to pear-shaped, with 1.45 ± 0.1 µm long and 1.02 ± 0.2 µm wide. Can occur as single cells or as rosettes and divide by budding. In solid medium, forms small, circular and white/beige colonies in the initial phase of incubation, that change to light pink-pigmented colonies. The temperature growth range is 15–30 °C, with optimal growth between 20 and 25 °C. Able to grow with 0–11% (w/v) NaCl, with optimal growth at 1%. Growth occurs from pH 5.0 to 9.5, with optimal growth at pH 7.0–7.5. Can grow without vitamins. Uses as nitrogen sources l-arginine, l-tyrosine, l-threonine, l-glutamine, l-isoleucine, l-aspartic acid, peptone, yeast extract, NAG and nitrate. Optimal growth was obtained with yeast extract. Can use as carbon source d-arabinose, cellobiose, glycerol, myo-inositol, lactose, maltose, d-manitol, d-sorbitol, saccharose, trehalose, d-xylose, d-glucose, raffinose and dextran. Optimal growth was obtained with dextran. Maximal doubling time is 17.2 h in M13 + NAG medium. Major fatty acids are C16:0 and C18:1ω9c Can grow under microaerophilic conditions, but not under anaerobic conditions. The genome size is 7.77 Mb with a DNA G + C content of 56.3%. The type strain is TO1_6T (= CECT 30432T, = LMG 32465T), which was isolated from a river water sample from Tagus river in Portugal.
Data availability
The 16S rRNA gene sequence is deposited at NCBI’s GenBank database under accession number OK103954. The whole shotgun genome sequence is also deposited at NCBI’s GenBank under the accession number JAMYFE000000000.
References
Abed RM, Musat N, Musat F, Mussmann M (2011) Structure of microbial communities and hydrocarbon-dependent sulfate reduction in the anoxic layer of a polluted microbial mat. Mar Pollut Bull 62(3):539–546. https://doi.org/10.1016/j.marpolbul.2010.11.030
Akob DM, Mills HJ, Kostka JE (2007) Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS Microbiol Ecol 59(1):95–107. https://doi.org/10.1111/j.1574-6941.2006.00203.x
Alanjary M, Steinke K, Ziemert N (2019) AutoMLST: an automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res 47(W1):W276–W282. https://doi.org/10.1093/nar/gkz282
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen AV, Cheng AA, Liu S, Min SY, Miroshnichenko A, Tran HK, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B, Faltyn M, Hernandez-Koutoucheva A, Sharma AN, Bordeleau E, Pawlowski AC, Zubyk HL, Dooley D, Griffiths E, Maguire F, Winsor GL, Beiko RG, Brinkman FSL, Hsiao WWL, Domselaar GV, McArthur AG (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48(D1):D517–D525. https://doi.org/10.1093/nar/gkz935
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2013) GenBank. Nucleic Acids Res 41:36–42. https://doi.org/10.1093/nar/gks1195
Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13(1):42–51. https://doi.org/10.1038/nrmicro3380
Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T (2021) antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49(W1):W29–W35. https://doi.org/10.1093/nar/gkab335
Boedeker C, Schuler M, Reintjes G, Jeske O, van Teeseling MC, Jogler M, Rast P, Borchert D, Devos DP, Kucklick M, Schaffer M, Kolter R, van Niftrik L, Engelmann S, Amann R, Rohde M, Engelhardt H, Jogler C (2017) Determining the bacterial cell biology of planctomycetes. Nat Commun 8:14853. https://doi.org/10.1038/ncomms14853
Bondoso J, Harder J, Lage OM (2013) rpoB gene as a novel molecular marker to infer phylogeny in Planctomycetales. Antonie Van Leeuwenhoek 104(4):477–488. https://doi.org/10.1007/s10482-013-9980-7
Bondoso J, Balague V, Gasol JM, Lage OM (2014) Community composition of the planctomycetes associated with different macroalgae. FEMS Microbiol Ecol 88(3):445–456. https://doi.org/10.1111/1574-6941.12258
Bondoso J, Godoy-Vitorino F, Balague V, Gasol JM, Harder J, Lage OM (2017) Epiphytic planctomycetes communities associated with three main groups of macroalgae. FEMS Microbiol Ecol 93(3):9. https://doi.org/10.1093/femsec/fiw255
Calisto R, Sæbø EF, Storesund JE, Øvreås L, Herfindal L, Lage OM (2019) Anticancer activity in planctomycetes. Front Mar Sci 5:499. https://doi.org/10.3389/fmars.2018.00499
Cayrou C, Raoult D, Drancourt M (2010) Broad-spectrum antibiotic resistance of planctomycetes organisms determined by Etest. J Antimicrob Chemother 65(10):2119–2122. https://doi.org/10.1093/jac/dkq290
Cohen-Bazire G, Sistrom WR, Stanier RY (1957) Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol 49(1):25–68. https://doi.org/10.1002/jcp.1030490104
Coyne S, Rosenfeld N, Lambert T, Courvalin P, Perichon B (2010) Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 54(10):4389–4393. https://doi.org/10.1128/AAC.00155-10
Dedysh SN, Ivanova AA (2019) Planctomycetes in boreal and subarctic wetlands: diversity patterns and potential ecological functions. FEMS Microbiol Ecol 95(2):10. https://doi.org/10.1093/femsec/fiy227
Gimranov E, Santos JDN, Vitorino I, Martín J, Reyes F, Moura L, Tavares F, Santos C, Mariz-Ponte N, Lage OM (2022) Marine bacterial activity against phytopathogenic pseudomonas show high efficiency of planctomycetes extracts. Eur J Plant Pathol 162(4):843–854. https://doi.org/10.1007/s10658-021-02441-2
Godinho O, Calisto R, Ovreas L, Quinteira S, Lage OM (2019) Antibiotic susceptibility of marine planctomycetes. Antonie Van Leeuwenhoek 112(8):1273–1280. https://doi.org/10.1007/s10482-019-01259-7
Godinho O, Botelho R, Albuquerque L, Wiegand S, Kallscheuer N, da Costa MS, Lobo-da-Cunha A, Jogler C, Lage OM (2021) Bremerella alba sp. nov., a novel planctomycete isolated from the surface of the macroalga Fucus spiralis. Syst Appl Microbiol 44(3):126189. https://doi.org/10.1016/j.syapm.2021.126189
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57(Pt 1):81–91. https://doi.org/10.1099/ijs.0.64483-0
Graca AP, Calisto R, Lage OM (2016) Planctomycetes as novel source of bioactive molecules. Front Microbiol 7:1241. https://doi.org/10.3389/fmicb.2016.01241
Halter D, Cordi A, Gribaldo S, Gallien S, Goulhen-Chollet F, Heinrich-Salmeron A, Carapito C, Pagnout C, Montaut D, Seby F, Van Dorsselaer A, Schaeffer C, Bertin PN, Bauda P, Arsene-Ploetze F (2011) Taxonomic and functional prokaryote diversity in mildly arsenic-contaminated sediments. Res Microbiol 162(9):877–887. https://doi.org/10.1016/j.resmic.2011.06.001
Harrison PJ, Waters RE, Taylor FJR (1980) A broad spectrum artificial sea water medium for coastal and open ocean phytoplankton1. J Phycol 16(1):28–35. https://doi.org/10.1111/j.0022-3646.1980.00028.x
Hu Z, van Alen T, Jetten MS, Kartal B (2013) Lysozyme and penicillin inhibit the growth of anaerobic ammonium-oxidizing planctomycetes. Appl Environ Microbiol 79(24):7763–7769. https://doi.org/10.1128/AEM.02467-13
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform 11(1):119. https://doi.org/10.1186/1471-2105-11-119
Ivanova AA, Miroshnikov KK, Oshkin IY (2021) Exploring antibiotic susceptibility, resistome and mobilome structure of planctomycetes from Gemmataceae family. Sustainability 13:9. https://doi.org/10.3390/su13095031
Izumi H, Sagulenko E, Webb RI, Fuerst JA (2013) Isolation and diversity of planctomycetes from the sponge Niphates sp., seawater, and sediment of Moreton Bay, Australia. Antonie Van Leeuwenhoek 104(4):533–546. https://doi.org/10.1007/s10482-013-0003-5
Jeske O, Jogler M, Petersen J, Sikorski J, Jogler C (2013) From genome mining to phenotypic microarrays: planctomycetes as source for novel bioactive molecules. Antonie Van Leeuwenhoek 104(4):551–567. https://doi.org/10.1007/s10482-013-0007-1
Jeske O, Surup F, Ketteniss M, Rast P, Forster B, Jogler M, Wink J, Jogler C (2016) Developing techniques for the utilization of planctomycetes as producers of bioactive molecules. Front Microbiol 7:1242. https://doi.org/10.3389/fmicb.2016.01242
Jogler C, Waldmann J, Huang X, Jogler M, Glockner FO, Mascher T, Kolter R (2012) Identification of proteins likely to be involved in morphogenesis, cell division, and signal transduction in planctomycetes by comparative genomics. J Bacteriol 194(23):6419–6430. https://doi.org/10.1128/JB.01325-12
Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36:5–9. https://doi.org/10.1093/nar/gkn201
Kallscheuer N, Jeske O, Sandargo B, Boedeker C, Wiegand S, Bartling P, Jogler M, Rohde M, Petersen J, Medema MH, Surup F, Jogler C (2020a) The planctomycete Stieleria maiorica Mal15(T) employs stieleriacines to alter the species composition in marine biofilms. Commun Biol 3(1):303. https://doi.org/10.1038/s42003-020-0993-2
Kallscheuer N, Wiegand S, Kohn T, Boedeker C, Jeske O, Rast P, Muller RW, Brummer F, Heuer A, Jetten MSM, Rohde M, Jogler M, Jogler C (2020b) Cultivation-independent analysis of the bacterial community associated with the calcareous sponge Clathrina clathrus and isolation of Poriferisphaera corsica gen. nov., sp. nov., belonging to the barely studied class Phycisphaerae in the phylum Planctomycetes. Front Microbiol 11:602250. https://doi.org/10.3389/fmicb.2020.602250
Kim M, Oh HS, Park SC, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64(Pt 2):346–351. https://doi.org/10.1099/ijs.0.059774-0
Kohn T, Wiegand S, Boedeker C, Rast P, Heuer A, Jetten MSM, Schuler M, Becker S, Rohde C, Muller RW, Brummer F, Rohde M, Engelhardt H, Jogler M, Jogler C (2020) Planctopirus ephydatiae, a novel Planctomycete isolated from a freshwater sponge. Syst Appl Microbiol 43(1):126022. https://doi.org/10.1016/j.syapm.2019.126022
König E, Schlesner H, Hirsch P (1984) Cell wall studies on budding bacteria of the Planctomyces/Pasteuria group and on a Prosthecomicrobium sp. Arch Microbiol 138(3):200–205. https://doi.org/10.1007/bf00402120
Konstantinidis KT, Tiedje JM (2005) Towards a genome-based taxonomy for prokaryotes. J Bacteriol 187(18):6258–6264. https://doi.org/10.1128/JB.187.18.6258-6264.2005
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Kumar D, Kumar G, Uppada J, Ahmed S, Sasikala C, Venkata Ramana C (2021) Descriptions of Roseiconus nitratireducens gen. nov. sp. nov. and Roseiconus lacunae sp. nov. Arch Microbiol 203(2):741–754. https://doi.org/10.1007/s00203-020-02078-5
Lage OM, Bondoso J (2011) Planctomycetes diversity associated with macroalgae. FEMS Microbiol Ecol 78(2):366–375. https://doi.org/10.1111/j.1574-6941.2011.01168.x
Lage OM, Bondoso J (2014) Planctomycetes and macroalgae, a striking association. Front Microbiol 5:267. https://doi.org/10.3389/fmicb.2014.00267
Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics, pp 115–175
Letunic I, Bork P (2019) Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47(W1):W256–W259. https://doi.org/10.1093/nar/gkz239
Lonhienne TG, Sagulenko E, Webb RI, Lee KC, Franke J, Devos DP, Nouwens A, Carroll BJ, Fuerst JA (2010) Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci U S A 107(29):12883–12888. https://doi.org/10.1073/pnas.1001085107
Marinho MC, Lage OM, Catita J, Antunes SC (2018) Adequacy of planctomycetes as supplementary food source for Daphnia magna. Antonie Van Leeuwenhoek 111(6):825–840. https://doi.org/10.1007/s10482-017-0997-1
Marinho MC, Lage OM, Sousa CD, Catita J, Antunes SC (2019) Assessment of Rhodopirellula rubra as a supplementary and nutritional food source to the microcrustacean Daphnia magna. Antonie Van Leeuwenhoek 112(8):1231–1243. https://doi.org/10.1007/s10482-019-01255-x
MIDI (2017) Sherlock™ microbial ID system: bacterial ID by fatty acid analysis
Oberbeckmann S, Kreikemeyer B, Labrenz M (2017) Environmental factors support the formation of specific bacterial assemblages on microplastics. Front Microbiol 8:2709. https://doi.org/10.3389/fmicb.2017.02709
Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25(7):1043–1055. https://doi.org/10.1101/gr.186072.114
Qin QL, Xie BB, Zhang XY, Chen XL, Zhou BC, Zhou J, Oren A, Zhang YZ (2014) A proposed genus boundary for the prokaryotes based on genomic insights. J Bacteriol 196(12):2210–2215. https://doi.org/10.1128/JB.01688-14
Rivas-Marin E, Canosa I, Devos DP (2016) Evolutionary cell biology of division mode in the bacterial Planctomycetes-Verrucomicrobia-Chlamydiae superphylum. Front Microbiol 7:1964. https://doi.org/10.3389/fmicb.2016.01964
Rivas-Marin E, Peeters SH, Claret Fernandez L, Jogler C, van Niftrik L, Wiegand S, Devos DP (2020) Non-essentiality of canonical cell division genes in the planctomycete Planctopirus limnophila. Sci Rep 10(1):66. https://doi.org/10.1038/s41598-019-56978-8
Rodriguez-R LM, Konstantinidis KT (2016) The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ (Preprints)
Rodriguez-R LM, Konstantinidis KT (2014) Bypassing cultivation to identify bacterial species. Microbe 9(3):111–118
Sandargo B, Jeske O, Boedeker C, Wiegand S, Wennrich JP, Kallscheuer N, Jogler M, Rohde M, Jogler C, Surup F (2020) Stieleriacines, N-acyl dehydrotyrosines from the marine planctomycete Stieleria neptunia sp. nov. Front Microbiol 11:1408. https://doi.org/10.3389/fmicb.2020.01408
Santarella-Mellwig R, Franke J, Jaedicke A, Gorjanacz M, Bauer U, Budd A, Mattaj IW, Devos DP (2010) The compartmentalized bacteria of the Planctomycetes-Verrucomicrobia-Chlamydiae superphylum have membrane coat-like proteins. PLoS Biol 8(1):e1000281. https://doi.org/10.1371/journal.pbio.1000281
Santarella-Mellwig R, Pruggnaller S, Roos N, Mattaj IW, Devos DP (2013) Three-dimensional reconstruction of bacteria with a complex endomembrane system. PLoS Biol 11(5):e1001565. https://doi.org/10.1371/journal.pbio.1001565
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI Inc, Newark
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–2069. https://doi.org/10.1093/bioinformatics/btu153
Storesund JE, Ovreas L (2013) Diversity of planctomycetes in iron-hydroxide deposits from the arctic mid ocean ridge (AMOR) and description of Bythopirellula goksoyri gen. nov., sp. nov., a novel planctomycete from deep sea iron-hydroxide deposits. Antonie Van Leeuwenhoek 104(4):569–584. https://doi.org/10.1007/s10482-013-0019-x
Storesund JE, Lanzen A, Garcia-Moyano A, Reysenbach AL, Ovreas L (2018) Diversity patterns and isolation of planctomycetes associated with metalliferous deposits from hydrothermal vent fields along the Valu Fa Ridge (SW Pacific). Antonie Van Leeuwenhoek 111(6):841–858. https://doi.org/10.1007/s10482-018-1026-8
Surup F, Wiegand S, Boedeker C, Heuer A, Peeters SH, Jogler M, Jetten MSM, Rohde M, Jogler C, Kallscheuer N (2020) Stieleria varia sp. nov., isolated from wood particles in the Baltic Sea, constitutes a novel species in the family Pirellulaceae within the phylum Planctomycetes. Antonie Van Leeuwenhoek 113(12):1953–1963. https://doi.org/10.1007/s10482-020-01456-9
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Vitorino IR, Lage OM (2022) The Planctomycetia: an overview of the currently largest class within the phylum Planctomycetes. Antonie Van Leeuwenhoek 115(2):169–201. https://doi.org/10.1007/s10482-021-01699-0
Vitorino I, Santos JDN, Godinho O, Vicente F, Vasconcelos V, Lage OM (2021) Novel and conventional isolation techniques to obtain planctomycetes from marine environments. Microorganisms 9(10):2078. https://doi.org/10.3390/microorganisms9102078
Vitorino I, Klimek D, Calusinska M, Lobo-da-Cunha A, Vasconcelos V, Lage O (2022) Stieleria sedimenti sp. nov., a novel member of the family Pirellulaceae with antimicrobial activity isolated in Portugal from brackish sediments. Microorganisms 10:11. https://doi.org/10.3390/microorganisms10112151
Watson SW, Bock E, Valois FW, Waterbury JB, Schlosser U (1986) Nitrospira marina gen. nov. sp. nov.: a chemolithotrophic nitrite-oxidizing bacterium. Arch Microbiol 144(1):1–7. https://doi.org/10.1007/bf00454947
Wiegand S, Jogler M, Boedeker C, Heuer A, Rast P, Peeters SH, Jetten MSM, Kaster AK, Rohde M, Kallscheuer N, Jogler C (2020a) Additions to the genus Gimesia: description of Gimesia alba sp. nov. Gimesia algae sp. nov., Gimesia aquarii sp. nov., Gimesia aquatilis sp. nov., Gimesia fumaroli sp. nov., and Gimesia panareensis sp. nov., isolated from aquatic habitats of the Northern Hemisphere. Antonie Van Leeuwenhoek 113(12):1999–2018. https://doi.org/10.1007/s10482-020-01489-0
Wiegand S, Jogler M, Boedeker C, Pinto D, Vollmers J, Rivas-Marin E, Kohn T, Peeters SH, Heuer A, Rast P, Oberbeckmann S, Bunk B, Jeske O, Meyerdierks A, Storesund JE, Kallscheuer N, Lucker S, Lage OM, Pohl T, Merkel BJ, Hornburger P, Muller RW, Brummer F, Labrenz M, Spormann AM, Op den Camp HJM, Overmann J, Amann R, Jetten MSM, Mascher T, Medema MH, Devos DP, Kaster AK, Ovreas L, Rohde M, Galperin MY, Jogler C (2020b) Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat Microbiol 5(1):126–140. https://doi.org/10.1038/s41564-019-0588-1
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017a) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67(5):1613–1617. https://doi.org/10.1099/ijsem.0.001755
Yoon SH, Ha SM, Lim J, Kwon S, Chun J (2017b) A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110(10):1281–1286. https://doi.org/10.1007/s10482-017-0844-4
Zaicnikova MV, Berestovskaya YY, Akimov VN, Kostrikina NA, Vasilieva LV (2011) Singulispaera mucilagenosa sp. nov., a novel acid-tolerant representative of the order Planctomycetales. Microbiology 80(1):101–107. https://doi.org/10.1134/s002626171101019x
Acknowledgements
We thank Prof. Dr. Aharon Oren for his contribution concerning the nomenclature and etymology of the novel taxon. We also thank to Ângela Alves for sample preparation for electron microscopy.
Funding
Open access funding provided by FCT|FCCN (b-on). This research was supported by national funds through FCT—Fundação para a Ciência e Tecnologia within the scope of UIDB/04423/2020 and UIDP/04423/2020. This research was partially supported by FCT (Ph.D. grants SFRH/BD/144289/2019, SFRH/BD/145577/2019, SFRH/BD/145576/2019, SFRH/BD/125527/2016), and by the Luxembourg National Research Fund (FNR).
Author information
Authors and Affiliations
Contributions
OG—Investigation, Methodology, Writing—original draft, DK—Investigation, Writing—review & editing, AJ—Investigation, BG—Investigation, EA—Investigation, Methodology, Writing—review & editing, RC- Investigation, Writing—review & editing, IRV—Investigation, Writing—review & editing, JDNS—Investigation, Writing—review & editing, IG- Investigation, Writing—review & editing, Resources, ALC—Investigation, Resources, MC—Resources, Writing—review & editing, SQ—Methodology, Writing—review & editing, OML—Supervision, Investigation, Methodology, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Godinho, O., Klimek, D., Jackiewicz, A. et al. Stieleria tagensis sp. nov., a novel member of the phylum Planctomycetota isolated from Tagus River in Portugal. Antonie van Leeuwenhoek 116, 1209–1225 (2023). https://doi.org/10.1007/s10482-023-01877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-023-01877-2