Abstract
Human immunodeficiency virus (HIV) viral load (VL) monitoring was likely interrupted during the Coronavirus disease 2019 (COVID-19) pandemic. We used routine data on repeat VL testing among 667 prevention of vertical HIV transmission (PVT) clients in Ehlanzeni district, to determine compliance to VL testing recommendations and associated factors during different time periods: pre-COVID-19, transition, and COVID-19. Descriptive and multivariable Poisson regression analyses were conducted, with and without including revised PVT-guidelines rolled out in January-2020. Among 405 women with ≥ 2 VL tests, the overall median age was 30 years (interquartile range: 26–35 years). Compliance to recommended VL testing guidelines ranged between 81.5% (172/211) and 92.3% (191/207) at different time periods. Across all three periods and when revised PVT-guidelines were used, being compliant was significantly reduced among those with earliest VL = 50–999 copies/ml (incidence rate ratio (IRR) = 0.71 [95% confidence interval (CI) 0.61–0.82], p value < 0.001) and VL ≥ 1000 copies/ml (IRR = 0.18 [95% CI 0.09–0.36], p value < 0.001). When guideline revisions were excluded, compliance was only significantly reduced among those with VL ≥ 1000 copies/ml (IRR = 0.14 [95% CI 0.06–0.32], p value < 0.001) and increased during the COVID-19 period versus pre-COVID-19 (IRR = 1.10 [95% CI 1.05–1.15], p value < 0.001). Similar significant associations between compliance and VL level were observed when the COVID-19 period was analyzed separately. Significantly increased compliance to VL testing among the 25–34 years age-group versus younger women was also observed across all periods. These results highlight the importance of strengthening strategies such as short message service reminders and educational messaging, reaching all age-groups, to fast-track implementation targets for VL monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) recommended viral load (VL) testing for monitoring the effectiveness of antiretroviral treatment (ART) in human immunodeficiency virus (HIV) positive persons in 2013 [1]. VL monitoring is vital to tracking individual responses to ART, population-level effectiveness of ART, and progress towards United Nations Acquired Immunodeficiency Syndrome (UNAIDS) global targets for combating the HIV epidemic [2]. The UNAIDS’s third target in its 95-95-95 goals is to ensure that 95% of people on ART are virally suppressed by 2030 [3, 4]. However, progress towards the third target has been slow. In 2022, about 71% of people with HIV (PWHIV) globally were virally suppressed [5]. New interim targets for 2025 were developed to accelerate progress towards the 2030 goals. One of the main 2025 targets is to have 95% of pregnant and breastfeeding women with HIV (PBWHIV) having suppressed VL for elimination of vertical HIV transmission (EVT) [5,6,7,8,9,10]. The 2015 South African guidelines for Prevention of vertical HIV transmission (PVT) recommended a repeat VL in 1 month if VL ≥ 1000 copies/ml and within 6 months when VL < 1000 copies/ml, while the 2019 guidelines reduced the window period for repeat VL testing when VL = 50–999 copies/ml to 8–10 weeks and recommended the use of PVT codes on the National Health Laboratory Service (NHLS) VL requisition forms to electronically identify and permit frequent VL performed in PBWHIV at antenatal care (ANC), delivery, and postpartum, for rapid follow-up and care [11, 12].
Progress towards EVT has been slow and new infections continue to occur due to existing gaps in PVT; (i) new HIV infections during pregnancy, (ii) late HIV diagnosis and initiation of ART, and (iii) sub-optimal retention in care and viral suppression [13,14,15,16,17,18,19,20,21]. VL monitoring in this population has been sub-optimal, and compliance to repeat VL testing guidelines is not documented in South Africa [22,23,24]. Documenting compliance to VL testing in low-middle income countries is not yet common practice, yet it is crucial for understanding progress towards EVT. One example in Kenya reported that between 2016 and 2018 only 6% of PBWHIV who used public hospitals achieved a repeat VL test within the recommended schedule [25]. Additionally, PVT program activities are expected to have been impacted by the Coronavirus disease 2019 (COVID-19) pandemic and the restrictions on mobility and trade that were imposed to curb it.
In many countries, HIV services were interrupted, resources shifted towards the pandemic, and healthcare workers were allocated to COVID-19 tasks. Additionally, lockdown restrictions and fear of contracting COVID-19 made it difficult for people including pregnant and breastfeeding women to access healthcare services, and conducting face-to-face encounters was near impossible [10, 26, 27]. This may explain the decline of 25% or more in testing for HIV in six countries (Jamaica, Nigeria, Rwanda, Mozambique, Cameroon, Sierra Leone, and Botswana) among pregnant women [10]. In the 2022 UNICEF report, pregnant women from the Republic of Moldova missed and delayed HIV-related laboratories tests during the COVID-19 pandemic [28]. Reports of delays in VL monitoring among PWHIV when clinics and laboratories were functioning sub-optimally during COVID-19 have also been noted [29, 30]. Considering the existing gaps and effects of COVID-19, VL monitoring for timely management of HIV infection among PBWHIV was likely to be negatively affected, increasing the potential for vertical transmission. For these reasons, we conducted an exploratory study aimed at determining compliance (i.e., timely tests within guideline recommended number of weeks/months) to VL testing guidelines, and associated factors among PVT clients, before and during the COVID-19 pandemic in Ehlanzeni District, South Africa, within a context of changing PVT-guidelines.
Methods
Study Design and Setting
This was a retrospective cohort analysis of pregnant women and postpartum mothers, who were previously included in a facility-based cross-sectional study (the PHANGISA study). The PHANGISA study took place in the eight largest community healthcare centers of the Ehlanzeni District, located in rural Mpumalanga province of South Africa, from September to December 2019. Ehlanzeni’s HIV prevalence during pregnancy is the highest in the province and is one of the districts with the highest HIV prevalence (20%) in the general population nationally [31,32,33]. The PHANGISA study was designed to measure the prevalence of maternal VL non-suppression among pregnant or postpartum women with HIV and is described in detail in Ngandu et al. [34].
The 667 HIV-positive mothers who were enrolled in the PHANGISA study were retrospectively and virtually followed up using routine VL testing data from the NHLS’ Corporate Data Warehouse (NHLS-DW). Study participant names, surnames and dates of birth were used to link study participants with their respective HIV VL test results entered in the NHLS-DW from 1-April-2019 until 30-September-2021 (the study period). Once test results were linked on demographic data, the NHLS-DW unique identifier- created to assign multiple tests to a single person- was used to extract additional VL tests performed on the PHANGISA study participants within the study district and nationally.
Sampling and Sample Size Determination
Sample precision was calculated assuming that all participants (N = 667) enrolled during the PHANGISA study would be included in the analysis. Based on this, the study had 100% power to detect assumed VL testing compliance of 38 ± 5% (based on the Mpumalanga province's known compliance) OR 90.2% power to detect compliance of 38 ± 3% OR 58.7% power to detect compliance of 38 ± 2% with 5% level of significance [35].
Inclusion Criteria and Exclusion Criteria
The inclusion criteria for this current study were: all HIV-positive pregnant and postpartum mothers (i) aged 15 years and above enrolled in the primary study, (ii) with at least two VL test results in the NHLS-DW during the study period, and (iii) who reached 24 months postpartum only after the start of COVID-19 lockdown period (i.e., after 25-March-2020). Mothers with no NHLS-DW data or insufficient NHLS-DW data i.e., only one VL test throughout the study period, and those who reached 24 months postpartum before the COVID-19 period were excluded.
Variables and Outcomes
Primary Outcome
The primary outcome measure was compliance to repeat VL testing within each period (i.e., pre-COVID-19, transition, or COVID-19), and was defined as the proportion of participants with a timely test (i.e., within guideline recommended number of weeks/months) out of the sample expected to have a repeat test during that period. The samples for each period were determined as follows: pre-COVID-19 sample (Fig. 1-a step-2)- included all participants with the earliest VL test (pre-COVID-19 earliest) and expected repeat VL test falling before the start of lockdown (i.e., before 25-March-2020); Transition sample (Fig. 1-b step-2)- included all participants with the latest observed VL test (transition earliest) in the pre-COVID-19 period and the expected repeat VL test falling during the COVID-19 period; COVID-19 period sample (Fig. 1-c step-2)- included all participants with the earliest observed VL test (COVID-19 earliest) and the expected repeat VL test both observed during the COVID-19 period.
The numerators for timing of repeat tests are defined in Fig. 1-step-3 and Table 1. The difference between the calculated expected date and the actual observed VL test date was used to determine the timing of the VL test (on-time, delayed or missed), within each of the three COVID-19 periods using 2015 and 2019 guidelines [10, 11].
Two sets of calculations were done for the primary outcome: one accounted for the rollout of the revised 2019 guidelines from January-2020 onwards wherein the time to a repeat VL test when earliest VL = 50–999 copies/ml was shortened from 6-monthly to 8–10 weekly (referred to hereafter as the 2015/2019-PVT analysis); the other set assumed a potential delay in the rollout of the revised guidelines and thus used the 2015 recommendations throughout the study period (i.e., the 2015-PVT analysis).
Main Exposures
The first main exposure variable was the time periods: pre-COVID-19, transition, and COVID-19, defined by when VL tests were performed (Fig. 1). The second main exposure variable was the COVID-19 lockdown levels (1 through to 5) at the repeat VL test which changed differentially over the course of the COVID-19 period. This second exposure was only applied to the COVID-19 period.
Other Independent (Exposure) Variables
Sociodemographic factors collected during the PHANGISA study recruitment, perceived to influence healthcare uptake and HIV indicators were evaluated [34]. These included the participant’s PVT stage at recruitment (pregnant or postpartum), maternal age in years and further categorized (15–24, 25–34, 35–49 years) in secondary analysis to understand outcomes among the historically known HIV high-risk adolescent and young women age-group, level of education (ranging from no formal schooling to higher learning), marital status (never married or never lived with a partner, married or living together, widowed, divorced or separated), source of income (employed, spouse/partner, depend on others and government grant/no income), household monthly income categorized according to the national poverty line household cut-off (≤ R3200 or > R3200) [36], body mass index (BMI) (obese, overweight, normal, underweight) [37], partner's HIV status (positive, negative, unknown), condom use frequency (never, sometimes, always), planned pregnancy (yes or no), gestation at first ANC visit (0–12 weeks, 13–20 weeks, 21–40 weeks) and number of ANC visits (0–4 visits, 5–12 visits) were categorized according to the World Health Organization four-visit focused ANC model [38], timing of HIV diagnosis and ART initiation (before recent pregnancy, before 28 weeks gestation, after 28 weeks gestation, around labour/delivery, at postnatal before or after 6 months), current ART regimen (first-, second-, third- line, unknown), missed an ART dose last 7 days (yes or no), and facing any ART adherence challenges (yes or no).
Data Management and Analysis
The primary outcome variable and exposure variables were generated using definitions explained earlier. Data were analyzed in Stata/BE-basic edition 17.0 [StataCorp, College Station, Texas USA] and R version 4.2.0 [2022-04-22 ucrt]. The socio-demographic data and the longitudinal VL tests data were merged. Data were cleaned to exclude participants not meeting the inclusion criteria for this analysis. Proportions were used to describe population characteristics and to report compliance to repeat VL testing. The outcome variable was also presented as a binary of on-time versus delayed or missed for the rest of the analyses. The distribution of the outcome by various exposures was assessed using a Chi-square test, for the pre-COVID-19, transition, and COVID-19 periods, separately. A partially overlapping samples z-test which compares proportions for two dichotomous samples was used to compare compliance to repeat VL testing between pre-COVID-19 and transition periods, pre-COVID-19 and COVID-19 periods, and transition and COVID-19 periods. Data in each comparison comprised a combination of paired (within patient) and unpaired observations [39]. Univariable and multivariable Poisson regression (with robust error variance) analyses were conducted to identify exposure factors associated with compliance to repeat VL testing across the three periods (including all repeat VL tests) and during the COVID-19 period separately (sub-analysis). Exposure variables with an overall univariable Poisson p value < 0.200 were selected for each multivariable model. Factors- age, education level, and income were kept in both overall multivariable analysis and sub-analysis a priori. A significance level of 0.050 was used in the multivariable analysis to indicate a significant association with compliance to VL testing. All descriptive and inferential statistics involving the outcome variable were conducted twice, i.e., for the 2015/2019-PVT analysis and the 2015-PVT analysis.
Ethical Considerations
Ethics approval for the study was obtained from the South African Medical Research Council (SAMRC) ethics committee (EC021-6/2020). All PHANGISA participants gave consent for their routine data to be accessed and used for other related research in the future. Permission from the NHLS-DW gatekeeper was obtained to provide VL results matched to PHANGISA study participants.
Results
Sample Size Realization
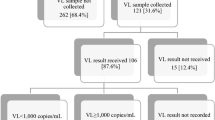
Out of the target sample of 667, data for 526 (78.9%) participants were successfully extracted from the NHLS-DW using personal identifiers. A further 23% (121/526) of participants were excluded from the study using the study exclusion criteria, resulting in a final sample of 405 participants (Fig. 2). There were insufficient VL data for 243/667 (36.4%) participants. With the achieved final sample size (n = 405), the study had 89% power to detect VL testing compliance of 86 ± 3% with 5% level of significance. The number of participants included in the pre-COVID-19, transition, and COVID-19 exposure samples were 282, 211, and 207, respectively in the 2015/2019-PVT analysis and 280, 208, and 207, respectively, in the 2015-PVT analysis.
Population Characteristics
Socio-demographics
Of the 405 included in the analysis, the overall median age of study participants was 30 years (interquartile range: 26–35) (Table 2). Over 90% of participants had at least grade 8 education level. About 60% of the participants were receiving less than R3200 of income monthly and approximately 35% did not know the HIV status of their male partner.
PVT-Related Factors
A majority (70%) of the participants were in the postpartum period at recruitment into the PHANGISA study (Table 3). Most (63.2%) attended their first ANC visit within 0–12 weeks of pregnancy, and around 75% were diagnosed with HIV before their current or recent pregnancy and had initiated ART.
Compliance to Repeat VL Testing During Pre-COVID-19, Transition, and COVID-19 Periods
Compliance to VL testing guidelines during pre-COVID-19, transition, and COVID-19 was determined, overall and by earliest VL status (Fig. 3, 2015/2019-PVT analysis). During the pre-COVID-19 period the proportion of women who were compliant to the VL test schedule, i.e., on time, was 82.6% (233/282). Compliance was 81.5% (172/211) during transition and 86.0% (178/207) during COVID-19. Compliance to repeat VL testing was compared between two periods at a time and there was no significant difference (all z-test p values > 0.05) between periods. In contrast, the 2015-PVT analysis (Fig. 4), a significant difference was observed between the pre-COVID-19 [82.9% (232/280)] and the COVID-19 [92.3% (191/207)] periods (difference in proportion: 9.4% [95% confidence interval (CI) 3.3–15.5], z-test p value < 0.001).
Compliance to Repeat VL Testing by Exposure Variables During Pre-COVID-19, Transition, and COVID-19 Periods
Table S1(2015/2019-PVT analysis) and S2(2015-PVT analysis) show the distribution of compliance to repeat VL testing by baseline socio-demographic (S1A/S2A) and PVT-related (S1B/S2B) factors during different periods. During pre-COVID-19 compliance to the recommended VL testing schedule was higher—among older age groups, ≥ 25 years (≥ 81.3%), compared to the younger group, 15–24 years (2015/2019-PVT analysis: 68.2%, chi squared p value = 0.012) or (2015-PVT analysis: 67.4%, chi squared p value = 0.007). In all three time periods (pre-COVID-19, transition, and COVID-19), compliance was higher when earliest VL < 50 versus VL ≥ 1000 copies/ml, irrespective of PVT guidelines used (91.4% versus 12.5%, chi squared p values < 0.001, 86.6% (83.7% 2015-PVT analysis) versus 50.0% (0.0% 2015-PVT analysis), chi squared p value < 0.001 (chi squared p value = 0.003 2015-PVT analysis), and 97.7% versus 14.3%, chi squared p values < 0.001, respectively). However, compliance varied when earliest VL = 50–999 copies/ml. In the 2015/2019-PVT analysis, compliance when earliest VL = 50–999 versus VL ≥ 1000 copies/ml was higher only during pre-COVID-19 (S1B, ~ 81.7% versus ~ 12.5%), yet in the 2015-PVT analysis it was higher in all three periods (S2B, ~ 82.8–100.0% versus 0–14.3%). Compliance to VL testing also appeared to be lower among underweight participants during COVID-19 (S1A) and those on first line ART regimen during transition period (S1B), but only in the 2015/2019-PVT analysis.
Factors Associated with Compliance to Repeat VL Testing
In both the 2015 and 2019-PVT analyses, compliance to VL testing was significantly reduced among those with earliest VL = 50–999 copies/ml or VL ≥ 1000 copies/ml versus VL < 50 copies/ml, irrespective of the time-period (VL = 50–999 Incidence Rate Ratio (IRR) = 0.70 [95% CI 0.61–0.81], Poisson p value < 0.001 and VL ≥ 1000 IRR = 0.18 [95% CI 0.09–0.36], Poisson p value < 0.001 across all three periods—Table 4; and VL = 50–999 IRR = 0.40 [95% CI 0.23–0.67], Poisson p value = 0.001 and VL ≥ 1000 IRR = 0.14 [95% CI 0.04–0.51], Poisson p value = 0.003 during the COVID-19 period—Table 5).
In the 2015-PVT analysis and irrespective of the time-period, the likelihood of being compliant was significantly reduced only among those with earliest VL ≥ 1000 copies/ml (versus VL < 50 copies/ml) (IRR = 0.14 [95% CI 0.06–0.32], Poisson p value < 0.001 across all periods—Table 6; and IRR = 0.15 [95% CI 0.04–0.52], Poisson p value = 0.003 during the COVID-19 period—Table 7). There was significant overall increased compliance during the COVID-19 versus pre-COVID-19 period IRR = 1.10 [95% CI 1.04–1.15], Poisson p value < 0.001, Table 6.
When age was categorized to investigate the differences between the historically known high-risk age group (15–24 years) and older age groups, all results remained the same except for age, across the three stages and irrespective of PVT guidelines. There was significant overall increased compliance among the 25–34 years age-group versus younger (15–24 years) women (IRR = 1.12 [95% CI 1.01–1.23], Poisson p value = 0.027) in the 2015/2019-PVT analysis-Table S3; and (IRR = 1.10 [95% CI 1.00–1.20], Poisson p value = 0.035) in the 2015-PVT analysis-Table S4).
Discussion
This study documented compliance to VL testing in PBWHIV before and during the COVID-19 pandemic in a rural district in South Africa, during April-2019 to September-2021. Given the study period overlapped with the rollout of the 2019-PVT guidelines in January-2020, three months before the COVID-19 hard lockdown in South Africa, we considered a scenario with and without the use of the revised PVT guidelines to delineate the effect of the pandemic in the context of changing guideline practices. Our results indicated that compliance to VL testing was largely affected by the frequency of recommended repeat tests irrespective of the COVID-19 pandemic status and that the COVID-19 pandemic lockdown delayed the implementation of the revised PVT-guidelines.
The increased likelihood of being compliant during the COVID-19 period compared to the pre-COVID-19 period, observed only in the 2015-PVT analyses where frequent visits for low-level viraemia were not applied, supports the delayed implementation of the revised guidelines. The higher proportion of delayed/missed VL testing visits among those on first-line ART regimen during the transition period when frequent visits were applied, also supports delayed implementation of the revised guidelines, given the majority of those with increasing VL would be expected to be on first-line regimen. This likely reflected the sub-group with low-level viraemia who might have been prescribed the 6-monthly repeat visit instead of the 8–10 weekly visit. The association between first-line ART regimen and high VL was also confirmed in the baseline cross-sectional point prevalence study, supporting a delay in treatment intervention for those with detectable viraemia [34]. These observations confirm that the COVID-19 pandemic interrupted the training of healthcare workers for the implementation of the 2019-PVT guidelines. Training should have been resumed with urgency after the pandemic restrictions were eased.
The second major finding of this study raises a conundrum over the implementation of the revised and previous guidelines alike. According to this study, compliance to more frequent VL testing visits, which are prescribed for those at highest risk of VHT, was sub-optimal even before the COVID-19 pandemic. In both PVT-guideline scenarios and across the entire study period, compliance to VL testing was significantly reduced among those with high-level viraemia VL ≥ 1000 copies/ml, who were routinely prescribed 4–6 weekly clinic visits. Compliance was significantly reduced when VL = 50–999 copies/ml (i.e., low-level viraemia), only when the revised guidelines were used, wherein low-level viraemia clients are prescribed 8–10 weekly clinic visits instead of the previous 6-monthly visits. Shortening time to repeat clinic visits for those with low-level viraemia was motivated by growing evidence of high risk for VHT [40]. The revised guidelines imply an increase in the population of those requiring frequent visits amid sub-optimal prior implementation. Implementation interventions need to be prioritized urgently.
To improve VL monitoring compliance, continuous evaluation and strengthening of the existing interventions, such as short message service (SMS) appointment reminders sent to PVT clients as part of Accelerating Programme Achievements to Control the Epidemic program is vital [41]. SMS reminders have previously been shown to improve retention and viral suppression among PVT clients, early infant diagnosis follow-up testing, and uptake of repeat HIV testing [42,43,44]. Recommendations for improving outcomes include (i) eLABS, that deliver VL results to healthcare workers digitally as soon as the results are ready and are piloting SMS reminders for patients [45] and (ii) Results for Action (RfA) reports, that collate HIV VL results weekly and identify high VL belonging to PBWHIV for clinical action, using codes introduced in the 2019-PVT guidelines [11, 12, 46, 47]. Furthermore, some previously proposed interventions that have shown potential to improve and encourage VL testing include educational efforts on the benefits of VL testing, using point-of-care VL testing for quick result turnaround times and enhanced access to VL testing in resource-limited settings, enhanced adherence counselling, compensating patients, and services aimed at young PBWHIV as they are less likely to receive VL testing [33, 48,49,50,51]. Periodic evaluation will also help in understanding the gaps that exist around implementation of guidelines and assist in addressing the impact of natural disasters such as COVID-19 pandemic on PWHIV.
The better compliance to VL testing observed among older compared to younger women, observed when age was categorized in a secondary analysis, is not unexpected, given known challenges in achieving viral suppression, uptake of care and VHT risk in younger women [34, 50,51,52,53]. The insignificance of age in the COVID-19 period sub-analyses, regardless of PVT-guidelines used, could be due to the universally enforced COVID-19 movement restrictions. Other participant sociodemographic factors such as education and income were not risk factors unlike in other related PVT indicators [50, 51, 54, 55].
This study had limitations and strengths. Although the message of delayed implementation of guidelines is undeniable, the observed levels of compliance to VL testing are best interpreted as an over-estimation. This study had a higher (~ 82%) compliance to repeat VL testing among PVT clients during pre-COVID-19 compared to what was reported for the general population during 2016 in South Africa (47.7–56.4%) [35], and what was seen among PVT clients in Kenya (only 6% of repeat VL testing within recommended schedule) [25] and Republic of Moldova during the COVID-19 pandemic among PWHIV (28.6%) [28], but comparable to observations of a similarly small PWHIV sub-population in southwestern Uganda (80.3%) [55]. A high proportion of women were excluded because of only one VL test observed (102/667) in the NHLS-DW, despite searching nation-wide routine data to account for women’s mobility. Deaths of study participants due to COVID-19 or loss-to-follow-up may have contributed to the exclusions. However, the realized study sample was highly powered for the analyses of repeated measures and sufficiently portrayed changes (or lack thereof) over time. In contrast, the sub-samples used for reporting point prevalence of compliance to VL testing at each time-period were insufficient for external validity. However, the distribution of age in the remaining sample is similar to that in the baseline sample as well as the national VL monitoring survey, indicating that the sample that could not be traced on the NHLS-DW (mostly due to inconsistent use of patient demographics and the absence of a unique health identifier) was random [22, 34]. This qualifies the appropriateness and reliability of the rest of the results attained from the repeated measures regression analyses.
The sociodemographic factors were collected at one time-point, thus the effect of time-changing factors was not measured.
Although there are a few limitations, the study managed to combine observations of routine VL testing among PBWHIV over a period of 30 months, including 12 months before the onset of the pandemic in South Africa, with an assessment of changes in PVT-guidelines within that period, and raised an alarm for implementation priorities. Given that there have been extremely limited assessments of compliance to VL testing for PBWHIV in the past, the implementation and evaluation of the 2019 recommendation to use PVT codes on the NHLS laboratory requisition forms to identify and track VL performed in PBWHIV at ANC, delivery and postpartum, needs to be prioritized.
Conclusions
Although the point prevalence of compliance to repeat VL testing were over-estimated, the study had high power to accurately monitor VL testing over a 30-month period and delineate the effect of the COVID-19 pandemic within a context of rolling out revised PVT-guidelines. Two major observations were made: Compliance to repeat VL testing was significantly reduced among those with viraemia and requiring frequent visits, both before and during the COVID-19 pandemic; and the rollout of the revised PVT-guidelines for shortening time to the next clinic visit for a low-level viraemia, was delayed due to the COVID-19 pandemic restrictions initiated barely three months into the rollout. To improve compliance to repeat VL testing, especially among those with detectable VL and young women, there is a need to strengthen interventions by (i) training healthcare workers on the new guidelines, (ii) educational messaging on VL testing benefits, (iii) SMS appointment reminders, and (iv) adolescent-PBWHIV friendly services, all which have shown a potential to improve uptake and timeous linkage for appropriate treatment and care.
References
World Health Organization. HIV treatment and care: what's new in treatment monitoring: viral load and CD4 testing: information note. Geneva: World Health Organization; 2017. Contract No.: WHO/HIV/2017.22.
World Health Organization. Technical update: considerations for developing a monitoring and evaluation framework for viral load testing: collecting and using data for scale-up and outcomes. Geneva: World Health Organization; 2019. Contract No.: WHO/CDS/HIV/19.5.
Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic 2014. https://www.unaids.org/en/resources/909090.
Joint United Nations Programme on HIV/AIDS. Global report. UNAIDS report on the global AIDS epidemic. 2014.
Joint United Nations Programme on HIV/AIDS. THE PATH THAT ENDS AIDS. 2023.
Joint United Nations Programme on HIV/AIDS. Start free, stay free, AIDS free: 2017 progress report. 2017.
Goga AE, Lombard C, Jackson D, Ramokolo V, Ngandu NK, Sherman G, et al. Impact of breastfeeding, maternal antiretroviral treatment and health service factors on 18-month vertical transmission of HIV and HIV-free survival: results from a nationally representative HIV-exposed infant cohort, South Africa. J Epidemiol Commun Health. 2020;74(12):1069–77.
World Health Organization. HIV diagnosis and ARV use in HIV-exposed infants: a programmatic update. World Health, Organization; 2018.
Myer L, Essajee S, Broyles LN, Watts DH, Lesosky M, El-Sadr WM, et al. Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med. 2017;14(8):e1002375.
Joint United Nations Programme on HIV/AIDS. Prevailing against pandemics by putting people at the centre—World AIDS Day report 2020.
National Department of Health South Africa. ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. National Department of Health Pretoria; 2019.
National Department of Health SA. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults: NDoH Pretoria; 2015. https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf.
Technau K-G, Kalk E, Coovadia A, Black V, Pickerill S, Mellins CA, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2014;65(5):e170.
Kendall C, Claessens L, Dorward J, Mfeka G, Gate K. Reasons for failure of prevention of mother-to-child HIV transmission in a rural South African district hospital. South Afr J HIV Med 2015. 2015;16(1).
Myer L, Dunning L, Lesosky M, Hsiao NY, Phillips T, Petro G, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2017;64(4):422–7.
Moyo F, Mazanderani AH, Sherman G, Kufa T. Population-level risk factors for vertical transmission of HIV in the national prevention of mother-to-child transmission programme in South Africa: an ecological analysis. S Afr Med J. 2022;112(3):219–26.
Chetty T, Vandormael A, Thorne C, Coutsoudis A. Incident HIV during pregnancy and early postpartum period: a population-based cohort study in a rural area in KwaZulu-Natal, South Africa. BMC Pregnancy Childbirth. 2017;17(1):1–10.
Mofenson LM. Risk of HIV acquisition during pregnancy and postpartum: a call for action. Oxford University Press US; 2018. p. 1–4.
Joint United Nations Programme on HIV/AIDS. Global data on HIV epidemiology and response: AIDSinfo | UNAIDS; 2020. https://aidsinfo.unaids.org/.
Goga AE, Dinh TH, Jackson DJ, Lombard CJ, Puren A, Sherman G, et al. Population–level effectiveness of PMTCT Option A on early mother–to–child (MTCT) transmission of HIV in South Africa: implications for eliminating MTCT. Journal of global health. 2016;6(2).
Woldesenbet S, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, et al. The 2017 National antenatal sentinel HIV survey. National Department of Health: South Africa. 2019.
Woldesenbet SA, Kufa-Chakezha T, Lombard C, Manda S, Cheyip M, Ayalew K, et al. Coverage of maternal viral load monitoring during pregnancy in South Africa: results from the 2019 national Antenatal HIV Sentinel Survey. HIV Med. 2021;22(9):805–15.
Sherman G, Mazanderani H, Barron P, Bhardwaj S, Ronelle N, Okobi M, et al. Toward elimination of mother–to–child transmission of HIV in South Africa: how best to monitor early infant infections within the Prevention of Mother–to–Child Transmission Program.
Penazzato M, Lule F, Essajee S. Paediatric HIV: the unfinished business. Lancet HIV. 2017;4(10):e425–7.
Sandbulte M, Brown M, Wexler C, Maloba M, Gautney B, Goggin K, et al. Maternal viral load monitoring: coverage and clinical action at 4 Kenyan hospitals. PLoS ONE. 2020;15(5):e0232358.
Vrazo AC, Golin R, Fernando NB, Killam WP, Sharifi S, Phelps BR, et al. Adapting HIV services for pregnant and breastfeeding women, infants, children, adolescents and families in resource-constrained settings during the COVID-19 pandemic. J Int AIDS Soc. 2020;23(9):e25622.
Naidoo K, Hoque M, Buckus S, Hoque M, Jagernath K. Prevention-of-mother-to-child-transmission (PMTCT) program outcomes in South Africa in the pre-COVID and COVID eras. BMC Public Health. 2023;23(1):1395.
United Nations Children's Fund. Assessing the impact of COVID-19 on people living with HIV, including pregnant women and children. 2022.
El-Nahal WG, Shen NM, Keruly JC, Jones JL, Fojo AT, Manabe YC, et al. Time Between Viral Loads for People with HIV during the COVID-19 Pandemic. JAIDS J Acquir Immune Defic Syndr. 9900:1. https://doi.org/10.1097/qai.0000000000003026
Madhi SA, Gray GE, Ismail N, Izu A, Mendelson M, Cassim N, et al. COVID-19 lockdowns in low- and middle-income countries: success against COVID-19 at the price of greater costs. S Afr Med J. 2020;110(8):724–6.
Woldesenbet SA, Kufa T, Barron P, Ayalew K, Cheyip M, Chirombo BC, et al. Assessment of readiness to transition from antenatal HIV surveillance surveys to PMTCT programme data-based HIV surveillance in South Africa: the 2017 Antenatal Sentinel HIV Survey. Int J Infect Dis. 2020;91:50–6.
Simbayi L, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: towards achieving the UNAIDS 90-90-90 targets. 2019.
Nyakura J, Shewade HD, Ade S, Mushavi A, Mukungunugwa SH, Chimwaza A, et al. Viral load testing among women on “option B+” in Mazowe, Zimbabwe: how well are we doing? PLoS ONE. 2019;14(12):e0225476.
Ngandu NK, Lombard CJ, Mbira TE, Puren A, Waitt C, Prendergast AJ, et al. HIV viral load non-suppression and associated factors among pregnant and postpartum women in rural northeastern South Africa: a cross-sectional survey. BMJ Open. 2022;12(3):e058347.
Fox MP, Brennan AT, Nattey C, MacLeod WB, Harlow A, Mlisana K, et al. Delays in repeat HIV viral load testing for those with elevated viral loads: a national perspective from South Africa. J Int AIDS Soc. 2020;23(7):e25542.
Department of Employment and Labour. National Minimum Wage Act No. 9 of 2018 investigation into the national minimum wage. 2021. Contract No.: No. 45649.
Weir CB, Jan A. BMI classification percentile and cut off points: StatPearls Publishing, Treasure Island (FL); 2022 2022.
World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience: World Health Organization; 2016.
Derrick B. How to compare the means of two samples that include paired observations and independent observations: a companion to Derrick, Russ, Toher and White (2017). Quant Methods Psychol. 2017;13(2):120–6.
Landes M, van Lettow M, Nkhoma E, Tippett Barr B, Truwah Z, Shouten E, et al. Low detectable postpartum viral load is associated with HIV transmission in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. 2019;22(6):e25290.
Right to Care. The APACE (Accelerating Programme Achievements to Control the Epidemic) Programme. https://www.righttocare.org/projects/the-apace/.
Kinuthia J, Ronen K, Unger JA, Jiang W, Matemo D, Perrier T, et al. SMS messaging to improve retention and viral suppression in prevention of mother-to-child HIV transmission (PMTCT) programs in Kenya: a 3-arm randomized clinical trial. PLoS Med. 2021;18(5):e1003650.
Mugo PM, Wahome EW, Gichuru EN, Mwashigadi GM, Thiong’o AN, Prins HA, et al. Effect of text message, phone call, and in-person appointment reminders on uptake of repeat HIV testing among outpatients screened for acute HIV infection in Kenya: a randomized controlled trial. PLoS ONE. 2016;11(4):e0153612.
Dube-Pule A, Zanoni BC, Connolly C, Shabangu M, Archary M. Evaluation of an SMS-based mHealth intervention to enhance early infant diagnosis follow-up testing and assessment of postnatal prophylaxis. South Afr J HIV Med. 2021;22(1):1–8.
Mezzanine. eLABS: Faster lab result turnaround time, better patient care. Accepted 19 Aug 2022. https://mezzanineware.com/digital-productivity-technology/healthcare-technology-solutions/laboratory-improvement-technology/.
Health NDo. The South African antiretroviral treatment guidelines. Pretoria. 2013.
National Department of Health. CLINICAL GUIDELINES: PMTCT (Prevention of Mother-to-Child Transmission). Pretoria2010.
Drain PK, Dorward J, Violette LR, Quame-Amaglo J, Thomas KK, Samsunder N, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV. 2020;7(4):e229–37.
Nkengasong JN, Yao K, Onyebujoh P. Laboratory medicine in low-income and middle-income countries: progress and challenges. The Lancet. 2018;391(10133):1873–5.
Nakalega R, Mukiza N, Kiwanuka G, Makanga-Kakumba R, Menge R, Kataike H, et al. Non-uptake of viral load testing among people receiving HIV treatment in Gomba district, rural Uganda. BMC Infect Dis. 2020;20(1):727.
Thinn KK, Thekkur P, Kyaw NTT, Aye NS, Zaw TM, Soan P, et al. Uptake of routine viral load testing among people living with HIV and its implementation challenges in Yangon region of Myanmar: a mixed-methods study. BMJ Open. 2019;9(12):e032678.
Larsen A, Magasana V, Dinh TH, Ngandu N, Lombard C, Cheyip M, et al. Longitudinal adherence to maternal antiretroviral therapy and infant Nevirapine prophylaxis from 6 weeks to 18 months postpartum amongst a cohort of mothers and infants in South Africa. BMC Infect Dis. 2019;19(Suppl 1):789.
Woldesenbet SA, Lombard C, Manda S, Kufa T, Ayalew K, Cheyip M, et al. The 2019 National Antenatal Sentinel HIV Survey, South Africa.https://www.nicd.ac.za/wp-content/uploads/2021/11/Antenatal-survey-2019-report_FINAL_27April21.pdf: South Africa Department of Health; 2021 Accessed 12 Apr 2022.
Swannet S, Decroo T, de Castro S, Rose C, Giuliani R, Molfino L, et al. Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health. 2017;9(4):206–14.
Lubega P, Nalugya SJ, Kimuli AN, Twinokusiima M, Khasalamwa M, Kyomugisa R, et al. Adherence to viral load testing guidelines, barriers, and associated factors among persons living with HIV on ART in Southwestern Uganda: a mixed-methods study. BMC Public Health. 2022;22(1):1268.
Funding
Open access funding provided by South African Medical Research Council. The work is based on the research funded by the South African National Department of Health. The author: TEM was supported in part by the National Research Foundation of South Africa and the South African Medical Research Council, NKN by the South African Medical Research Council. This study acknowledges the support of the NHLS for provision of data, the PHANGISA study team, Ehlanzeni district PVT managers, participants, and partners. Disclaimer: Opinions, findings, conclusions, or recommendations expressed are those of the authors, not of the funders or organizations.
Author information
Authors and Affiliations
Consortia
Contributions
Overall study concept—NKN; facilitation of data extraction—GGS, NKN; interpretation of extracted routine data—GGS; manuscript data cleaning and statistical analyses—TEM; supervision of manuscript aims, methods and statistical analyses—TK, NKN; first draft of manuscript—TEM; revision of subsequent drafts—TEM, TK, GGS, NKN. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interest
All authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbira, T.E., Kufa, T., Sherman, G.G. et al. Compliance to Viral Load Monitoring Schedules Among Women Attending Prevention of Vertical HIV Transmission Services Before and During the COVID-19 Pandemic in Ehlanzeni District, Mpumalanga, South Africa. AIDS Behav 28, 868–885 (2024). https://doi.org/10.1007/s10461-023-04192-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-023-04192-z