Abstract
A Gram-strain positive, aerobic, endospore-forming bacterial strain (JJ-246T) was isolated from the rhizosphere of Zea mays. The 16S rRNA gene sequence similarity comparisons showed a most closely relationship to Paenibacillus oenotherae DT7-4T (98.4%) and Paenibacillus xanthinolyticus 11N27T (98.0%). The pairwise average nucleotide identity and digital DNA-DNA hybridisation values of the JJ-246T genome assembly against publicly available Paenibacillus type strain genomes were below 82% and 33%, respectively. The draft genome of JJ-246T shared many putative plant-beneficial functions contributing (PBFC) genes, related to plant root colonisation, oxidative stress protection, degradation of aromatic compounds, plant growth-promoting traits, disease resistance, drug and heavy metal resistance, and nutrient acquisition. The quinone system of strain JJ-246T, the polar lipid profile and the major fatty acids were congruent with those reported for members of the genus Paenibacillus. JJ-246T was shown to represent a novel species of the genus Paenibacillus, for which the name Paenibacillus plantiphilus sp. nov. is proposed, with JJ-246T (= LMG 32093T = CCM 9089T = CIP 111893T) as the type strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Paenibacillus is a still growing bacterial genus now accommodating more than 280 species (https://lpsn.dsmz.de/genus/paenibacillus) isolated from various sources. Initially proposed by Ash et al. (1993) on the basis of few species it is very clear, that species of Paenibacillus can be found in many different habitats, among them plants and their rhizosphere (Elo et al. 2001; Ma et al. 2007; Kim et al. 2009a,b, 2015a,b; Hong et al. 2009; Beneduzi et al. 2010; Zhang et al. 2013, 2015; Wang et al. 2014; Son et al. 2014; Han et al. 2015; Kämpfer et al. 2016, 2017a,b, 2021, 2022; Xin et al. 2017), seeds (Liu et al. 2015) or the phyllosphere (Rivas et al. 2005, 2006). They can be also found in plants as endophytes (Carro et al. 2013; Lai et al. 2015; Kittiwongwattana & Thawai 2015; Gao et al. 2015).
In this context, they may play an important role in their capacity to promote plant growth and form endospores (Grady et al. 2016). Several plant growth promoting traits have been confirmed for several members of the genus Paenibacillus (e.g. Wang et al. 2023). Plant growth promoting rhizobacteria (PGPR) are of increasing importance to enhance the efficiency and yield in sustainable agricultural crob production (Grady et al. 2016).
For this purpose we aimed to isolate further new Paenibacillus species colonising the rhizosphere and roots of agricultural crop plants which may support efficiently plant growth and agricultural yield production (de Andrade et al. 2023). In this context we characterised the new Paenibacillus sp. JJ-246T and studied its genome for the presence of different plant-beneficial function contributing (PBFC) genes, related to plant root colonisation, oxidative stress protection, degradation of aromatic compounds, plant growth-promoting traits, disease resistance, drug and heavy metal resistance, nutrient acquisition, nitrogen fixation, and phosphate solubilisation in addition for its capacity to produce indole-3-acetic acid (IAA).
Materials and methods
Isolation and culture conditions
A collection of 1,884 bacterial strains were obtained from field-grown maize plants (Zea mays) in Dunbar, Nebraska. The collection was assembled with the purpose of finding strains with beneficial plant properties. Fields in the US Midwest have a history of successfully supporting healthy crops and harvests, and it was surmised that the microbes in these fields may play a role in the overall health of these crops. Once in pure culture, strains could then be tested individually by seed-treatment in a greenhouse assay on agriculturally important crops. Toward this goal, two-week-old maize seedlings were manually uprooted. Roots, approximately 15 cm in length, were separated from the surrounding soil by vigorous shaking such that only the most tightly adhering soil remained. Bacteria were harvested from the root surface by immersing the root in sterile water followed by plating dilutions on Nutrient Agar (Sigma-Aldrich). Strain JJ-246T was initially isolated at Auburn University in this manner. Subsequent cultivation for detailed characterisation of JJ-246T was performed on tryptone soya agar (TSA, Oxoid) at 28 °C for 24 h. The medium was chosen because reference Paenibacillus strains used for comparative purpose were routinely cultured under the same conditions.
16S rRNA gene sequencing and analysis
For a first phylogenetic placement, the 16S rRNA gene of strain JJ-246T was PCR amplified with primer system Eub9f (5′-GAGTTTGATCMTGGCTCAG-3′) and Eub1492R (5′-ACGGYTACCTTGTTACGACTT-3′) (Lane 1991), and sequenced with the Sanger dideoxy sequencing technology using primers Eub9f and E786F (5′-GATTAGATACCCTGGTAG-3′), respectively, as described previously (Schauss et al. 2015). The sequence was manually processed and corrected based on the electropherograms using MEGA11 (Tamura et al. 2021).
A first phylogenetic assignment was performed by BLAST analysis against the EzBioCloud 16S rRNA gene sequence database including all type strain 16S rRNA gene sequences and blastn blast + v2.13.0 of the NCBI (https://blast.ncbi.nlm.nih.gov) against the curated 16S ribosomal RNA type strain database (update 2023/01/12). The 16S rRNA gene sequences of strain JJ-246T and non-included related type strains were subsequently added to LTP_2020 database of the “All-Species Living Tree'' Project (LTP; Yarza et al. 2008) using ARB release 5.2 (Ludwig et al. 2004). The updated database version LTP_04_2021 (released in September 2021) was used. The imported 16S rRNA gene sequences were aligned in the alignment explorer using the pt server generated for the respective database. The alignment was checked manually before subsequent analyses. The sequence was added to the database tree tree_Ltp_all_04_21 containing all type strain 16S rRNA gene sequences of species described until May 2021 using the parsimony quick ad marked tool of ARB and the gap95_q0_to_q5 filter.
All strains of the resulting cluster and the 16S rRNA gene sequence of the type strain of the type species P. polymyxa and next related type strains which clustered with that were included in the analysis. According to previous phylogenetic analysis of the genus Paenibacillus, two Cohnella type strains were used as outgroup (Kämpfer et al. 2022). The genus is assigned as a neighbouring monophyletic genus. Different phylogenetic trees were calculated which were all based on gene termini 95 to 1475 (Escherichia coli numbering, Brosius et al. 1978). This sequence range was covered by all compared sequences. A maximum-likelihood tree was calculated with RAxML v7.04 (Stamatakis 2006), GTR-GAMMA and rapid bootstrap analysis and a maximum parsimony tree with DNAPARS v 3.6 (Felsenstein 2005). Both trees were calculated with 100 re-samplings (bootstrap analysis; Felsenstein 1985) and based on 16S rRNA gene sequences between gene termini 95 to 1446 (Escherichia coli numbering, Brosius et al. 1978).
Genome sequencing and analyses
The whole genome sequencing of strain JJ-246T was carried out with a NextSeq500 instrument using the Nextera XT DNA library preparation kit (Illumina) and a 2 × 150 bp paired-end protocol, yielding 3,948,028 read pairs (192 × average sequencing depth, 361 bp average insert size). Read processing, genome assembly and gene annotation were performed using fq2dna v21.06 (https://gitlab.pasteur.fr/GIPhy/fq2dna).
Pairwise average nucleotide and amino acid identity (ANI and AAI, respectively) values were computed using OGRI_B v1.1 (https://gitlab.pasteur.fr/GIPhy/OGRI) between the draft genome of JJ-246T and every Paenibacillus type strain genome selected for the 16S rRNA phylogenetic analysis (when publicly available).
A phylogenetic classification of these genomes was inferred using JolyTree v2.1 (Criscuolo 2019, 2020). An alternative phylogenetic tree was inferred using the 120 conserved phylogenetic markers suggested by Parks et al. (2018). For each locus, gathered amino acid sequences were aligned using MAFFT v7.467 (Katoh and Standley 2013, 2016), and next processed using BMGE v2.0 (Criscuolo and Gribaldo 2010) to select aligned characters that are suited for phylogenetic inference (default options; see Text S1). Maximum likelihood phylogenetic inference from the concatenation of the resulting 120 multiple sequence alignments was carried out using IQ-TREE v2.2.2.2 (Minh et al. 2020) with evolutionary model LG + I + F + R5, derived by minimising the Bayesian information criterion as estimatedby default in IQ-TREE (Kalyaanamoorthy et al. 2017). All phylogenetic trees were drawn using FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree).
Plant-beneficial function contributing (PBFC) genes (as listed in Cherif-Silini et al. 2019) were searched using tblastn against the draft genome of JJ-246T. Biosynthetic gene clusters for secondary metabolites were inferred using antiSMASH v7.0.0beta1-67b538a9 (Blin et al. 2021).
Physiology and chemotaxonomy
A detailed phenotypic characterisation of strain JJ-246T was performed on the basis of the methods published previously (Kämpfer et al. 2016, 2017a,b, 2021). Cell morphology and motility was observed under a Zeiss light microscope at a magnification of 1000, using cells that had been grown for 3 days at 28 °C on TSA (Oxoid). Gram-staining was performed by the modified Hucker method according to Gerhardt et al. (1994). Oxidase activity was tested with a oxidase reagent text kit following the instructions of the manufacturer (bioMérieux, France). Growth was tested on different agar media including R2A (Oxoid), Nutrient broth agar (NB, Oxoid), TSA, Malt agar (Merck), PYE [0.3% (w/v) yeast extract, and 0.3% (w/v) casein peptone, respectively, 15 g agar L−1, pH 7.2], CASO agar (Carl Roth), K7 [0.1% (w/v) of yeast extract, peptone, and glucose, 15 g L−1 agar, pH 6.8], medium 65 (M65, according to DSMZ), DEV agar (DEV, Merck), Nutrient agar (NA, Becton Dickinson), Luria Bertani agar (LB, Sigma-Aldrich), Marine agar 2216 (MA, Becton Dickinson), Columbia agar with sheep blood (Oxoid), and MacConkey agar (Oxoid). Growth was evaluated after 48 h incubation at 28 °C. Temperature dependent growth was determined on Columbia agar with sheep blood at 4, 10, 15, 20, 25, 28, 30, 36, 45, 50, and 55 °C. pH and salinity dependent growth was tested in R2A broth incubated at 28 °C. The pH was adjusted to pH 4.5 to 10.5 (1 pH unit intervals incrementing) using HCl and NaOH. For salinity dependent growth 1 to 8% (w/v) NaCl was added (in 1% intervals incrementing). Growth was monitored after 72 h of incubation.
Further physiological characterisation of the strains was performed with the API 20NE and API ZYM test systems according to the instruction of the manufacturer (bioMérieux) and with methods described by Kämpfer et al. (1991, 1993) and Kämpfer (1990). All tests were incubated at 28 °C.
For analyses of quinones and polar lipids, cells were grown in trypton soya broth at 30 °C for 48 h. Quinones were extracted as described by Minnikin et al. (1984) and by Wiertz et al. (2013) and analysed with an Agilent 1260 infinity HPLC system. Polar lipids were extracted and analysed by thin-layer chromatography (TLC) according to Minnikin et al. (1984). TLC plates were stained with ninhydrin for detection of aminolipids and with molybdenum blue reagent for detection of phospholipids. All polar lipids were visualised with sulfuric acid and heating the plates on 140 °C.
The fatty acids were extracted and analysed as described by Kämpfer and Kroppenstedt (1996). Strains were grown under identical conditions (TSA after 72 h incubation at 28 °C) and the cells for extractions were taken from colonies of the same size. Fatty acids were identified with the Sherlock version 2.11, TSBA40 Rev. 4.1.
Results and discussion
Molecular and genome characteristics
The final corrected 16S rRNA gene sequence of strain JJ-246T (OQ300222) had a size of 1,479 nt and spanned gene termini 8 to 1,475 (numbering according to the Escherichia coli rrnB sequence published by Brosius et al. 1978). Based on the 16S rRNA gene sequences, strain JJ-246T was placed within the cluster of Paenibacillus type strains determined as the next related species by BLAST analyses. Strain JJ-246T showed highest 16S rRNA gene sequence similarity to the type strain of Paenibacillus oenotherae (98.4%), followed by the type strain of Paenibacillus xanthinilyticus (98.0%); sequence similarities to all other type strains were below 97%.
Several phylogenetic trees were calculated and several outgroup strains were tested. The genus Cohnella is monophyletic and well distinguishable to members of the paraphyletic genus Paenibacillus, for that reason it gave a well supported outgroup taxon. Independent of the applied treeing method, strain JJ-246T showed a tendency to cluster with the type strains of P. oenotherae and P. xanthinilyticus (70% bootstrap support; Fig. S1).
The draft genome of strain JJ-246T was made of 6,062,127 bps on 102 contigs (N50, 147,865) with G + C content of 49.25 mol%. A total of 5,293 coding sequences and 64 tRNA was inferred. Genome sequence authenticity was assessed by aligning the 16S rRNA segment derived from Sanger sequencing (OQ300222) against the de novo assembly (CAKMMF000000000) using blastn, leading to 99.73% pairwise sequence similarity.
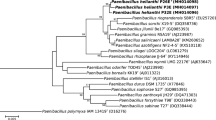
Average nucleotide and amino acid identity (ANI and AAI, respectively) values are reported in Table S1, together with the associated digital DNA-DNA hybridisation (dDDH) values (formula 2; https://ggdc.dsmz.de). All these estimated pairwise similarity values are far below the commonly admitted species delineation cutoffs (ANI, 95%; AAI, 96%; dDDH, 70%). Phylogenomic classifications confirmed that strain JJ-246T formed a stable cluster (100% branch support) with the type strain of P. oenotherae (Fig. 1; Fig. S2).
Whole-genome-based tree showing the phylogenetic placement of strain JJ-246T among type strains of closely related Paenibacillus species. This minimum evolution tree was inferred using JolyTree (https://gitlab.pasteur.fr/GIPhy/JolyTree). Two publicly available Cohnella type strain genomes were used as outgroup. The genome sequence accession is specified between parentheses next after each taxon name. Branch supports were assessed by the rate of elementary quartets, as estimated by JolyTree (only supports > 0.5 were specified). Bar, 0.025 nucleotide substitutions per site
The draft genome of JJ-246T shares different plant-beneficial function contributing (PBFC) genes, related to plant root colonisation (Table S2), oxidative stress protection (Table S3), degradation of aromatic compounds (Table S4), plant growth-promoting traits (Table S5), disease resistance (Table S6), drug and heavy metal resistance (Table S7), and nutrient acquisition (Table S8). Contrary to many Paenibacillus strains (e.g. Xie et al. 2014), the genome of JJ-246T does not seem to share particular genes related to nitrogen fixation. However, it contains genes related to phosphate solubilisation (Table S8), most of them being grouped into a unique cluster (CAH1206316-CAH1206402) comparable to a phosphate-specific transport (tsp) operon (e.g. Li et al. 2020). Phophorus (P) is an essential macronutrient for plants which is limited in bioaviable forms in soil. Solubilisation of P by PGPR is an sufficient process to enhance the P availability for the uptake by plant roots and improve thereby crob yield (Din et al. 2020). The genome of JJ-246T also shares a gene cluster trpEDCFBA (CAH1218260-CAH1218273; Table S5), related to the production of indole-3-acetic acid (IAA), an important phytohormone known to stimulate plant growth (e.g. Yuan et al. 2022; Shao et al. 2015).
Finally, a total of 16 biosynthetic gene clusters involved in secondary metabolite production were found, including eight non-ribosomal peptide synthetase, four polyketide synthase, three agrD-like cyclic lactone autoinducer peptides, two IucA/Iuc-like siderophores, one lasso peptide, one heterocyst glycolipid synthase-like polyketide synthase, one terpene and one resorcinol clusters (Table S9). Interestingly, two of them are quite similar to paenibacterin and paeninodin biosynthetic gene clusters (60% and 80% gene similarity, respectively) already described in other Paenibacillus strains and involved in the production of broad-spectrum antimicrobial compounds (Huang et al. 2014; Zhu et al. 2016).
Phenotype
The results of the physiological characterisation are given in Table 1 and in the species description. Strain JJ-246T can be differentiated from the type strains of the most closely related species with a battery of selected physiological tests. Strain JJ-246T was not able to hydrolyse starch and to utilise L-arabinose, sucrose, amygdalin in contrast to P. oenotherae DLE-12T and P. xanthinilyticus 11N27T, which both showed positive reactions in these tests. In addition, strain JJ-246T did not produce α-galactosidase which differentiated the strain from P. oenotherae DLE-12T and was able to grow at 37 °C which differentiated the strain from P. xanthinilyticus 11N27T. More differentiating tests are shown in Table 1. The polar lipid profile consisted of diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylserine (PS) and one additional unidentified aminophospholipid (APL) (Fig. S3). The type strain of P. oenotherae DLE-12T also revealed diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), phosphatidylglycerol (PG) as major lipids, but did not produce phosphatidylserine (PS), but instead three unidentified aminophospholipids, an unidentified aminolipid, an unidentified phospholipid and two unidentified lipids (Kim et al. 2015b). The polar lipids present in strain P. xanthinilyticus 11N27T were also phosphatidylethanolamine (PE), two unknown phospholipids, two unknown aminolipids, one unknown aminophospholipid, and two unknown polar lipids that were not stainable with any of the specific spray reagents applied to detect a phosphate, an amino-, or a sugar moiety (Kim et al. 2015a). It is quite likely that among the two unidentified phospholipids also diphosphatidylglycerol (DPG) and phosphatidylglycerol (PG) are produced, which are typical for Panibacillus species.
The dominating quinone of strain JJ-246T was menaquinone MK-7 (97%) Minor amounts of menaquinone MK-8 (3%) were also detected. Presence of menaquinone MK-7 as well as the polar lipid profile are in agreement with the characteristics of other species of the genus Paenibacillus, also for P. xanthinilyticus and P. oenotherae (Kim et al. 2015a,b).
The fatty acids comprised mainly iso- and anteiso-branched fatty acids, and the fatty acid profile was very similar to that of the most closely related Paenibacillus species, which is also quite typical for Paenibacillus species, which are quite similar with respect to fatty acid profiles. Strain JJ-246T was able to produce C15:0 which was not detected in P. oenotherae DLE-12T and P. xanthinilyticus 11N27T. For the majority of other fatty acids in most cases only minor quantitative differences could be found. The detailed fatty acid profile obtained from cells grown is shown in Table S10.
Conclusion
On the basis of genotypic and phenotypic features a novel species of the genus Paenibacillus is proposed for which the name Paenibacillus plantiphilus is proposed with JJ-246T as type strain. The strain was isolated from the rhizosphere of Zea mays indicating its potential to grow efficient in the rhizosphere of agricultural plants. Genome analyses showed the potential of the strain to represent a novel PGPR.
Description of Paenibacillus plantiphilus sp. nov.
Paenibacillus plantiphilus (plan.ti'phi.lus. L. fem. n. planta, a plant; Gr. masc. adj. philos, loving; N.L. masc. adj. plantiphilus, plant-loving).
Cells (with rounded ends) stain Gram-positive. No chains or filaments could be observed after growth on TSA at 28 °C for 48 h. Cells were 2.0-3.0 µm in length and 0.8-1.0 µm in width) and showed no motility. Endospores were not observed. No other cell inclusions could be detected. Colonies grown on TSA (Oxoid) at 28°C after 48 h of incubation are circular, convex and beige with a shiny appearance and an average diameter of 2 to 3 mm. Growth at 28 °C on R2A (Oxoid), CASO agar (Carl Roth), K7, M65, DEV agar (DEV, Merck), NA (Becton Dickinson), LB (Sigma-Aldrich), and Columbia agar with sheep blood (Oxoid). No growth on MA (Becton Dickinson), NB (Oxoid), Malt agar (Merck), and MacConkey agar (Oxoid).
Good growth on CASO agar between 10 and 45 °C (weak) (optimal growth at 25–30 °C), but not at 4 °C and 50 °C. Optimal pH for growth in R2A broth at 28 °C is pH 7–8; growth occurs between pH 5.5–10.5. Optimal growth in R2A broth at 28 °C without and in the presence of 1% NaCl, growth occurs between 0 to 1% NaCl, but not at 2% or above. Tests for catalase and oxidase activities are positive. According to API 20 NE, positive for the assimilation of D-glucose, L-arabinose and D-maltose, but negative in the activities of β-glucosidase (esculin hydrolysis), PNPG beta-galactosidase, indole production, D-glucose fermentation, arginine dihydrolase, urease, gelatin hydrolysis and the assimilation of D-mannitol, N-acetylglucosamine, D-maltose, capric acid, adipic acid, malic acid, trisodium citrate, and phenylacetic acid. According to API 20E and to tests of Kämpfer et al. (1991), negative in acid formation of D-glucose, L-arabinose, D-mannose, D-mannitol, N-acetylglucosamine, D-maltose, sucrose, amygdalin, inositol, melibiose.
According to API ZYM, positive for naphtol-AS-BI-phosphohydrolase and negative for leucine arylamidase, acid phosphatase, β-galactosidase, alkaline phosphatase, esterase (C4), esterase lipase (C8), lipase (C14), valine arylamidase, cystine arylamidase, trypsin, α-chymotrypsin, α-galactosidase, β-glucuronidase, α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase.
Some sugars or sugar-related compounds were utilised by strain JJ-246T according to the method of Kämpfer et al. (1991): L-arabinose (weak), arbutin, D-cellobiose, D-galactose, glucose (weak), D-maltose, D-melibiose, sucrose, D-trehalose, D-xylose and D-maltitol are utilised as sole sources of carbon. D-gluconate, ribose, D-fructose, i-inositol, D-mannose, salicin, D-sorbitol, acetate, N-acetyl-D-glucosamine, cis-aconitate, trans-aconitate, adipate, D-adonitol, 4-aminobutyrate, azelate, citrate, itaconate, malate, mesaconate, 2-oxoglutarate, propionate, putrescine, pyruvate and L-rhamnose are not utilised as sole carbon source.
The quinone system is dominated by menaquinone MK-7. The polar lipid profile is composed of the major lipids diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, phosphatidylserine and one additional unidentified aminophospholipid (APL).
Major fatty acids are anteiso C15:0 and iso C15:0. The genomic DNA G + C content is 49.25 mol% (based on the genome sequence).
The type stain JJ-246T (= LMG 32093T = CCM 9089T = CIP 111893T) was isolated from the root surface of a field-grown corn plant in Dunbar, Nebraska USA. The genome sequence of the type strain is available under accession number CAKMMF00000000, and the 16S rRNA gene sequence under accession number OQ300222.
Data availability
The 16S rRNA gene and the complete genome sequences of strain JJ-246T have been deposited under the GenBank/EMBL/DDBJ accession numbers OQ300222 and CAKMMF000000000, respectively.
References
Ash C, Priest FG, Collins MD (1993) Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie Van Leeuwenhoek 64:253–260. https://doi.org/10.1007/bf00873085
Beneduzi A, Costa PB, Parma M et al (2010) Paenibacillus riograndensis sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Triticum aestivum. Int J Syst Evol Microbiol 60:128–133. https://doi.org/10.1099/ijs.0.011973-0
Blin K, Shaw S, Kloosterman AM et al (2021) antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49:W29–W35
Brosius J, Palmer ML, Kennedy PJ et al (1978) Complete nucleotide-sequence of a 16S ribosomal-RNA gene from Escherichia coli. PNAS 75:4801–4805. https://doi.org/10.1073/pnas.75.10.4801
Carro L, Flores-Félix JD, Cerda-Castillo E et al (2013) Paenibacillus endophyticus sp. nov., isolated from nodules of Cicer arietinum. Int J Syst Evol Microbiol 63:4433–4438. https://doi.org/10.1099/ijs.0.050310-0
Cherif-Silini H, Thissera B, Chenari Bouket A et al (2019) Durum wheat stress tolerance induced by endophyte pantoea agglomerans with genes contributing to plant functions and secondary metabolite arsenal. Int J Mol Sci 20:3989. https://doi.org/10.3390/ijms20163989
Criscuolo A (2019) A fast alignment-free bioinformatics procedure to infer accurate distance-based phylogenetic trees from genome assemblies. Res Ideas Outcomes 5:e36178. https://doi.org/10.3897/rio.5.e36178
Criscuolo A (2020) On the transformation of MinHash-based uncorrected distances into proper evolutionary distances for phylogenetic inference. F1000Research 9:1309. https://doi.org/10.12688/f1000research.26930.1
Criscuolo A, Gribaldo S (2010) BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10:210. https://doi.org/10.1186/1471-2148-10-210
de Andrade LA, Santos CHB, Frezarin ET, Sales LR, Rigobelo EC (2023) Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 11(4):1088. https://doi.org/10.3390/microorganisms11041088
Din ARJM, Rosli MA, Azam ZM, Othman NZ, Sarmidi MR (2020) Paenibacillus polymyxa role involved in phosphate solubilization and growth promotion of Zea mays under abiotic stress condition. Proc Natl Acad Sci USA 90:63–71. https://doi.org/10.1007/s40011-019-01081-1
Elo S, Suominen I, Kämpfer P et al (2001) Paenibacillus borealis sp. nov., a nitrogen-fixing species isolated from spruce forest humus in Finland. Int J Syst Evol Microbiol 51:535–545
Felsenstein J (1985) Confidence limits of phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Gao JL, Lv FY, Wang XM et al (2015) Paenibacillus wenxiniae sp. nov., a nifH gene-harbouring endophytic bacterium isolated from maize. Antonie Van Leeuwenhoek 108:1015–1022. https://doi.org/10.1007/s10482-015-0554-8
Gerhardt P, Murray RGE, Wood W et al (1994) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC
Grady EN, MacDonald J, Liu L et al (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact 15:203. https://doi.org/10.1186/s12934-016-0603-7
Han T-Y, Tong X-M, Wang Y-W et al (2015) Paenibacillus populi sp. nov., a novel bacterium isolated from the rhizosphere of Populus alba. Antonie Van Leeuwenhoek 108:659–666. https://doi.org/10.1007/s10482-015-0521-4
Hong YY, Ma YC, Zhou YG et al (2009) Paenibacillus sonchi sp. nov., a nitrogen-fixing species isolated from the rhizosphere of Sonchus oleraceus. Int J Syst Evol Microbiol 59:2656–2661. https://doi.org/10.1099/ijs.0.009308-0
Huang E, Guo Y, Yousef AE (2014) Biosynthesis of the new broad-spectrum lipopeptide antibiotic paenibacterin in Paenibacillus thiaminolyticus OSY-SE. Res Microbiol 165:243–251. https://doi.org/10.1016/j.resmic.2014.02.002
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler V, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Kämpfer P (1990) Evaluation of the Titertek-Enterobac-Automated system (TTE-AS) for identification of Enterobacteriaceae. Zentbl Bakteriol 273:164–172. https://doi.org/10.1016/s0934-8840(11)80244-6
Kämpfer P, Steiof M, Becker P, Dott W (1993) Characterization of chemoheterotrophic bacteria associated with the in situ bioremediation of a waste-oil contaminated site. Microb Ecol 26:161–188. https://doi.org/10.1007/BF00177050
Kämpfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:989–1005. https://doi.org/10.1139/m96-128
Kämpfer P, Steiof M, Dott W (1991) Microbiological characterisation of a fuel-oil contaminated site including numerical identification of heterotrophic water and soil bacteria. Microbiol Ecol 21:227–243. https://doi.org/10.1007/bf02539156
Kämpfer P, Busse HJ, McInroy JA et al (2021) Paenibacillus allorhizosphaerae sp. Nov., from soil of the rhizosphere of Zea mays. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijsem.0.005051
Kämpfer P, Busse HJ, Kloepper JW et al (2016) Paenibacillus cucumis sp. nov., isolated from a cucumber plant. Int J Syst Evol Microbiol 66:2599–2603
Kämpfer P, Busse HJ, McInroy JA et al (2017a) Paenibacillus nebraskensis sp. nov., isolated from the root surface of field-grown maize. Int J Syst Evol Microbiol 67:4956–4961. https://doi.org/10.1099/ijsem.0.002357
Kämpfer P, Busse HJ, McInroy JA et al (2017b) Paenibacillus rhizoplanae sp. nov., isolated from the rhizosphere of Zea mays. Int J Syst Evol Microbiol 67:1058–1063. https://doi.org/10.1099/ijsem.0.001779
Kämpfer P, Lipski A, Lamothe L, Clermont D, Criscuolo A et al (2022) Paenibacillus allorhizoplanae sp. Nov. from the rhizoplane of a Zea mays root. Arch Microbiol 204:630. https://doi.org/10.1007/s00203-022-03225-w
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Katoh K, Standley DM (2016) A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 32:1933–1942. https://doi.org/10.1093/bioinformatics/btw108
Kim BC, Lee KH, Kim MN et al (2009a) Paenibacillus pini sp. nov., a cellulolytic bacterium isolated from the rhizosphere of pine tree. J Microbiol 47:699–704. https://doi.org/10.1007/s12275-009-0343-z
Kim BC, Lee KH, Kim MN et al (2009b) Paenibacillus pinihumi sp. nov., a cellulolytic bacterium isolated from the rhizosphere of Pinus densiflora. J Microbiol 47:530–535. https://doi.org/10.1007/s12275-009-0270-z
Kim Du, Kim SG, Lee H, Chun J, Cho JC et al (2015a) Paenibacillus xanthinilyticus sp. nov., isolated from agricultural soil. Int J Syst Evol Microbiol 65:2937–2942
Kim TS, Han JH, Joung Y, Kim SB (2015b) Paenibacillus oenotherae sp. nov. and Paenibacillus hemerocallicola sp. nov., isolated from the roots of herbaceous plants. Int J Syst Evol Microbiol 65:2717–2715
Kittiwongwattana C, Thawai C (2015) Paenibacillus lemnae sp. nov., an endophytic bacterium of duckweed (Lemna aequinoctialis). Int J Syst Evol Microbiol 65:107–112. https://doi.org/10.1099/ijs.0.067876-0
Lai WA, Hameed A, Lin SY et al (2015) Paenibacillus medicaginis sp. nov. a chitinolytic endophyte isolated from the root nodule of alfalfa (Medicago sativa L.). Int J Syst Evol Microbiol 65:3853–3860. https://doi.org/10.1099/ijsem.0.000505
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Li JY, Gao TT, Wang Q (2020) Comparative and functional analyses of two sequenced Paenibacillus polymyxa genomes provides insights into their potential genes related to plant growth-promoting features and biocontrol mechanisms. Front Genet 11:564939. https://doi.org/10.3389/fgene.2020.564939
Liu Y, Zhai L, Wang R et al (2015) Paenibacillus zeae sp. nov., isolated from maize (Zea mays L.) seeds. Int J Syst Evol Microbiol 65:4533–4538. https://doi.org/10.1099/ijsem.0.000608
Ludwig W, Strunk O, Westram R et al (2004) ARB: a software environment for sequence data. Nucleic Acid Res 32:1363–1371. https://doi.org/10.1093/nar/gkh293
Ma Y, Xia Z, Liu X et al (2007) Paenibacillus sabinae sp. nov., a nitrogen-fixing species isolated from the rhizosphere soils of shrubs. Int J Syst Evol Microbiol 57:6–11. https://doi.org/10.1099/ijs.0.64519-0
Minh BQ, Schmidt HA, Chernomor O et al (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37:1530–1534. https://doi.org/10.1093/molbev/msaa015
Minnikin DE, O’Donnell AG, Goodfellow M et al (1984) An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiological Method 2:233–241. https://doi.org/10.1016/0167-7012(84)90018-6
Parks DH, Chuvochina M, Waite DW et al (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. https://doi.org/10.1038/nbt.4229
Rivas R, García-Fraile P, Mateos PF et al (2006) Paenibacillus cellulosilyticus sp. nov., a cellulolytic and xylanolytic bacterium isolated from the bract phyllosphere of Phoenix dactylifera. Int J Syst Evol Microbiol 56:2777–2781. https://doi.org/10.1099/ijs.0.64480-0
Rivas R, Mateos PF, Martínez-Molina E et al (2005) Paenibacillus phyllosphaerae sp. nov., a xylanolytic bacterium isolated from the phyllosphere of Phoenix dactylifera. Int J Syst Evol Microbiol 55:743–746. https://doi.org/10.1099/ijs.0.63323-0
Schauss T, Busse HJ, Golke J et al (2015) Empedobacter stercoris sp. nov., isolated from an input sample of a biogas plant. Int J Syst Evol Microbiol 65:3746–3753. https://doi.org/10.1099/ijsem.0.000486
Shao J, Li S, Zhang N, Cui X, Zhou X, Zhang G, Shen Q, Zhang R (2015) Analysis and cloning of the synthetic pathway of the phytohormone indole-3-acetic acid in the plant-beneficial Bacillus amyloliquefaciens SQR9. Microb Cell Fact 14:130. https://doi.org/10.1186/s12934-015-0323-4
Son J-S, Kang H-U, Ghim S-Y (2014) Paenibacillus dongdonensis sp. nov., isolated from rhizospheric soil of Elymus tsukushiensis. Int J Syst Evol Microbiol 64:2865–2870. https://doi.org/10.1099/ijs.0.061077-0
Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Wang DS, Jiang YY, Wie XM et al (2014) Paenibacillus quercus sp. nov., isolated from rhizosphere of Quercus aliena var. acuteserrata. Antonie Van Leeuwenhoek 105:1173–1178. https://doi.org/10.1007/s10482-014-0178-4
Wang D, Poinsot V, Li W et al (2023) Genomic insights and functional analysis reveal plant growth promotion traits of Paenibacillus mucilaginosus G78. Genes (Basel) 14(2):392. https://doi.org/10.3390/genes14020392
Wiertz R, Schulz SC, Müller U et al (2013) Corynebacterium frankenforstense sp. nov. and Corynebacterium lactis sp. nov., isolated from raw cow milk. Int J Syst Evol Microbiol 63:4495–4501. https://doi.org/10.1099/ijs.0.050757-0
Xie J-B, Du Z, Bai L et al (2014) Comparative genomic analysis of N2-fixing and Non-N2-fixing Paenibacillus spp.: organization, evolution and expression of the nitrogen fixation genes. PLoS Genet 10(3):e1004231. https://doi.org/10.1371/journal.pgen.1004231
Xin K, Li M, Chen C et al (2017) Paenibacillus qinlingensis sp. nov., an indole-3-acetic acid-producing bacterium isolated from roots of Sinopodophyllum hexandrum (Royle) Ying. Int J Syst Evol Microbiol 67:589–595. https://doi.org/10.1099/ijsem.0.001666
Yarza P, Richter M, Peplies J et al (2008) The all-species living tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 31:241–250. https://doi.org/10.1016/j.syapm.2008.07.001
Yuan L, Jiang H, Jiang X et al (2022) Comparative genomic and functional analyses of Paenibacillus peoriae ZBSF16 with biocontrol potential against grapevine diseases, provide insights into its genes related to plant growth-promoting and biocontrol mechanisms. Front Microbiol 13:975344. https://doi.org/10.3389/fmicb.2022.975344
Zhang L, Gao JS, Zhang S et al (2015) Paenibacillus rhizoryzae sp. nov., isolated from rice rhizosphere. Int J Syst Evol Microbiol 65:3053–3059. https://doi.org/10.1099/ijs.0.000376
Zhang J, Wang ZT, Yu HM et al (2013) Paenibacillus catalpae sp. nov., isolated from the rhizosphere soil of Catalpa speciosa. Int J Syst Evol Microbiol 63:1776–1781. https://doi.org/10.1099/ijs.0.040659-0
Zhu S, Hegemann JD, Fage CD et al (2016) Insights into the Unique phosphorylation of the lasso peptide paeninodin. J Biol Chem 291(26):13662–13678. https://doi.org/10.1074/jbc.M116.722108
Acknowledgements
We acknowledge the help of the HPC Core Facility of the Institut Pasteur for this work. We also thank Bettina Becker and David Heidler von Heilborn for support concerning quinone and polar lipid analyses, and Prof. Aharon Oren for his help with the etymology of the species epithet.
Funding
Open Access funding enabled and organised by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
PK wrote the main manuscript text. SG, AL and PK conducted the experiments. DC and AC provided the genome sequence. AC and LL performed the genomic and phylogenomic analyses. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The manuscript is submitted with the consent of all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kämpfer, P., Lipski, A., Lamothe, L. et al. Paenibacillus plantiphilus sp. nov. from the plant environment of Zea mays. Antonie van Leeuwenhoek 116, 883–892 (2023). https://doi.org/10.1007/s10482-023-01852-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-023-01852-x