Abstract
Ramularia leaf spot is a major economic disease of barley caused by the dothidiomycete fungus Ramularia collo-cygni. The fungus has a complex life cycle which includes extensive late season spore release events and a seed-borne phase. Predicting disease epidemics during the growing season remains a difficult challenge. To better understand the interaction between spore movement and disease epidemics, spore samplers were set up in Germany (two sites over 4 years), Poland (seven sites over 2 years) and the UK (two sites over 9 years), where the disease has been observed. Spore concentrations were determined using a real time PCR assay, and meteorological data were obtained from co-located automatic stations. Spore release events were seen to peak in June on mainland Europe and July in the UK. The pattern of spore release was broadly similar across countries with earlier peaks in mainland Europe. A relationship was observed in the UK between July spore levels and disease in following winter barley crops. Rainfall and temperature were proposed as significant drivers of spore release in these months. The major environmental parameter associated with spore release across the two UK sites was crop surface wetness, although some site-specific interactions were noted for rainfall and wind movement. Regression analysis of spore patterns and disease epidemics indicates a relationship between spore levels 75–105 days pre harvest and final disease levels in UK winter barley crops. This relationship was not observed in spring barley. The implications on risk forecasts are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ramularia collo-cygni (R. collo-cygni) is the biotic factor responsible for the disease Ramularia leaf spot (RLS) of barley (Hordeum vulgare). It has been detected in an increasing number of barley growing countries across the world (Havis et al., 2015; Spencer et al., 2019) and is known to cause economic losses in many temperate countries (Dussart et al., 2020). The life cycle and epidemiology of the disease has only recently been elucidated (Havis et al., 2015). The initial proposed life cycle of the pathogen involved transmission from winter to spring barley crops and back again via airborne spores (Sachs, 2006; Walters et al, 2008). However, the development of molecular diagnostic tools indicated an additional seed borne phase in the fungal life cycle (Havis et al, 2006; Matusinsky et al, 2011). In the UK, seed borne infection was identified as the primary source of inoculum in barley crops (Havis et al., 2014), although this has not been confirmed in a recent study in Estonia (Mäe et al., 2018). Schützendübel et al. (2008) found a closer relationship between spore movement and RLS disease epidemics in trials in Germany and supported the original life cycle. This life cycle proposed a role for spore movement in disease epidemiology and proposed a green bridge between winter sown and spring sown crops, i.e. all year round presence of barley crops for the pathogen to colonise (Sachs, 2006). Zamani-Noor (2011) found that spore release from winter crops contributed to disease levels in spring crops.
Fungi from the different classes are known to have varying methods of spore dispersal (Ingold, 1984). Ascomycetes and many of the Deuteromycetes have an inert dispersal mechanism when the spores are disturbed by a combination of factors on the leaf surface, e.g. air movement and rainfall. Air is important in short to medium distance dispersal of plant pathogens (Brown & Hovmøller, 2002). The movement of fungal spores has been studied by the use of spore traps and microscopy; however, modern molecular testing methods, such as qPCR assays, have now become the standard method to identify and quantify spore levels of agricultural pathogens in the environment (Duvivier et al., 2013; Fountaine et al., 2010; Kaczmarek et al., 2009). Indeed, spores of R. collo-cygni are known to be small and difficult to distinguish from other fungal spores (Sutton & Waller, 1988). Ramularia collo-cygni has been shown to sporulate profusely with numbers estimated at 30 billion per hectare during heavy disease outbreaks (Huss, 2004). This offers the potential for rapid colonisation of nearby crops by heavy spore colonisation and the rapid spread of resistant strains. Other studies examining the release of fungal spores and meteorological data have indicated that the distribution patterns vary depending on the spore type and conditions (Troutt and Levetin, 2001). The movement of the phoma stem canker causing pathogens (Leptosphaeria biglobosa and L. maculans, currently re-named as Plenodomus biglobosus and P. lingam) in Poland and England was studied over growing seasons (Huang et al., 2005; Kaczmarek et al., 2012, 2014). Differences in spore release were observed between countries and between sites within countries. Rainfall was needed to induce spore release but temperature had no effect on spore movement unless below 5 °C or above 20 °C. The Ramularia species which attacks sugar beet (R. beticola) has been shown to release spores which can be detected above the crop canopy. In this pathosystem, spore production was related to high relative humidity and temperatures of 17 to 20 °C in the crop in the previous 24 h (Hestjberg & Dissing, 1995).
The previous studies looking into the interaction between environmental conditions and Ramularia leaf spot epidemics reported that a high humidity level (measured as % RH) was crucial for the initiation of the epidemic, while radiation intensity (measured in watts m−2) was reported as being of minor importance (Formayer et al., 2004). This observation is interesting as the damage related to Ramularia infection was believed to be due to the activation of anthraquinone compounds, called rubellins, within the fungus (Heiser et al., 2004). However, recent work has indicated that the fungus also produces further secondary metabolites which are involved in the damage to the host plant (Dussart et al, 2018). The interaction between the fungus and host plant is now being elucidated at the molecular level (Lemcke et al., 2021; Sjokvist et al., 2019). Marik et al. (2011) found that stronger symptomatic expression was positively affected by a higher number of rainy days in the three weeks post flowering. These authors also reported that higher temperatures and lower rainfall post flowering reduced disease levels in the Czech Republic. Climate projections suggest an increase in susceptibility of crops to R. collo-cygni (West et al., 2012). A recent study by Hoheneder et al. (2021) suggested that long lasting drought periods were unfavourable conditions for RLS development and that the extent and duration of leaf wetness is a key factor for RLS epidemics.
The aims of this study were threefold; (i) to compare spore release patterns from countries with varying climates (ii) to map spore movement for the fungus R. collo-cygni over the full year and identify the environmental parameters which influence spore movement from infected leaves; and (iii) to elucidate a role for R. collo-cygni spore movement on disease outbreaks within and between cropping season. This study represents the first report of Ramularia spore movement across a number of European countries and also over the complete cropping year.
2 Materials and methods
2.1 Airborne inoculum quantification
Burkard 7 day volumetric spore samplers (Burkard Manufacturing, UK) were set up at two sites in Germany, Frankendorf, Bavaria (50.9683° N, 11.4501° E) and Weihenstephan, Bavaria (48.3962° N, 11.7247° E) over a number of seasons, at seven sites in Poland, Charbielin (53.0962° N, 17.6719° E), Glebokie (49.4655° N, 20.7013° E), Krościna Mała (51.3764° N, 16.9446° E), Lublin (51.2465° N, 22.5684° E), Poznan (52.4064° N, 16.9252° E), Radostowo (53.4526° N, 21.2133° E) and Rarwino (53.9531° N, 15.7928° E) over 2013 and 2014 and at two SRUC field trial sites in Scotland; the Bush Estate, Midlothian (55.8603° N, 3.2079° W) and Drumalbin Farm, Lanarkshire (55°39′16″ N, 3°43′34″ W) Identical methodology for spore capture and quantification was undertaken in each country. The Hirst spore sampler machines used in the study sampled air from the environment at a rate of 10 L/hour. The air was drawn through a small aperture and passed over coated Mellinex tape. After 7 days the tape was removed and divided into segments which correspond to 24 h periods. These were then halved lengthways and stored at − 20 °C. DNA was extracted from the tape using the method described in Fountaine et al. (2007). R. collo-cygni DNA levels were quantified using a previously reported black hole quencher probe PCR assay (Taylor et al., 2010). Levels were expressed as picograms per sample. For the purpose of the statistical analysis, R. collo-cygni DNA figures are considered as airborne inoculum. The threshold for the qPCR changed in 2009 as the assay was refined, allowing lower DNA concentrations to be quantified.
Some pre-processing was carried out on the data for inoculum levels. Two inoculum measurements were commonly given per sample, as two wells were used for each tape sample in the qPCR assay. The values were highly correlated by Pearson testing (data not shown) and as a result the mean of these two values was used in the analysis. To handle the highly skewed data set, a log transformation was applied to spore levels, prior to modelling, with an offset corresponding to half the 1st percentile of the non-censored counts.
2.2 Comparison of different locations
At the two UK sites, Bush and Lanark, data were collected almost all year round for 8 and 4.5 years, respectively. The data from Germany and Poland ranged from late Spring to early Summer, reflecting the shorter growing period for barley crops in central Europe. In total data represented 2 years in most cases, with one site having 4. The shorter time period within each year forced us to adopt an exploratory methodology where we compared the sites by plotting and contrasting airborne inoculum levels and averages of the environmental variables that are available at both locations.
2.3 Investigating environmental parameters impacting spore movement
Weather data were collected from all the sites in the study. Daily environmental data were collected from all the sites in the study. The data from Germany were collected by Agrarmeteorologie Bayern (www.wetter-by.de) for the stations 008 Freising and 045 Frankendorf, which are fully equipped for climate measurements in the vicinity of the trials (< 1 km). The data from Poland were collected from seven weather stations located onsite at seven places of the experiment (Charbielin, Glebokie, Krościna Mała, Lublin, Poznań, Radostowo and Rarwino). The data from the UK sites were collected from Delta-T automatic weather stations at the two trial sites. Sensors were used to detect air temperature (°C), soil temperature (°C), surface wetness (%), humidity (%), wind direction (deg), solar radiation (KW m−2) and rainfall (mm). Data were downloaded by from each site by modem as excel files and collated into yearly files.
To connect environmental variables to inoculum release, the influence of a lagged effect was taken into consideration. This was instigated as it is important to separate effects, attributable to a specific variable, from the fact that both these environmental variables and inoculum release alters over the course of a year. To overcome this, the seasonal effect was removed from both the environmental variables and airborne inoculum level.
To remove season effects from each environmental variable, we fitted a GAM modelling its changes as a seasonal periodic pattern—so that each measurement is a function only of its Julian day of the year. This allows us to compute a variable anomaly as each measurement’s difference from its expected value.
Similarly, to the analysis methods outlined in 2.2, we model the transformed spore counts and then, for each possible lag, fitted Tobit GAMM models to the transformed inoculum levels, using a model of the form Y(i,t) = max(f_baseline(t) + vareffect_lag * variable_anomaly(i,t-lag) + yeareffect_i + error, detection_limit(i,t)).
This model is fitted for choices of lag ranging from 0 (representing comparing spore counts to the environmental variables on that day) and 50 days before the measurement.
Here, year effect is a random variable that relates to unaccounted for effects each year and f_baseline again describes the baseline pattern of spore counts over the course of a year. Thus, the interaction between the two, i.e. named variable x effect_lag is the estimated effect of the variable anomaly at that lag, in terms of changing the transformed spore count from its baseline value. This value was calculated to investigate the size of the effect in terms of statistical significance. All computations are done in R using the mgcv package.
2.4 Determining relationship of spore movement with disease outbreaks
Data were taken from field trials carried out on spring and winter barley crops at the two UK trial sites over a ten-year study period. Regular assessments of visual RLS infection in the barley crops over the course of the trials were carried out using a recently published scoring key devised at SRUC (AHDB, 2018). A final disease score was taken in the crops at GS 80 (Zadoks et al., 1974), and this was used as our measure of final disease level.
The relatively small number of years of disease measurements limits the investigation of the association between RLS and inoculum. No significant relationships were found when using disease levels as a dependent variable in simple exploratory analyses.
Instead, we proceeded by the opposite direction and attempted to see what elements of the annual pattern of airborne inoculum can be related to variability in disease levels. This can point to both spores released as an effect of high disease levels and periods of high inoculum that appear ahead of high disease crops, and so might suggest that the airborne inoculum cause the disease to occur. Our methodology for modelling airborne inoculum is based on the use of generalised additive mixed models (GAMMs), together with an implementation of the Tobit I model (Tobin, 1958) via Wood et al. (2016). A significant issue with analysing airborne inoculum is the presence of a detection limit—when airborne inoculum is sufficiently low, the actual level in the environment cannot be precisely determined, only the fact that they are below a set level. Instead of using an ad hoc replacement for these values, the Tobit model used in this study assumes the existence of a true unknown level with normally distributed error, which is censored by the detection limit. Thus, use of this model involves estimating these unknown values simultaneously with the rest of the model and allows us to appropriately incorporate these low values in a model that can include nonlinear components. The use of this methodology allowed more reliable hypothesis test values and estimates of the degree of uncertainty.
The model used in the study can be expressed in the following formula, where the log-transformed airborne inoculum Y in year i, day t can be expressed as follows:
Y(i,t) = max(f_baseline(t) + f_spring(t) * spring_disease_level(i) + f_winter(t) * winter_disease_level(i) + error, detection_limit_i,t).
This is then a nonlinear Tobit I model that is left censored at the detection limit, with three additive nonlinear components corresponding to a seasonal pattern unrelated to disease (f_baseline), and parts associated with spring (f_spring) and winter (f_winter) barley disease levels, respectively. The disease level here is expressed as the difference from the average disease level experienced in the study period.
This model is fitted twice, once setting spring barley sowing each year to equal day t = 0 and considering the year of inoculum quantification following that and again setting winter barley sowing to be t = 0 and using the year afterwards. These two fits were used to produce estimates of the inoculum movement that can be associated with the spring and winter barley disease level, respectively. For the spring barley disease this is given as f_baseline(t) + f_spring(t) * spring_disease_level(i), with corresponding values for winter.
3 Results
3.1 Comparison of German, Polish and UK sites
German data were recorded in two sites—Weihenstephan and Frankendorf, in the south of Bavaria. Of these two sites, Weihenstephan was the main research site and had more complete data, from years 2008, 2010, 2011 and 2014, while Frankendorf had data from years 2010 and 2011.
Measurements of spore movement began in April and ran until July, apart from two years at Weihenstephan, where sampling continued till September. Analysis of the German data showed infrequent detectable R. collo-cygni spore movement after June or July. This contrasts to the UK sites, where some of the highest spore levels were recorded in June and July (Fig. 2). The shorter sampling period in Germany and Poland compared to the UK could be a consequence from differences in the vegetation but does present some constraints on further analysis. Hence, although weather data were available at all years (analysis of the relationship to environmental variables cannot be reasonably done). Surface wetness was only available for the Weihenstephan site.
The airborne R. collo-cygni inoculum measurements from Poland were composed of two years of recordings (2013 and 2014) from seven sites (Rarwino, Radostowo, Poznan, Glebokie, Kroscina Mala, Charbielin and Lublin). From each site, 80 days (from April to June) of airborne inoculum concentrations and environmental data were generated. The weather data included temperature, precipitation, wind speed and humidity readings 2014 were the only year which yielded significant levels of airborne R. collo-cygni DNA.
In terms of the environmental variables measured, the German sites appeared to be warmer and wetter than Bush during similar time periods. Average temperature and surface wetness were higher in Germany compared to the UK, e.g. 17 °C compared to 12 °C and 37.3%, versus 16.6%. However, the data for the full summer months suggested a similar relationship between the environment and levels of airborne R. collo-cygni at the German and UK sites.
At the Polish experimental sites, significant amounts of Rcc DNA were found only in 2014 at two sites, Rarwino and Radostowo (Supplementary Figure 1). For those two sites in year 2014, the concentrations of Rcc DNA were high relative to Bush during the same period of the year, an average of 16.0 pgrams against 5.40 pgrams. Rcc DNA levels also showed somewhat lower variability. In year 2013 R. collo-cygni DNA was substantially lower than in 2014. R. collo-cygni spore movement, measured as quantified fungal DNA, peaked earlier than in the UK and more comparable with the pattern observed in Germany.
Climatic conditions are noticeably different between the countries at the studied sites. At the Bush site, April to June temperatures over the years of the study period averaged 8.6 degrees, whereas at the Polish sites temperatures ranged from 11.4 to 16.7 °C. Radostowo 2014 (11.4 °C) and Rarwino 2014 (12.6 °C) were amongst the coolest of the Polish sites, although there may be insufficient data to draw firm conclusions on the effect of temperature as a significant driver of airborne R. collo-cygni inoculum patterns. Differences in precipitation were relatively small. The Polish sites were typically drier with levels 0.95–3.00 mm of rain per day recorded, against an average at Bush of 2.48 mm, and a maximum of 5.30 mm. Radostowo had 1.07 mm of rainfall per day while Rarwino had 2.19 mm.
The highest spore levels recorded in May–June were in Germany with Weihenstephan slightly higher than Frankendorf (104 ngrams; Fig. 1). Winter barley crops are nearing full maturity by late June in Bavaria, and spring crops are 4 weeks behind (M. Hess pers communication). Crops are at a similarly advanced stage in Poland in May and June (M Jedryczka, pers communication). In contrast, at the UK sites the crops are at least two to three weeks behind the barley crops in the other countries.
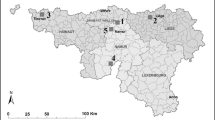
Contour map comparing R. collo-cygni airborne inoculum concentrations in May and June across German (two sites), Polish (seven sites) and UK (two sites) in all years of study and rainfall and temperatures. Each circle represents one site in one year. The size of the circle denotes the level of spores captured. For identification of site see Figure insert
3.2 R. collo-cygni inoculum levels at UK sites
The longer sampling period at the UK sites allowed data to be summarised over the full calendar year (Fig. 2).
A summary plot of weekly average R. collo-cygni airborne inoculum levels at Bush (black) and Lanark (red) locations. The graphs indicate spore release events are focussed on mid to late summer onwards and the fungus does not seem to be polycyclic over the cropping year. Values are computed using raw dataset values, with no available count set as zero. Offset log scale is used for the Y-axis. The shaded area at the bottom of the graph shows the detection limit of the qPCR assay
The spore count showed a seasonal pattern, peaking during summer and being lower during spring, though levels did change from year to year. Spore patterns in Lanark were similar in the available data to Bush—the levels were somewhat higher in Lanark in year 2009, and higher in Bush in year 2010.
Not included in Fig. 2 is year 2012, (Supplementary graphs A2 and A3) which showed an unusual pattern of airborne inoculum movement, with a single peak early in the year in contrast to summer like typical years. This was especially pronounced at the Lanark site, which also exhibited low inoculum levels during summer. Due to the unusual pattern for this single year, we chose to exclude it from all the analyses. This unusual pattern was believed to be related to the very unusual early spring conditions, with temperatures of 28 °C recorded in the last few days of March at the two UK sites. A peak in late March was recorded but then levels returned to zero shortly after.
3.3 Relationship of spore movement with disease
Disease levels of about 10% on average over the period have been observed (Fig. 3). They were lower in year 2010 and year 2011 for all crops at both sites in the UK, but generally disease patterns in the two crops and the two sites were quite variable, E.g. only in year 2009 were levels in w barley higher than spring barley at both sites.
The Tobit I methodology outlined in Sect. 2.3 was applied to decompose airborne inoculum patterns at Bush. This fitting gave highly significant components for the baseline as well as spring and winter barley disease, suggesting an association between the pattern of spore release and disease levels. At Lanark there was an insufficient number of years with both disease and spore data for the fitting to be carried out, as the spore sampler was only moved to that site in Feb 2007, therefore the results of the Bush fitting only are presented in Fig. 4. Variability in spore counts was significant, and there is autocorrelation present that is difficult to account for. Hence these findings are best described as exploratory at this point.
Components of R. collo-cygni airborne inoculum measurements at Bush experiment site, Scotland, UK, which can be associated with winter and spring barley. Curves give fitted values each year, with the top graph giving components associated with each year’s winter barley disease level (keeping spring barley disease constant), and the bottom conversely showing trends associated with spring barley disease. The x-axis is chosen so that the two graphs are approximately aligned in calendar time, i.e. with 100 days since winter sowing approximately equal to 1 January. The vertical lines marked W and S mark the average timing of winter and spring barley harvest (in practice the actual day of harvest can vary from this average by up to 20 days). As before, the shaded area shows the detection limit. The colour and density of the lines denote the corresponding disease levels, the corresponding years and given in the legend
For both spring and winter barley, high disease was associated with high levels or early appearance of airborne inoculum both before and after harvest. In addition, for winter barley, high disease in the subsequent crop is associated with high airborne inoculum levels around the time of sowing. This may suggest a green bridge effect, though these airborne inoculum levels were found very soon after sowing. As remarked in Sect. 2.3, attempts to directly correlate disease levels to inoculum levels in the 2 months after sowing did not show a significant relationship (p = 0.146), but this may be a consequence of the small number of years considered. Examining in more detail a 30-day period starting 75 days prior to harvest, high inoculum levels were correlated to disease levels in winter barley (R2 = 54%, p = 0.036), but there was no clear relationship for spring barley (p = 0.34).
3.4 Environmental factors on spore movement
Table 1 summarises findings from associating environmental variables with varying lags to spore movement, both considered relative to their seasonal baseline as shown in Sect. 2.4. The table gives a median p value as a measure of statistical significance, a maximum absolute coefficient giving a peak effect size, and a maximum lag giving a measure of the duration of the variable’s effect on spore movement.
The table indicates that of the variables considered, only surface wetness showed a consistently highly significant impact on spore movement across both sites. Coefficients across both sites were comparable (0.009 for Bush; 0.012 for Lanark, Table 1). Rainfall may also be a significant factor in initiating spore movement, although the significant finding for the Bush site (p = 0.076, Table1) presented here may be suspect due to the highly skewed distribution of rainfall levels.
4 Discussion
The history of Ramularia leaf spot in the three countries in this study varies greatly. In Germany, the disease was identified in barley crops in Bavaria in 1986 (Huss et al., 2003) and has been a significant issue since then (Hess et al., 2006). The pathogen has been detected in plant samples from Poland for around a decade but symptom levels in crops remain low compared to the other countries in the study (Karolewski et al., 2012). In the UK, the disease was not identified in barley crops until the end of the twentieth century by Dr Edelgard Sachs (Walters et al., 2008) but has risen in importance to become a major disease in barley. (Havis et al., 2015).
No studies of R. collo-cygni spore movement across countries have been carried out previously, although national studies have been carried out in Germany (Schützendübel et al., 2008; Zamani-Noor, 2011) and earlier work in Norway suggested long distance dispersal by wind (Salamati & Reitan, 2006).
To answer objective one, spore release patterns across three countries were compared. An examination of the airborne inoculum release patterns in this study indicates a single peak during the growing season, rather than multiple peaks. Testing of spore samples from Poland has shown airborne R. collo-cygni DNA present between April and June in both 2013 and 2014). Spore testing in the UK and Poland has shown that airborne R. collo-cygni DNA can be detected in areas where symptoms are rarely seen (N. Havis, M Jedryczka, unpublished data). The patterns of the peaks indicate an earlier response of the fungus to environmental conditions in Poland, compared to the UK. This may also be related to earlier crop development in Poland due to higher temperatures from spring onwards.
Another study performed in Poland, examining Cladosporium spore movement, indicated warm and dry weather during the growing season accelerated both spore release intensity and duration (Kasprzyk et al., 2016). This in turn, resulted in an increase in disease incidence and severity. This effect of the environment on spore movement and disease severity has also been reported for spores of Leptospheria sp. and the symptoms of phoma leaf spotting and stem canker (Kaczmarek et al., 2016).
RLS in the UK varies greatly between season, with the disease far more prevalent in 2014 than 2013. The weather patterns in 2013 and 2014 were very different. In 2013, heavy rains across much of central Europe caused flooding in parts of Bavaria and Poland at the end of May/early June. In 2014, much of Europe experienced one its warmest years on record. The contour maps presented in Fig. 1 indicate the effect of increasing temperature and rainfall on R. collo-cygni spore release across the three countries in May and June. Previous work in the UK suggested RLS levels in spring barley crops were related to higher rainfall and temperatures experienced by the crop from sowing to GS 59 (ear fully emerged) (Havis et al., 2018). The spore levels were much lower in the UK compared to Germany and Poland, but this could be related to slower crop development (due to lower temperatures in the crop). Previous work in Germany suggested multiple spore release events in these two months (Schützendübel et al., 2008). The authors did not find any correlation with rainfall in the crop and reported spore movement preceded disease development.
The contour graph (Fig. 1) indicates spore levels were much higher in Germany than in Poland. This could be related to levels of barley production in the two countries. Germany is the world’s second largest barley producer at just over 10 million tonnes while Poland’s production is only 3 million tonnes (FAOSTAT, 2016). There is also regional variation in barley production hectarage and climate, which could have affected spore levels recorded.
A conservative approach was adopted for the statistical analysis. The elimination of season effects from both the environmental variables and airborne inoculum eliminated the consideration that the seasonal pattern of spore release is itself due to the seasonal pattern of weather. Instead, the relationship between differences in environmental values and differences in inoculum levels, measured as R. collo-cygni DNA was examined. In addition, the inclusion of the year random effect meant it was comparatively more difficult to attribute the yearly variation in overall airborne inoculum levels to overall shifts in the yearly average temperatures and wetness. However, both these steps were necessary to avoid confusing the impact of environmental variables with other such effects.
The data from Germany presented in this paper suggest spore levels peak at approximately 200 days post sowing (Supplementary Figure 1). This pattern is more reminiscent of the one from the UK sites, which showed major peaks towards the end of the growing season towards late summer (Fig. 1). The data from the UK sites suggest the fungus does not sporulate significantly in the early season. The fungus has been detected in plants well before symptom expression (Frei et al., 2007; Havis et al., 2014). The results from the spore analysis support the theory that the initial inoculum comes from seed via vertical transmission.
To complete the second and third objectives of the study on the pattern and influence of spore movement on disease levels within the growing season, Rcc DNA from the UK sites and disease data from co-located trials were analysed. The results indicated a correlation between spore release levels from spring barley crops and disease levels in winter barley crops in the following year (Figs. 3 and 4). This supports the green bridge life cycle for the fungus proposed by Sachs (2006). Although, the crops only rarely share a temporal link there may be spore movement from crop debris from spring barley which allows transmission to winter crops. The potential of reverse transmission also occurs in the summer months. Indeed, a link between RLS in winter barley and the appearance of the fungus in spring barley was also suggested by Frei et al. (2007). These authors suggested that sporulation in winter barley was the prerequisite to the appearance of the pathogen in spring crops. A study to examine the spatio-temporal distribution of the related dothidiomycete fungus, Zymoseptoria tritici, in Belgium over two seasons indicated multiple spore production peaks during each growing season (Duvivier et al., 2013). The authors reported important between site variations in this study and a correlation between spore levels and observed disease levels in adjacent crops. The presence of infected stubble from previous crops was shown to be a predictor of spore release, as was temperature and rainfall. The analysis in this study shows association between disease levels and airborne inoculum patterns, indicating interaction between these two variables in UK data. The data sets from Germany and Poland indicated similar spore release patterns and that spore levels were similar in Bavaria over the course of the study. The spore levels were generally lower in Poland but variation was seen between sites (Fig. 1, Supplementary Figure 1).
Movement of fungal spores has been studied for many years, and the prediction of plant disease through pathogen movement has been investigated in various pathosystems (Pan et al., 2010). Preliminary analysis on R. collo-cygni DNA levels from spore tapes and meteorological data from the Bush site using three years data highlighted a significant correlation between prolonged levels of leaf surface wetness in July and spore dispersal (Havis et al., 2009). An increase in spore release was also observed when ambient temperature increased from 5 to 15 °C, indicating temperature may be a factor for this process. This response to environmental conditions has also been observed with other fungi (Toscano-Underwood et al., 2013). One of the drawbacks in controlling RLS in barley crops is the lack of any robust risk forecast. Disease control has relied on the application of effective fungicides prior to symptom expression. However, the influence of environmental conditions on epidemics is only being slowly uncovered, and the impact of leaf wetness at barley stem extension on disease epidemics has been reported previously (Salamati & Reitan, 2006). Interestingly, recent work has indicated this relationship over seasons might not be robust enough to construct a risk forecast and that additional environmental and host physiology parameters may need to be considered in forecast construction (Havis et al., 2018). A recent study in Germany indicated that periods of continued drought reduced disease severity in barley crops (Hoheneder et al., 2021). Here, we have observed a relationship between R. collo-cygni spore levels, 75–105 days prior to harvest and final winter barley disease levels. This period approximates to the second spray timing in winter barley crops in the UK (GS49) (Zadoks et al., 1974). This raises the possibility that an accurate quantified figure for R.collo-cygni spore numbers during the growing season could be incorporated into a risk forecast for barley growers.
It is worth noting that many other factors could influence RLS development including the presence of other diseases, crop development and the rate of crop senescence. Studies to establish a link between disease and inoculum release at locations outside of the UK will require a standardised methodology to derive significant associations. Recent work in Germany has suggested environmental stress followed by leaf wetness in the crop reduce symptom expression and sporulation but not necessarily disease levels (M Hess, unpublished). Wind movement and gusts have been described as a major influence on movement of airborne fungal spores in crops (McCartney et al., 2006). However, in our analysis wind only influenced spore release at one UK site, Bush Estate (Table 1). The correlation pattern was different between the two UK sites with surface wetness the only common environmental factor significantly influencing spore release at both sites.
The only environmental parameters which affect R.collo-cygni inoculum release at both UK sites is surface wetness (Table1). The recent findings from Hoheneder et al (2021) also highlight the importance of leaf area duration on RLS epidemic and disease development in Germany. Solar radiation had an effect at one site, as did precipitation. If temperature has an effect it may possibly be over a longer term than shown in our analysis. Leaf wetness duration has been associated with the release of other plant pathogens, e.g. Alternaria alternata (Timmer et al., 1998). In this pathosystem field trapping showed that spore release was not related to rainfall amounts or average wind speed but weakly associated with duration of leaf area wetness (Table 1). For R. collo-cygni the structure of the conidiophores with their characteristic swan neck spores emerging from stomata suggests an important role for turgor pressure in spore development and release (Sutton & Waller, 1988). A number or dispersal mechanisms have been reported for fungal spores (Lacy, 1985). Alternaria species are known to have an active dispersal mechanism, and there may be a similar mechanism for R. collo-cygni. Fungi have a minimum wetting period requirement to promote sporulation, and there is a sigmoidal increase in some spores if this period is exceeded (Lacy, 1985). The data on R. collo-cygni airborne inoculum release suggest that extended leaf wetness in barley crops does initiate significant spore release from the crop (Table 1). The influence of other factors on airborne inoculum release remains uncertain as the results from the two UK sites show an influence of differing factors. This might be related to the influence of other diseases and environmental conditions on crop physiology and senescence.
This first report of Ramularia spore movement over the entire cropping season helps to better understand of their contribution to disease epidemics. The detailed analysis on the data from the UK sites indicates the use of spore numbers during the season for risk forecast and suggests methods in which future datasets may be analysed to demonstrate the relationship between the fungus, environment and the expression of disease in the crop. More research and the development of a robust model to predict spore levels and disease will aid researchers and agronomists in helping design control programmes for Ramularia leaf spot.
References
AHDB (2018). Identification and field scoring guide for Ramularia leaf spot. Accessed online https://cereals.ahdb.org.uk/media/1340591/is60a-ramularia-guide-january-2018-v2.pdf.
Brown, J. K. M., & Hovmøller, M. S. (2002). Aerial dispersal of fungi on the global and continental scales and its consequence for plant disease. Science, 297, 537–541.
Dussart, F., Creissen, H. E., & Havis, N. D. (2020). Ramularia collo-cygni—An enemy in waiting. eLS (pp. 1–8). John Wiley & Sons Ltd.
Dussart, F., Douglas, R., Sjokvist, E., Hoebe, P. N., Spoel, S. H., & McGrann, G. R. D. (2018). Genome-based discovery of polyketide-derived secondary metabolism pathways in the barley pathogen Ramularia collo-cygni. Molecular Plant-Microbe Interactions, 31, 962–975.
Duvivier, M., Dedeurwaerder, G., De Proft, M., Moreau, J. M., & Legrève, A. (2013). Real-time PCR quantification and spatio-temporal distribution of airborne inoculum of Mycosphaerella graminicola in Belgium. European Journal of Plant Pathology, 137(2), 325–341.
FAOSTAT (2016). Crops. Available online http://www.fao.org/faostat/en/#data/QC/.
Formayer, H., Huss, H., & Kromb-Kolb, H. (2004). Influence of climatic factors on the formation of symptoms of Ramularia collo-cygni. In: A. H. Yahyaoui, L. Brader, A. Tekauz, H. Wallwork, & B. Steffenson (Eds.), Proceedings of the second international workshop on barley leaf blights (pp. 329–330).
Fountaine, J. M., Shaw, M. W., Napier, B., Ward, E., & Fraaije, B. A. (2007). Application of real-time and multiplex PCR assays to study leafblotch epidemics in barley. Phytopathology, 97, 297–303.
Fountaine, J. M., Shaw, M. W., Ward, E., & Fraaije, B. A. (2010). The role of seeds and airborne inoculum in the initiation of leaf blotch (Rhynchosporium secalis) epidemics in winter barley. Plant Pathology, 59(2), 330–337.
Frei, P., Gindro, K., Richter, H., & Schürch, S. (2007). Direct-PCR detection and epidemiology of Ramularia collo-cygni associated with barley necrotic leaf spots. Journal of Phytopathology, 155, 281–288.
Havis, N. D., Brown, J. K. M., Clemente, G., Frei, P., Jedryczka, M., Kaczmarek, J., Kaczmarek, M., Matusinsky, P., McGrann, G. R. D., Pereyra, S., Piotrowska, M., Sghyer, H., Tellier, A., & Hess, M. (2015). Ramularia collo-cygni—An emerging pathogen of barley crops. Phytopathology, 105, 895–904.
Havis, N. D., Evans, N., & Hughes, G. (2018). Development of a UK wide risk forecast for Ramularia leaf spot in barley. AHDB Report 600. Available online https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Cereals%20and%20Oilseed/final-report-pr600.pdf.
Havis, N. D., Nyman, M., & Oxley, S. J. P. (2014). Evidence for seed transmission and asymptomatic growth of Ramularia collo-cygni in barley (Hordeum vulgare). Plant Pathology, 63, 929–936.
Havis, N. D., Oxley, S. J. P., Piper, S. R., & Langrell, S. R. H. (2006). Rapid nested PCR-based detection of Ramularia collo-cygni direct from barley. FEMS Microbiology Letters, 256, 217–223.
Havis, N. D., Taylor, J. M. G., Nyman, M., & Oxley, S. J. P. (2009). Epidemiology of Ramularia collo-cygni. Aspects of Applied Biology, 92, 1–7.
Heiser, I., Hess, M., Schmidtke, K.-U., Vogler, U., Miethbauer, S., & Liebermann, B. (2004). Fatty acid peroxidation by rubellin B, C and D, phytotoxins produced b Ramularia collo-cygni (Sutton & Waller). Physiological Molecular Plant Pathology, 64, 135–143.
Hess, M., Habeker, R., Hausladen, H., & Heiser, I. (2006). Monitoring the epidemics of Ramularia collo-cygni: Comparison of varieties, sites, years and methods. In Proceedings 1st European Ramularia workshop March 2006, Göttingen, Germany (pp. 47–52).
Hestjberg, H., & Dissing, H. (1995). Studies on the concentration of Ramularia beticola conidia in the air above sugar beet fields in Denmark. Journal of Phytopathology, 143(5), 269–273.
Hoheneder, F., Hofer, K., Groth, J., Herz, M., Hess, M., & Hückelhoven, R. (2021). Ramularia leaf spot disease of barley is highly host genotype-dependent and suppressed by continuous drought stress in the field. Journal of Plant Disease and Protection, 128, 749–767.
Huang, Y. J., Fitt, B. D. L., Jedryczka, M., Dakowska, S., West, J. S., Gladders, P., Steed, J. M., & Li, Z. Q. (2005). Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). European Journal of Plant Pathology, 111, 263–277.
Huss, H. (2004). The biology of Ramularia collo-cygni. In: A. Ya hyaou, L. Brader, A. Tekauz, H. Wallwork, B. Steffenson (Eds.), Proceedings of the second international workshop on barley leaf blights, 7–11 April 2002 (pp. 321–328). ICARDA.
Huss, H., Formayer, H., & Stabentheimer, E. (2003). Die Sprenkelkrankheit – weiter auf dem Vormarsch. Pflanzenschutz, 5, 8–11.
Ingold, C. T. (1984). The biology of fungi (5th ed.). London: Hutchinson and Co.
Kaczmarek, J., Jedryczka, M., Cools, H., Fitt, B. D. L., Lucas, J. A., & Latunde-Dada, A. O. (2012). Quantitative PCR analysis of abundance of airborne propagules of Leptosphaeria species in air samples from different regions of Poland. Aerobiologia, 28, 199–212.
Kaczmarek, J., Jędryczka, M., Fitt, B. D. L., Lucas, J. A., & Latunde-Dada, A. O. (2009). Analyses of air samples for ascospores of Leptosphaeria maculans and L. biglobosa with light microscopic and molecular techniques. Journal of Applied Genetics, 50(4), 411–419.
Kaczmarek, J., Kedziora, A., Brachaczek, A., Latunde-Dada, A. O., Dakowska, S., Karg, G., & Jedryczka, M. (2016). Effect of climate change on sporulation of the teleomorphs of Leptosphaeria species causing stem canker of brassicas. Aerobiologia, 32, 39–51.
Kaczmarek, J., Latunde-Dada, A. O., Irzykowski, W., Cools, H. J., Stonard, J. F., Brachaczek, A., & Jedryczka, M. (2014). Molecular screening for avirulence alleles AvrLm1 and AvrLm6 in airborne inoculum of Leptosphaeria maculans and winter oilseed rape (Brassica napus) plants from Poland and the UK. Journal of Applied Genetics, 55, 529–539.
Karolewski, Z., Kaczmarek, J., Jedryczka, M., Cools, H. J., Fraaije, B. A., Lucas, J. A., & Latunde-Dada, A. O. (2012). Detection and quantification of airborne inoculum of Pyrenopeziza brassicae in Polish and UK winter oilseed rape crops by real-time PCR assays. Grana, 51, 270–279.
Kasprzyk, I., Kaszewski, B. M., Weryszko-Chmielewska, E., Nowak, M., Sulborska, A., Kaczmarek, J., Szymanska, A., Haratym, W., & Jedryczka, M. (2016). Warm and dry weather accelerates and elongates Cladosporium spore seasons in Poland. Aerobiologia, 32, 109–126.
Lacy, J. (1985). Water availability and fungal reproduction: Patterns of spore production liberation and dispersal. In P. G. Ayres & L. Boddy (Eds.), Water fungi and plants (pp. 65–86). Cambridge University Press.
Lemcke, R., Sjökvist, E., Visentin, S., Kamble, M., James, E. K., Hjørtshøj, R., Wright, K. M., Avrova, A., Newton, A. C., Havis, N. D., Radutoiu, S., & Lyngkjær, M. F. (2021). Deciphering molecular host-pathogen interactions during Ramularia collo-cygni Infection on Barley. Frontiers in Plant Science, 12, 747661.
Mäe, A., Sooväli, P., & Põllumaa, L. (2018). Ramularia collo-cygni: A new pathogen spreading in barley fields in Estonia. Agricultural and Food Science, 27(2), 138–145.
Marik, P., Snejdar, Z., & Matusinsky, P. (2011). Expression of resistance to Ramularia leaf spot in winter barely cultivars grown in conditions of the Czech Republic. Czech Journal of Genetics of Plant Breeding, 47, 37–40.
Matusinsky, P., Leisova-Svobodova, L., Gubis, J., Hudcovicova, M., Klcova, L., Gubisova, M., Marik, P., Tvaruzek, L., & Minarikova, V. (2011). Impact of the seed-borne stage of Ramularia collo-cygni in barley seed. Journal of Plant Pathology, 93, 679–689.
McCartney, H. A., Fitt, B. D. L., & West, J. S. (2006). Dispersal of foliar plant pathogens: Mechanisms, gradients and spatial patterns. In B. M. Cooke, D. Gareth-Jones, & B. Kaye (Eds.), The epidemiology of plant diseases (2nd ed., pp. 159–186). Springer, Netherlands.
Pan, Z., Li, X., Yang, X. B., Andrade, D., Xue, L., & McKinney, N. (2010). Prediction of plant disease through modelling and monitoring airborne pathogen dispersal. CAB Reviews Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 5(18), 1–11.
Sachs, E. (2006). The history of research into Ramularia leaf spot on barley. In Proceedings of the first European Ramularia workshop (pp. 9–15).
Salamati, S., & Reitan, L. (2006). Ramularia collo-cygni on spring barley, an overview of its biology and epidemiology. In Proceedings of the first European Ramularia workshop (pp. 19–35).
Schützendübel, A., Stadler, M., Wallner, D., & von Tiedemann, A. (2008). A hypothesis on physiological alterations during plant ontogenesis governing susceptibility of winter barley to Ramularia leaf spot. Plant Pathology, 57, 518–526.
Sjokvist, E., Lemcke, R., Kamble, M., Turner, F., Blaxter, M., Havis, N. D., Lyngkjær, M. F., & Radutoiu, S. (2019). Dissection of ramularia leaf spot disease by integrated analysis of barley and Ramularia collo-cygni transcriptome responses. Molecular Plant-Microbe Interactions, 32(2), 176–193.
Spencer, M., Havis, N. D., & Creissen, H. E. (2019). Ramularia detection dates by country. Zenodo. https://doi.org/10.5281/zenodo.3375540
Sutton, B., & Waller, J. (1988). Taxonomy of Ophicladium hordei causing leaf lesions on Triticale and other Graminutesae. Transactions of the British Mycological Society, 90, 55–61.
Taylor, J. M. G., Paterson, L., & Havis, N. D. (2010). A quantitative real-time PCR assay for the detection of Ramularia collo-cygni from barley (Hordeum vulgare). Letters in Applied Microbiology, 50, 493–499.
Timmer, L. W., Solel, Z., Gottwald, T. R., Ibañez, A. M., & Zitko, S. E. (1998). Environmental factors affecting production, release, and field populations of conidia of Alternaria alternata, the cause of brown spot of citrus. Phytopathology, 88(11), 1218–1223.
Tobin, J. (1958). Estimation of relationships for limited dependent variables. Econometrica, 26(1), 24–36.
Toscano-Underwood, C., Huang, Y. J., Fitt, B. D. L., & Hall, A. M. (2013). Effects of temperature on maturation of pseudothecia of Leptosphaeria maculans and L. biglobosa on oilseed rape stem debris. Plant Pathology, 52(6), 726–736.
Troutt, C., & Levetin, E. (2001). Correlation of spring spore concentrations and meteorological conditions in Tulsa, Oklahoma. International Journal of Biometeorology, 45, 64–74.
Walters, D. R., Havis, N. D., & Oxley, S. J. P. (2008). Ramularia collo-cygni: The biology of an emerging pathogen of barley. FEMS Microbiology Letters, 279, 1–7.
West, J. S., Townsend, J. A., Stevens, M., & Fitt, B. D. L. (2012). Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. European Journal of Plant Pathology, 133(1), 315–331.
Wood, S. N., Pya, N., & Säfken, B. (2016). Smoothing Parameter and model selection for general smooth models. Journal of the American Statistical Association, 111(516), 1548–1563.
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421.
Zamani-Noor, N. (2011). Studies on Ramularia leaf spots on barley—resistance phenotyping, epidemiology and pathogenicity. PhD thesis, Georg-August-University Göttingen, Germany
Acknowledgements
We would like to thank the dedicated field trial teams and technical staff at SRUC, Institute of Plant Genetics PAS, TUM and LfL Bayern. We would like to acknowledge the advice and guidance given by Professor Gareth Hughes in the analysis of the data.
Funding
The work carried out in the UK was financially supported by the Scottish Government Strategic Research Programme. The work in Poland was funded by DuPont Poland (Corteva Agricience). The experiments in Germany were funded by the Bayerisches Staatsministerium für Ernährung, Landwirtschaft und Forsten (StMELF) (Project KL/08/07 and KL/12/07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Havis, N.D., Kaczmarek, J., Jedryczka, M. et al. Spore dispersal patterns of the ascomycete fungus Ramularia collo-cygni and their influence on disease epidemics. Aerobiologia 39, 105–118 (2023). https://doi.org/10.1007/s10453-022-09778-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-022-09778-z