Abstract
Objective (s)
Circulating angiogenic factors are used for prediction of placenta-related complications, but their associations with first-trimester placental development is unknown. This study investigates associations between maternal angiogenic factors and utero-placental vascular volume (uPVV) and utero-placental vascular skeleton (uPVS) as novel imaging markers of volumetric and morphologic (branching) development of the first-trimester utero-placental vasculature.
Methods
In 185 ongoing pregnancies from the VIRTUAL Placenta study, a subcohort of the ongoing prospective Rotterdam Periconception cohort, three-dimensional power Doppler ultrasounds of the placenta were obtained at 7–9–11 weeks gestational age (GA). The uPVV was measured as a parameter of volumetric development and reported the vascular quantity in cm3. The uPVS was generated as a parameter of morphologic (branching) development and reported the number of end-, bifurcation- crossing- or vessel points and total vascular length. At 11 weeks GA, maternal serum biomarkers suggested to reflect placental (vascular) development were assessed: placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng). sFlt-1/PlGF and sEng/PlGF ratios were calculated. Multivariable linear regression with adjustments was used to estimate associations between serum biomarkers and uPVV and uPVS trajectories.
Results
Serum PlGF was positively associated with uPVV and uPVS development (uPVV: β = 0.39, 95% CI = 0.15;0.64; bifurcation points: β = 4.64, 95% CI = 0.04;9.25; crossing points: β = 4.01, 95% CI = 0.65;7.37; total vascular length: β = 13.33, 95% CI = 3.09;23.58, all p-values < 0.05). sEng/PlGF ratio was negatively associated with uPVV and uPVS development. We observed no associations between sFlt-1, sEng or sFlt-1/PlGF ratio and uPVV and uPVS development.
Conclusion(s)
Higher first-trimester maternal serum PlGF concentration is associated with increased first-trimester utero-placental vascular development as reflected by uPVV and uPVS.
Clinical trial registration number Dutch Trial Register NTR6854.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The placenta plays a critical role in health and disease, not only during pregnancy, but also later in life [1]. Healthy placental development and function are highly dependent on adequate establishment of the utero-placental vasculature in early pregnancy [2]. Aberrant vascular development is associated with placenta-related complications, including fetal growth restriction (FGR), preeclampsia (PE) and preterm birth (PTB) [3, 4]. To secure adequate development of the utero-placental vasculature, the maternal uterine vasculature undergoes a variety of adaptations, including formation, dilatation and funneling of spiral arteries [5]. Consequently, markers that monitor development of the utero-placental vasculature in early pregnancy could provide unique insights in the pathophysiology of placental development and placenta-related complications.
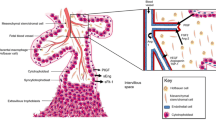
The placenta produces pro- and anti-angiogenic factors that impact maternal vascular adaptation to pregnancy, including placental growth factor (PlGF), soluble fms-like tyrosine kinase (sFlt-1), and soluble endoglin (sEng) [6]. PlGF stimulates vasodilatation and angiogenesis in the utero-placental and feto-placental circulations by binding to the vascular endothelial growth factor receptor-1 (VEGFR-1) [6]. sFlt-1, a soluble variant of VEGFR-1, binds to circulating PlGF, thereby suppressing PlGF bioavailability and consequently inhibiting its proangiogenic functions [7]. Similar to sFlt-1, sEng binds and neutralizes free transforming growth factor beta (TGFβ), another molecule involved in vasorelaxation and angiogenesis (Fig. 1) [8, 9]. Circulating levels of these angiogenic factors are directly linked to vascular development and an imbalance in the second and third trimester plays an essential role in the pathogenesis of preeclampsia [7,8,9,10,11]. Yet, how circulating angiogenic factors relate to utero-placental vascular development in the first trimester remains to be fully established.
Pro-and anti-angiogenic factors that impact maternal vascular adaptation to pregnancy. PlGF stimulates angiogenesis in the utero-placental and feto-placental circulations by binding to the VEGF R1. TGFβ stimulates vasodilatation and angiogenesis via binding to the TGFβ R. sFlt-1, a soluble variant of VEGFR-1, binds to circulating PlGF thereby suppressing PlGF bioavailability and consequently inhibiting its proangiogenic functions. Similar to sFlt-1, sEng binds and neutralizes TGFβ. PlGF Placenta Growth Factor, TGFβ transforming growth factor beta, sFlt-1 soluble fms-like tyrosine kinase-1, sEng soluble endoglin, VEGF R1 vascular endothelial growth factor receptor-1, TGFβ R transforming growth factor beta receptor. This image was created in BioRender.com
We recently proposed the utero-placental vascular volume (uPVV) and utero-placental vascular skeleton (uPVS) as non-invasive in-vivo imaging markers of first-trimester utero-placental vascular volumetric and morphologic development, using 3-dimensional (3D) power Doppler ultrasound, virtual reality (VR) and skeletonization [12, 13]. Increased absolute volumetric and morphologic development of the utero-placental vasculature might be favorable for pregnancy outcomes, as previous studies demonstrated that the uPVV and uPVS associate positively with embryonic and fetal growth and birth weight percentiles [14, 15]. Moreover, increased uPVS development is associated with lower incidence of placenta-related complications, including FGR, PE, and PTB [12]. Further, increased density of vascular branching is considered unfavorable as it is associated with decreased embryonic and fetal growth and an increased incidence of placenta-related complications [12, 14]. Therefore, we postulate that the uPVV and uPVS prove valuable to investigate associations between circulating angiogenic factors and utero-placental vascular development in the first trimester of pregnancy.
The aim of this study was to investigate whether increased levels of PlGF, decreased levels sFlt-1 and sEng, or decreased sFlt-1/PlGF and sEng/PlGF ratios are associated with increased development of the first-trimester utero-placental vasculature, measured with the uPVV and uPVS. Secondly, we investigated whether these parameters associate with placental volume (PV) as a measure of trophoblast tissue, and whether the associations are influenced by conception method, fetal sex and the occurrence of placenta-related complications.
Methods
Study design
We used data form the VIRTUAL Placenta cohort, embedded in the ongoing prospective Rotterdam Periconception Cohort (Predict Study) [16, 17]. Women were enrolled from a tertiary referral hospital between January 2017 and March 2018, if they were at least 18 years old, carried a singleton pregnancy of less than 10 weeks gestational age (GA), and gave written informed consent. Naturally conceived pregnancies as well as pregnancies achieved via in vitro fertilization (IVF) with or without intracytoplasmic sperm injection (ICSI) were eligible for inclusion. Women were excluded from analysis in case of oocyte donation and/or miscarriage. Upon inclusion, participants were required to complete a questionnaire detailing their general characteristics, medical and obstetrical history and lifestyle behaviors. Geographical origin was defined by country of birth according to the classification of Statistics Netherlands (CBS) as ‘Netherlands, ‘Europe’ and ‘Non-Europe’ [18].
Each participant visited at least twice in the first trimester at 7, 9 and 11 weeks GA, during which transvaginal 3D PD ultrasound scans of the whole gestational sac including the placenta and utero-placental vasculature were acquired using a GE Voluson E8 (GE, Zipf, Austria). The standardized ultrasound settings used were previously described (quality: max; pulse repetition frequency (PRF): 0.6; wall motion filter (WMF): low1; compound resolution imaging (CRI): off; power Doppler (PD) gain: − 8.0) [13]. Ultrasound examinations were performed according to international guidelines on safe use of Doppler ultrasound in the first trimester of pregnancy (ALARA-principle) [19].
Body mass index (BMI) was determined during the first study visit according to protocol for the measurements of height and weight. Pregnancy outcome data was retrieved from from medical delivery records.
At 11 weeks GA, maternal venous blood samples were drawn according to the study protocol [16, 17].
Pregnancy dating
For naturally conceived pregnancies in regular cycles with a duration between 25 and 35 days, GA was calculated from the first day of last menstrual period (LMP). In case of unknown LMP or irregular cycle, GA was calculated from the Crown-Rump-Length (CRL). If the two methods varied > 6 days, the CRL-based GA was assumed the true GA. For fresh IVF/ICSI conceived pregnancies, GA was calculated from oocyte pick-up day + 14 days. In case of cryopreserved embryo transfer, GA was calculated from transfer date + 19 days.
Definitions of placenta-related complications
Placenta-related complications were specified as pregnancy induced hypertension (PIH), PE and/or FGR, small-for-gestational-age (SGA) or PTB. PIH was defined as new-onset hypertension after 20 weeks GA with systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg [20]. PE was defined as PIH with presence of ≥ 300 mg proteinuria in a 24-h period [20]. FGR was defined as a fetal abdominal circumference and/or EFW < 10th percentile on the Hadlock curve, or > 20 percentile decrease compared to a previous measurement with a minimal timespan of two weeks [21]. SGA was defined as birth weight < 10th percentile on growth curves specific for GA at birth, parity and fetal sex [22]. PTB was defined as a GA at birth < 37 + 0 weeks [23].
Imaging markers of the first-trimester utero-placental vascular development
Image quality was assessed on a four-point scale ranging between zero (optimal) and three (unusable) based on the presence of artefacts, the ability to discriminate between myometrium and trophoblastic tissue, and completeness of the placenta. Images with a quality score of three were excluded from the analyses. The placental volume (PV in cm3) was measured using VOCAL software according to the previously published study protocol [24]. The utero-placental vascular volume (uPVV in cm3) was measured using a virtual reality (VR) desktop system with the V-Scope volume rendering application according to the previously published study protocol [13]. Figure 2A depicts the uPVV.
Overview of utero-placental (vascular) imaging markers; the placental volume (PV), utero-placental vascular volume (uPVV) and utero-placental vascular skeleton (uPVS) of a first-trimester pregnancy. A: Utero-placental vascular volume (uPVV) and slice view of a three-dimensional (3D) power Doppler (PD) ultrasound of a first trimester pregnancy (bottom right). The yellow-highlighted section in the bottom right image indicates placental tissue, which is confined by the placental-myometrial interface on the outside and the gestational sac on the inside. These anatomical boundaries are used to calculate the placental volume (PV). Using virtual reality-based segmentation, all PD signals outside the PV-segment were erased. The uPVV is calculated by summation of the thresholded 3D PD voxels (3D pixels) within the PV-segment. B: Utero-placental vascular skeleton (uPVS) and slice view of a three-dimensional (3D) power Doppler (PD) ultrasound of a first trimester pregnancy (bottom right). A skeletonization algorithm has been applied to the uPVV (from panel A), resulting in the uPVS. The skeletonization algorithm repeatedly peels off the outermost layer of voxels from the uPVV, reducing the diameter of the PD signal at each point in the vascular network until one central voxel remains, thereby creating a network-like structure representing the vascular morphology. C: A magnified portion of the uPVS and overview of the uPVS including the magnified portion (yellow-highlighted section) at the bottom right. Each voxel of the uPVS is automatically assigned a morphologic characteristic based on the number of adjacent voxels: red = endpoint (1 adjacent voxel); white = vessel point (2 adjacent voxels); green = bifurcation point (3 adjacent voxels); blue = crossing point (4 adjacent voxels). Two other characteristics are derived from the uPVS: 1. Total network length was calculated by summation of the total number of voxels in the skeleton, multiplied by 1 voxel length (mm). 2. Average vessel thickness was calculated by the average number of voxel-layers that were peeled off from the uPVV to reach the central voxel of the uPVS, multiplied by 1 voxel length (mm)
The utero-placental vascular skeleton (uPVS) was generated by applying a skeletonization algorithm to the uPVV segmentation [12]. According to the previously published study protocol, the skeletonization algorithm repeatedly peels off the outermost layer of voxels from the uPVV, reducing the diameter of the PD signal at each point in the vascular network until one central voxel remains, thereby creating a network-like structure representing the vascular morphology. The uPVS is depicted in Fig. 2B. Following the construction of the network, the skeletonization algorithm generates 6 uPVS characteristics to represent absolute morphologic development of the utero-placental vasculature: endpoints (n), bifurcation points (n), crossing points (n), normal vessel points (n), total vascular length (mm) and average vascular thickness (mm), which is illustrated in Fig. 2C. Finally, we calculated ratios of the uPVS end-, bifurcation- and crossing points to the uPVV as to identify 3 imaging markers representing density of vascular branching in the utero-placental vascular volume. Women who had no PV, uPVV or uPVS measurement available, were excluded from analysis.
Given the small contribution of embryonic vascular structures to the VR-based segmentation and the restricted presence of embryonic flow in the first trimester, we assume the contribution of the embryonic blood space to the uPVV and the uPVS is limited. Therefore, we conclude that the uPVV and uPVS mainly include the distal segments of the maternal spiral and basal arteries and communicating anastomoses (arteriovenous shunts) [12, 13].
Assessment of PlGF, sFlt-1 and sEng in maternal serum
Directly after collection, all maternal serum samples were centrifuged and stored at − 80 °C until analysis. PlGF (pg/mL) and sFlt-1 (pg/mL) were measured using commercially available assays on the Elecsys platform (Roche Diagnostics), as previously described [25]. An enzyme-linked immunosorbent assay (ELISA) was performed for the determination of sEng (ng/mL) using the Quantikine® Elisa Human Endoglin/CD 105 commercial kit (R & D Systems, Abingdon, UK). For each sample, the sFlt-1/PlGF ratio and sEng/PlGF ratio were calculated [7, 26].
Statistical analysis
Baseline characteristics are presented as mean and standard deviation or median and interquartile range (IQR). If needed, non-volumetric parameters were transformed using a square root transformation to approximate a normal distribution. For volumetric parameters and ratios a cubic root and natural log transformation were used respectively.
We performed linear mixed models to estimate the associations between maternal serum biomarkers and their ratios at 11 weeks GA (PlGF, sFlt-1, sEng, sFlt-1/PlGF ratio and sEng/PlGF ratio) and longitudinal measurements of the imaging markers of utero-placental vascular development (PV, uPVV and uPVS), using a quadratic relation with GA. We constructed model 1 (adjusted for GA) and model 2 (additionally adjusted for maternal age, BMI, parity, conception mode, fetal sex and periconceptional alcohol consumption, smoking and folic acid supplement use). These possible confounders were selected using a covariate-correlation matrix and supplemented with covariates based literature after discussion amongst authors.
All analyses were performed using SPSS (version 25.0; SPSS Inc., Chicago, IL, USA) and R (version 4.0.2, R Core Team, Vienna, Austria, 2020), p-values < 0.05 were considered statistically significant.
Results
Study population
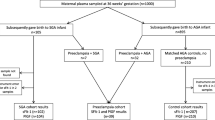
A total of 241 women were enrolled in the VIRTUAL Placenta study. A flow chart of the study population is depicted in Fig. 3. We excluded 56 women from the analyses because of withdrawal (n = 1), miscarriage (n = 22), oocyte donation (n = 4), or missing maternal blood samples (n = 29). Finally, 185 women were included in this study. For each participant at least one PV or uPVV measurement was available. Table 1 shows the baseline characteristics of the study population. Participants were on average 32.3 years of age, 57.8% was nulliparous and 57.8% of pregnancies was achieved via IVF-ICSI. Table 2 summarizes serum concentrations of PlGF, sFlt-1 and sEng and the sFlt-1/PlGF and sEng/PlGF ratios and reference values obtained from the literature. Maternal serum concentrations of PlGF and sFlt-1 were significantly higher in pregnancies without placenta-related complications than in pregnancies with placenta-related complications (PlGF: Median 40.6 pg/mL (IQR 19.4) vs Median 36.3 pg/mL (IQR 17.5), p-value = 0.028; sFlt-1: Median 1337.0 pg/mL (IQR 485) vs Median 1082.5 pg/mL (IQR 400), p-value < 0.001). No statistically significant differences were observed for sEng, and the sFlt-1/PlGF or sEng/PlGF ratios between the two groups.
PlGF, sFlt-1, sEng
Table 3 shows positive associations between maternal serum PlGF at 11 weeks GA and trajectories of PV (β = 0.53, 95% CI = 0.34; 0.73), uPVV, (β = 0.39, 95% CI = 0.15; 0.64) and uPVS (bifurcation points: β = 4.64, 95% CI = 0.04; 9.25; crossing points: β = 4.01, 95% CI = 0.65; 7.37; total vascular length: β = 13.33, 95% CI = 3.09; 23.58; average thickness: β = 0.05, 95% CI = 0.01; 0.08) and negative associations with density of vascular branching (end points: β = − 1.73, 95%CI = − 3.19; − 0.28; bifurcation points: β = − 0.76, 95%CI = − 1.38; − 0.14), all p-values < 0.05. In summary, higher maternal serum PlGF at 11 weeks is associated with both increased placental volume and absolute volumetric and morphologic (branching) development of the utero-placental vasculature and decreased density of vascular branching in the first trimester. We observed no associations between sFlt-1 and sEng and first-trimester development of PV, uPVV, uPVS characteristics or density of vascular branching, see Table 3.
sFlt-1/PlGF ratio, sEng/PlGF ratio
Table 4 shows negative associations between the sFlt-1/PlGF ratio and the trajectory of PV (β = − 0.35, 95% CI = − 0.53; − 0.16, p-value < 0.001), but not with any of the 3D PD imaging markers of utero-placental vascular development. We observed negative associations between the sEng/PlGF ratio and trajectories of PV (β = − 0.26, 95% CI = − 0.44; − 0.08), uPVV, (β = − 0.30, 95% CI = − 0.52; − 0.09) and uPVS (bifurcation points: β = − 4.08, 95% CI = − 7.87; − 0.30; crossing points: β = − 2.80, 95% CI = − 5.59; − 0.01; vessel points: β = − 9.20, 95% CI = − 17.89; − 0.53; total vascular length: β = − 10.03, 95% CI = − 18.50; − 1.56; average thickness: β = − 0.03, 95% CI = − 0.06; − 0.01) and positive associations with density of vascular branching (end points: β = 1.38, 95% CI = 0.18; 2.58; bifurcation points: β = 0.54, 95% CI = 0.03; 1.06), all p-values < 0.05, see Table 4. To summarize, higher maternal serum sFlt-1/PlGF ratio at 11 weeks is associated with decreased first-trimester placental volume but not with vascular development. Higher maternal serum sEng/PlGF ratio is associated with both decreased placental volume and absolute volumetric and morphologic (branching) development of the utero-placental vasculature and increased density of vascular branching in the first trimester.
Stratification for conception method, fetal sex and the occurrence of placenta-related complications
Figure 4 depicts correlation plots of the maternal serum biomarkers and their ratios at 11 weeks GA and imaging markers of the first-trimester utero-placental vascular development stratified for conception method, fetal sex and the occurrence of placenta-related complications. There are no statistically significant differences between the subgroups.
Correlation plot of maternal serum biomarkers at 11 weeks GA and imaging markers of the first-trimester utero-placental vascular development stratified for conception method, fetal sex and the occurrence of placenta-related complications. Unadjusted correlation plot. a. Stratified for conception method. b. Stratified for fetal sex. c. Stratified for the occurrence of placenta-related complications. Placenta-related complications are specified as PIH or PE and/or FGR, PTB and SGA. There are no statistically significant differences between the stratified groups. uPVV utero-placental vascular volume, uPVS utero-placental vascular skeleton, PlGF placenta growth factor, sFlt-1 soluble fms-like tyrosine kinase-1, sEng soluble endoglin, PIH pregnancy induced hypertension, PE preeclampsia, FGR fetal growth restriction, SGA small-for-gestational-age, PTB preterm birth
Discussion
Adequate utero-placental vascular development in the first trimester of pregnancy is essential to ensure optimal placental development and function, and consequently to achieve a healthy pregnancy outcome. In this study, increased maternal serum PlGF at 11 weeks is associated with increased first-trimester PV development, reflecting trophoblast tissue development and increased uPVV and uPVS development, reflecting volumetric and morphologic (branching) development of the utero-placental vasculature, respectively. Moreover, increased maternal serum sEng/PlGF ratio is associated with decreased first-trimester PV and decreased uPVV and uPVS development. These associations are not affected by conception method, fetal sex or the occurrence of placenta-related complications.
The positive association between maternal serum PlGF at 11 weeks and the novel uPVV and uPVS imaging markers substantiates that uPVV and uPVS measurements reflect development of the utero-placental vasculature in the first trimester of pregnancy.
Interestingly, our study revealed that the participants with an uneventful pregnancy had higher serum concentrations of sFlt-1 than participants that later experienced a placenta-related complication.
Interpretation in light of other evidence
PlGF, a member of the vascular endothelial growth factor (VEGF)-family, stimulates angiogenesis and vasodilatation not only in the placenta, but also in other organs outside of gestation [32]. Animal and in vitro studies show PlGF is involved with branching angiogenesis (blood vessel formation) as well as non-branching angiogenesis (blood vessel elongation and enlargement) and plays an important role in the ability to employ compensatory mechanisms in pathologic conditions, such as ischemic tissue damage and tumor vascularization [32,33,34,35,36]. PlGF knockout mice exhibit aberrant utero-placental vascular development at gestational day 8.5, which corresponds to the late first-trimester in human pregnancy, including reduced branching [34]. Our study is the first to show the associations between circulating PlGF levels on first-trimester utero-placental vascular volumetric and morphologic development in vivo in humans.
Notably, we observed the strongest associations between PlGF and PV development. The PV measurements include but are not limited to the utero-placental vasculature. In fact, the PV measurements represent the whole placenta, which consists mainly of trophoblast cells. It is well known that trophoblast cells produce PlGF, which might explain why the association between PlGF and PV is stronger than associations with the uPVV or uPVS [37].
We did not observe any associations between sFlt-1, the soluble antiangiogenic receptor of PlGF, and first-trimester placental volume, utero-placental vascular volume or morphologic development. Although a few animal studies show increased early and mid-gestational levels of sFlt-1 are associated with impaired spiral artery remodeling [38, 39], these associations have not been studied in humans. One study found positive associations between sFlt-1 levels and the uterine artery pulsatility index, as an indirect measure of utero-placental vascular development, in the third trimester [40], but these associations were not observed in the first and second trimester [41, 42].
Studies comparing first-trimester sFlt-1 levels between healthy pregnancies and pregnancies with preeclampsia or fetal growth restriction report ambiguous results [43,44,45,46]. Importantly, clinically relevant rises of sFlt-1 are observed only from 5 weeks before the onset of preeclampsia [47]. As a consequence, elevated sFlt-1 levels are almost exclusively observed in the second and third trimester. Our study’s findings seem to confirm that circulating sFlt-1 does not have a substantial influence on utero-placental vascular development in the first trimester.
Remarkably, in our study we observed lower sFlt-1 serum concentrations in pregnancies that later developed a placenta-related complication compared to the group with an uneventful pregnancy, which is in line with previous findings by Schiffer et al. [31]. However, other studies found first-trimester sFlt-1 to be higher in pregnancies that later developed preeclampsia [29, 48]. These seemingly paradoxical results pose interesting conjecture for future research.
We observed no associations between sEng, a small receptor with anti-angiogenic properties by limiting the bioavailability of proangiogenic TGFβ, and first-trimester placental volume, utero-placental vascular volume or morphologic development. Although several studies have investigated the predictive value of sEng in the first trimester for the risk of preeclampsia or fetal growth restriction, no studies have researched its associations with utero-placental vascular development. We found one study that found a negative correlation between sEng levels and the uterine artery pulsatility index in the second trimester, but not in the first trimester, which is in accordance with our results [42].
In our study, there were no differences in sEng levels between women with and without placenta-related complications. Some studies show higher first-trimester levels of sEng in women ultimately developing preeclampsia [29, 49]. Notably, in our study, placenta-related complications comprised a heterogeneous group of clinical conditions defined as the presence of PIH, PE and/or FGR, SGA or PTB, which could explain why we observed no associations. Further, other studies show elevated sEng levels in pregnancies which later developed preeclampsia from 17 weeks onwards [50], which may also explain the lack of associations in our study.
We found no associations between the sFlt-1/PlGF ratio and utero-placental vascular development. Our results imply that, at least in the first trimester, free PlGF levels are relevant for physiological angiogenesis (utero-placental vascular development) irrespective of sFlt-1 levels. These findings are in line with previous research, which suggests free PlGF concentrations are diminished in patients with preeclampsia independent from sFlt-1 concentrations [51]. Our findings are further substantiated by a recent study that shows the imbalance of sFlt-1/PlGF ratio in preeclampsia is mainly caused by diminished PlGF production as opposed to increased sFlt-1 levels [52]. These findings give rise to the possibly that, in the first trimester, an increase of sFlt-1 in is accompanied by a proportional rise in PlGF, maintaining the concentration of free PlGF constant. In later pregnancy, during the late second and third trimester, excessive rises in sFlt-1 are no longer matched by PlGF, which leads to a sFlt-1/PlGF ratio imbalance and gives rise to placenta-related complications.
Although previous studies have found associations between the sEng/PlGF ratio and pregnancy outcomes [26], no studies have investigated its associations with angiogenesis. The sEng/PlGF ratio can be interpreted as a composite ratio reflecting both the VEGF- and TGFβ-pathways involved with angiogenesis. In our study, associations with PlGF are stronger than associations with the sEng/PlGF ratio. In addition, we found no associations with sEng. Therefore, the associations between the sEng/PlGF ratio and imaging markers of utero-placental vascular development are likely contributable to the effect of PlGF and we consider the possibility the TGFβ-pathways is involved with first-trimester utero-placental vascular development unconvincing.
Strengths and limitations
One of the major strengths in our study is the use of validated test kits for the analyses of PlGF, sFlt-1 and sEng. Further, we made use of longitudinal first-trimester 3D power Doppler ultrasounds to perform validated 3D virtual reality based segmentations for the measurements of uPVV and uPVS [12, 13].
Unfortunately, all serum biomarker concentrations were determined only once during these pregnancies. Some studies show longitudinal changes in PlGF, sFlt-1 and sEng are potentially more clinically relevant than absolute levels [30, 53]. Longitudinal analysis may provide additional insights in the interplay between serum biomarker concentrations and first-trimester imaging markers of utero-placental vascular development.
The number of imaging markers used in this study might introduce some questions regarding the issue of multiple testing. However, our research has repeatedly demonstrated positive strong correlations between the uPVV and uPVS and an inverse association with density of vascular branching. Therefore, we argue the consistency of the presence or absence of these relationships can be viewed as internal validation of our findings.
Implications and conclusion
This study’s findings suggest higher free PlGF concentrations likely contribute to increased first-trimester utero-placental vascular development as reflected by uPVV and uPVS, thereby providing new insights into potential mechanisms underlying placenta-related complications. Moreover, these results substantiate a role for PlGF in early prediction models for placenta-related complications. In contrast, sFlt-1 and sEng appear not to have a substantial influence on first-trimester utero-placental vascular development and therefore the added value of first-trimester sFlt-1 and sEng for early detection of placenta-related complications will be limited.
Importantly, the uPVV and uPVS can be used as 3D power Doppler imaging markers to monitor the volumetric and morphologic development of the utero-placental vasculature throughout the first trimester of pregnancy. Future application of the uPVV and uPVS measurements could benefit research on early vascular development to improve our understanding of the pathophysiology of placenta-related complications and the mechanisms underlying both therapeutic and preventative regimens, such as prophylactic administration of aspirin.
Finally, as maternal serum PlGF is currently used in multiple prediction models for preeclampsia, we recommend investigating the added value of the uPVV and uPVS as non-invasive and uncostly predictors for preeclampsia.
Data availability
Datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Burton GJ, Fowden AL, Thornburg KL (2016) Placental origins of chronic disease. Physiol Rev 96(4):1509–1565. https://doi.org/10.1152/physrev.00029.2015
Burton GJ, Fowden AL (2015) The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci 370(1663):20140066. https://doi.org/10.1098/rstb.2014.0066
Junaid TO et al (2014) Fetoplacental vascular alterations associated with fetal growth restriction. Placenta 35(10):808–815. https://doi.org/10.1016/j.placenta.2014.07.013
Sharma D, Shastri S, Sharma P (2016) Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr 10:67–83. https://doi.org/10.4137/CMPed.S40070
Sato Y (2020) Endovascular trophoblast and spiral artery remodeling. Mol Cell Endocrinol 503:110699. https://doi.org/10.1016/j.mce.2019.110699
Umapathy A, Chamley LW, James JL (2020) Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 23(2):105–117. https://doi.org/10.1007/s10456-019-09694-w
Herraiz I et al (2015) Angiogenesis-related biomarkers (sFlt-1/PLGF) in the prediction and diagnosis of placental dysfunction: an approach for clinical integration. Int J Mol Sci 16(8):19009–19026. https://doi.org/10.3390/ijms160819009
Venkatesha S et al (2006) Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12(6):642–649. https://doi.org/10.1038/nm1429
Margioula-Siarkou G et al (2022) The role of endoglin and its soluble form in pathogenesis of preeclampsia. Mol Cell Biochem 477(2):479–491. https://doi.org/10.1007/s11010-021-04294-z
Maynard SE et al (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111(5):649–658. https://doi.org/10.1172/JCI17189
Rana S, Burke SD, Karumanchi SA (2022) Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol 226(2):S1019–S1034. https://doi.org/10.1016/j.ajog.2020.10.022
de Vos ES et al (2022) Assessment of first-trimester utero-placental vascular morphology by 3D power doppler ultrasound image analysis using a skeletonization algorithm: the rotterdam periconception cohort. Hum Reprod 37:2532–2545
Reijnders IF et al (2018) New imaging markers for preconceptional and first-trimester utero-placental vascularization. Placenta 61:96–102. https://doi.org/10.1016/j.placenta.2017.11.013
De Vos ES et al (2024) Morphologic development of the first-trimester utero-placental vasculature is positively associated with embryonic and fetal growth: the rotterdam periconception cohort. Hum Reprod. https://doi.org/10.1093/humrep/deae056
Reijnders IF et al (2021) First-trimester utero-placental (vascular) development and embryonic and fetal growth: the rotterdam periconception cohort. Placenta 108:81–90. https://doi.org/10.1016/j.placenta.2021.03.017
Steegers-Theunissen RP et al (2016) Cohort profile: the rotterdam periconceptional cohort (predict study). Int J Epidemiol 45(2):374–381. https://doi.org/10.1093/ije/dyv147
Rousian M et al (2021) Cohort Profile Update: the Rotterdam Periconceptional Cohort and embryonic and fetal measurements using 3D ultrasound and virtual reality techniques. Int J Epidemiol. https://doi.org/10.1093/ije/dyab030
CBS, Statistics Netherlands (2022) New classification of population by origin: replacing classification based on migration background and the concepts western/non-western
Drukker L et al (2020) Safety indices of ultrasound: adherence to recommendations and awareness during routine obstetric ultrasound scanning sicherheitsindizes im ultraschall: einhaltung der empfehlungen und aufmerksamkeit beim routine-ultraschall in der geburtshilfe. Ultraschall Med 41(2):138–145. https://doi.org/10.1055/a-1074-0722
Croke L (2019) Gestational hypertension and preeclampsia: a practice bulletin from ACOG. Am Fam Physician 100(10):649–650
NVOG (2017) NVOG-richtlijn Foetale groeirestrictie (FGR)
Zeve D et al (2016) Small at birth, but how small? the definition of sga revisited. Horm Res Paediatr 86(5):357–360. https://doi.org/10.1159/000449275
Vogel JP et al (2018) The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol 52:3–12. https://doi.org/10.1016/j.bpobgyn.2018.04.003
Reus AD et al (2013) Early first-trimester trophoblast volume in pregnancies that result in live birth or miscarriage. Ultrasound Obstet Gynecol 42(5):577–584. https://doi.org/10.1002/uog.13197
Schiettecatte J et al (2010) Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 43(9):768–770. https://doi.org/10.1016/j.clinbiochem.2010.02.010
Darmochwal-Kolarz D, Chara A (2023) The association of IL-17 and PlGF/sENG ratio in pre-eclampsia and adverse pregnancy outcomes. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph20010768
McLaughlin K et al (2022) Circulating maternal placental growth factor responses to low-molecular-weight heparin in pregnant patients at risk of placental dysfunction. Am J Obstet Gynecol 226:S1145–S1156
Verlohren S et al (2010) An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2009.09.016
Baumann MU et al (2008) First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2008.06.069
Erez O et al (2008) The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Maternal-Fetal Neonatal Med 21(5):279–287. https://doi.org/10.1080/14767050802034545
Schiffer V et al (2021) The association between first trimester placental biomarkers and placental lesions of maternal vascular malperfusion. Placenta 103:206–213
De Falco S (2012) The discovery of placenta growth factor and its biological activity. Exp Mol Med 44(1):1–9. https://doi.org/10.3858/emm.2012.44.1.025
Chau K, Hennessy A, Makris A (2017) Placental growth factor and pre-eclampsia. J Hum Hypertens 31(12):782–786. https://doi.org/10.1038/jhh.2017.61
Ratsep MT et al (2014) Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta 35(9):772–775. https://doi.org/10.1016/j.placenta.2014.07.006
Ziche M et al (1997) Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Invest 76(4):517–531
Luttun A et al (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8(8):831–840. https://doi.org/10.1038/nm731
Wang Y, Zhao S (2010) Vascular Biology of the Placenta. Morgan & Claypool Life Sciences, San Rafael. https://doi.org/10.4199/C00016ED1V01Y201008ISP009
Aberdeen GW et al (2022) Placental sFlt-1 gene delivery in early primate pregnancy suppresses uterine spiral artery remodeling. Endocrinology. https://doi.org/10.1210/endocr/bqac012
Vogtmann R et al (2021) Circulating maternal sFLT1 (soluble fms-like tyrosine kinase-1) Is sufficient to impair spiral arterial remodeling in a preeclampsia mouse model. Hypertension 78(4):1067–1079. https://doi.org/10.1161/Hypertensionaha.121.17567
Li JY et al (2019) Relationships of serum placental growth factor and soluble fms-like tyrosine kinase-1 with fetal and uterine artery Doppler indices in pre-eclampsia. Int J Gynecol Obstet 145(2):176–181. https://doi.org/10.1002/ijgo.12796
Noori M et al (2010) Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122(5):478–487. https://doi.org/10.1161/Circulationaha.109.895458
Petzold K et al (2011) Relation between maternal angiogenic factors and utero-placental resistance in normal first-and second-trimester pregnancies. Hypertens Pregnancy 30(4):401–407. https://doi.org/10.3109/10641955.2010.506234
Jacobs M et al (2011) Levels of soluble fms-like tyrosine kinase one in first trimester and outcomes of pregnancy: a systematic review. Reprod Biol Endocrinol. https://doi.org/10.1186/1477-7827-9-77
Bian Z, Shixia C, Duan T (2015) First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS ONE. https://doi.org/10.1371/journal.pone.0124684
Chaiyasit N et al (2022) Prospective evaluation of international prediction of pregnancy complications collaborative network models for prediction of preeclampsia: role of serum sFlt-1 at 11–13 weeks’ gestation. Hypertension 79(2):314–322. https://doi.org/10.1161/Hypertensionaha.121.18021
Ecker J et al (2003) First-trimester placental growth factor, SFLT-1, and risk for preeclampsia. Am J Obstet Gynecol 189(6):S60–S60. https://doi.org/10.1016/j.ajog.2003.10.013
Levine RJ et al (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350(7):672–683. https://doi.org/10.1056/NEJMoa031884
Rana S et al (2007) Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension 50(1):137–142. https://doi.org/10.1161/Hypertensionaha.107.087700
Allen RE et al (2014) Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstetr Gynecol Reprod Biol 182:194–201. https://doi.org/10.1016/j.ejogrb.2014.09.027
Levine RJ et al (2006) Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355(10):992–1005. https://doi.org/10.1056/NEJMoa055352
Neuman RI et al (2021) Accurate prediction of total PlGF (placental growth factor) from free PlGF and sFlt-1 (soluble Fms-like tyrosine kinase-1): evidence for markedly elevated PlGF levels in women with acute fatty liver of pregnancy. Hypertension 78(2):489–498. https://doi.org/10.1161/Hypertensionaha.121.17258
Kluivers ACM et al (2023) Angiogenic imbalance in pre-eclampsia and fetal growth restriction: enhanced soluble fms-like tyrosine kinase-1 binding or diminished production of placental growth factor? Ultrasound Obstet Gynecol 61(4):466–473
Myatt L et al (2013) Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG 120(10):1183–1191
Acknowledgements
The authors gratefully acknowledge all participants of the VIRTUAL Placenta study. Further, we thank Dr. Igna Reijnders for her involvement with the development and completion of the PV and uPVV measurements. In addition, we thank Ans Kluivers for help with logistics and Ingrid Garrelds-van den Berg for performing the serum biomarker analyses. Finally, we thank the Strong Babies Foundation for their financial support.
Funding
This research was funded by the International Society for the Study of Hypertension in Pregnancy—Young Investigator Grant No SB-JO-5, which was subsidized by the Strong Babies Foundation, and by the Department of Obstetrics and Gynecology, Erasmus MC University Medical Centre, Rotterdam, The Netherlands.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Eline de Vos. Analyses were performed by Eline de Vos, supervised by Sten Willemsen. The first draft of the manuscript was written by Eline de Vos. All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors report no conflicts of interest. This research was conducted in accordance with the ethical principles for medical research set out in the Declaration of Helsinki and was approved by the Institutional Review Board of the Erasmus Medical Centre on 2 June 2015 (MEC 2015–494).This study was registered at the Dutch Trial Register: NTR6854.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Vos, E.S., Danser, A.H.J., Koning, A.H.J. et al. Maternal serum PlGF associates with 3D power doppler ultrasound markers of utero-placental vascular development in the first trimester: the rotterdam periconception cohort. Angiogenesis (2024). https://doi.org/10.1007/s10456-024-09939-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10456-024-09939-3