Abstract
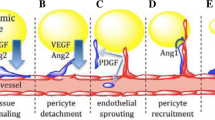
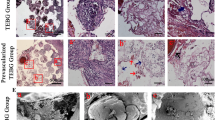
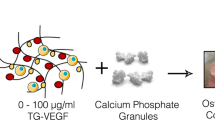
Clinical trials of therapeutic angiogenesis for the treatment of cardiovascular ischemia have failed to meet the expectations with the use of single growth factors, namely VEGF and bFGF. We show here that a bovine bone-derived growth factor mixture (GFM) of TGFβs, BMPs, and no more than 0.1% aFGF can initiate a dose-dependent angiogenic response in subcutaneously implanted Growth Factor Reduced Matrigel plugs that includes abundant smooth muscle actin positive (SMA+) tubes and functional CD31+, red blood cell filled, capillaries. Tube forming activity of the single factors, recombinant bFGF and bone-derived TGF-β2, were comparable to GFM, but only the bone-derived factors were able to create a larger fraction of SMA+ tubes than Matrigel alone at an equal dose. Basic FGF formed a greater number of RBC-filled capillaries within the plugs than GFM or TGF-β2 at the highest doses, although GFM created RBC-filled capillaries that penetrated deeper into the plugs than bFGF. However, bFGF showed the greatest number of non-cell-lined, RBC-filled pools, suggestive of vessel rupture, and the largest number of plugs showing signs of fluid accumulation in the form of large, cell-lined clefts in the implants. TGF-β2 showed less RBC-filled pools, but a significant number of implants with signs of fluid accumulation. At high doses of GFM penetration by blood vessels and mesenchymal cells was obstructed by cartilage development within the plugs accompanied by a prominent band of SMA+ granulation tissue with abundant RBC-filled capillaries encapsulating the implants. Thus, GFM is also capable of dramatically remodeling the vascular system in the interstitial space surrounding the plug. These results show that GFM is capable of inducing the formation of a more mature vascular system than that formed by the single factors bFGF and TGFβ-2. Natural mixtures of TGFβs, BMPs, and FGFs may have superior clinical utility in therapeutic angiogenesis applications.
Similar content being viewed by others
Abbreviations
- aFGF:
-
acidic fibroblast growth factor
- bFGF:
-
basic fibroblast growth factor
- BMP:
-
bone morphogenetic protein
- GFM:
-
growth factor mixture
- GFR Matrigel:
-
growth factor reduced Matrigel
- PDGF:
-
platelet-derived growth factor
- RBC:
-
red blood cell
- SMA:
-
smooth muscle actin
- TGFβ:
-
transforming growth factor beta
- VEGF:
-
vascular endothelial growth factor
References
Annex BH, Simons M. (2005). Growth factor-induced angiogenesis in the heart: Protein therapy. Cardiovasc Res 65: 649–55
Cao Y, Hong A, Schulten H, Post MJ. (2005). Update on therapeutic neovascularization. Cardiovasc Res 65: 639–48
Lebrin F, Deckers M, Bertolino P, ten Dijke P. (2005). TGF-β Receptor function in the endothelium. Cardiovasc Res 65: 599–608
Roethy W, et al. (2001). A growth factor mixture that significantly enhances angiogenesis in vivo. JPET 299: 494–500
Jain RK. (2003). Molecular regulation of vessel maturation. Nature Med 9(6): 685–93
Carano RAD, Filvaroff EH. (2003). Angiogenesis and bone repair. Drug Discovery Today 8(21): 980–89
Deckers MM, van Bezooijen RL, van der Horst G et al. (2002). Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived Vascular Endothelial Growth Factor-A. Endocrinology 143(4): 1545–53
Marrony S, Bassilana F, Seuwen K, Keller H. (2003). Bone morphogenetic protein 2 induces placental growth factor in mesenchymal cells. Bone 33(3): 426–33
Tokuda H, Hatakeyama D, Akamatsu S et al. (2003). Involvement of MAP kinases in TGF-beta-stimulated vascular endothelial growth factor synthesis in osteoblasts. Arch Biochem Biophys 415(1): 117–25
Yeh LC, Lee JC. (1999). Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol Cell Endocrinol 153(1–2): 113–24
Fujimura K, Bessho K, Okubo Y et al. (2002). The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Arch Oral Biol 47(8): 577–84
Nakamura Y, Tensho K, Nakaya H et al. (2005). Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 36(3): 399–407
Kubota K, Iseki S, Kuroda S et al. (2002). Synergistic effect of fibroblast growth factor-4 in ectopic bone formation induced by bone morphogenetic protein-2. Bone 31(4): 465–71
King KE, Iyemere VP, Weissberg PL et al. (2003). Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and Transforming Growth Factor β1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem 278(13): 11661–9
Kowanetz M, Valcourt U, Bergstrom R et al. (2004). Id2 and Id3 Define the potency of cell proliferation and differentiation responses to Transforming Growth Factor β and bone morphogenetic protein. Mol Cell Biol 24(10): 4241–54
Miyazono K and Miyazawa K. Id: A Target of BMP Signaling. Sci STKE 2002; 151: PE40
Goumans M-J, Lebrin F, Valdimarsdottir G. (2003). Controlling the angiogenic switch: A balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovascular Med 13: 301–7
Yancopoulos GD, Davis S, Gale NW et al. (2000). Vascular-specific growth factors and blood vessel formation. Nature 407: 242–8
Passaniti A, Taylor RM, Pili R, et al. (1992). Methods in laboratory investigation: A simple quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 67(4): 519–28
Ohashi K, Yokoyama T, Nakajima Y et al. Technical Bulletin #455: Methods for implantation of BD Matrigel matrix into mice and tissue fixation. http://www.bdbiosciences.com
Isaji M, Miyata H, Ajisawa Y et al. (1997). Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J Pharmacol 122(6): 1061–6
Murayama T, Tepper OM, Silver M et al. (2002). Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 30(8): 967–72
Langenfeld EM, Langenfeld J. (2004). Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res 2(3): 141–9
Huang SP, Wu MS, Shun CT et al. (2004). Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci 11(4): 517–27
25. Maekawa H, Oike Y, Kanda S et al. (2003). Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler Thromb Vasc Biol 23(11): 2008–14
Silvestre JS, Tamarat R, Ebrahimian TG et al. (2003). Vascular Endothelial Growth Factor-B promotes in vivo angiogenesis. Circ Res 93(2): 114–23
Kim HS, Shin HS, Kwak HJ et al. (2003). Betacellulin induces angiogenesis through activation of mitogen-activated protein kinase and phosphatidylinositol 3’kinase in endothelial cell. FASEB J 17(2): 318–20
Salani D, Taraboletti G, Rosano L et al. (2000). Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates angiogenesis in vivo. Am J Pathol 157(5): 1703–11
Salcedo R, Ponce ML, Young HA et al. (2000). Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood 96(1): 34–40
Salcedo R, Young HA, Ponce ML et al. (2001). Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol 166(12): 7571–8
Lee OH, Kim YM, Lee YM et al. (1999). Sphingosine 1-phosphate induces angiogenesis: Its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun 264(3): 743–50
Hartlapp I, Abe R, Saeed RW et al. (2001). Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15: 2215–24
Al-Khaldi A et al. (2003). Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Therapy 10: 621–9
Bompais H, Chagraoui J, Canron X et al. (2004). Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 103: 2577–84
Jain RK. (2005). Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 307(5706): 58–62
Damien CJ, Grob D, Boden SD et al. (2002). Purified bovine BMP extract and collagen for spine arthrodesis – preclinical safety and efficacy. SPINE 27(16S): S50-8
Sampath TK, Rashka KE, Doctor JS et al. (1993). Drosophila Transforming Growth Factorβ Superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci 90: 6004–8
Huffer WE, Lewis M, Barnes T, Benedict JJ. (2001). Strategies for tailoring bone and connective tissue regeneration using bone protein. In: Davies JE (eds). Bone Engineering: An International Workshop. University of Toronto Press, Toronto, pp. 408–20
Buschmann I, Heil M, Jost M, Schaper W. (2003). Influence of inflammatory cytokines on arteriogenesis. Microcirculation 10: 371–9
Helisch A, Schaper W. (2003). Arteriogenesis: The development and growth of collateral arteries. Microcirculation 10: 83–97
Sorescu GP, Sykes M, Weiss D et al. (2003). Bone morphogenetic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278(33): 31128–35
Fajardo LF, Prionas SD, Kwan HH et al. (1996). Transforming Growth Factor β1 induces angiogenesis in vivo with a threshold pattern. Lab Invest 74: 600–8
Bahamonde ME and Lyons KM. (2001). BMP3: To be of not to be a BMP. J Bone Joint Surg Am 83: 56–62
Ramoshebi LN, Ripamonti U. (2000). Osteogenic protein-1, a bone morphogenetic protein, induces angiogenesis in the chick chorioallantoic membrane and synergizes with basic fibroblast growth factor and Transforming Growth Factor-β1. Anat Rec 259: 97–107
Koike N, Fukumura D, Gralla O, et al. (2004). Creation of long-lasting blood vessels. Nature 428: 138–9
Hogan BL. (1996). Bone morphogenetic proteins in development. Curr Opin Genet Dev 6(4): 432–8
Chen D, Zhao M, Harris SE, Mi Z. (2004). Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci 9: 349–58
Roelen BAJ, ten Dijke P. (2003). Controlling mesenchymal stem cell differentiation by TGFβ family members. J Orthop Sci 8: 740–8
Sipe JB, Zhang J, Waits C et al. (2004). Localization of bone morphogenetic proteins (BMPs)-2, −4, and −6 within megakaryocytes and platelets. Bone 35(6): 1316–22
Acknowledgements
The authors thank Steven Simske, PhD for helpful discussion and editing. GFM was provided by Sulzer Biologics, Inc. This work was funded by NIH grant number HL07171.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roedersheimer, M., West, J., Huffer, W. et al. A bone-derived mixture of TGFβ-superfamily members forms a more mature vascular network than bFGF or TGF-β2 in vivo . Angiogenesis 8, 327–338 (2006). https://doi.org/10.1007/s10456-005-9022-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-005-9022-9