Abstract
Inhalation of olive pollen (Olea europaea L.) is one of the main causes of allergy in Mediterranean countries and some areas of North America. The response to allergens consists in the production of inflammatory cytokines which is mediated by the deregulation of Ca2+ signals. In this study, the biological activity of the material released in olive pollen hydration (PMR) was tested on Ca2+ cytosolic of PE/CA-PJ15 cells (PJ-15). Ca2+ cytosolic was determined by fluorometric assay with the cell line PE/CA-PJ15 (PJ-15) labeled with the fluorescent probe FURA 2 AM. The material released in olive pollen hydration (PMR) was analyzed by HPLC for the determination of phenolic acids. PMR was subjected to fractionation by gel filtration, and the fractions with Ca2+-chelating activity were tested with SDS-PAGE and the single bands characterized by proteomic analysis. PMR showed high Ca2+-chelating activity and is able of blocking the increase Ca2+-cytosolic produced by thapsigargin (TG). PMR then restored Ca2+ homeostasis in PJ-15 cells deregulated by the endoplasmic reticulum Ca2+-ATPases inhibitor. It is therefore possible that PMR can antagonize the effects of allergens on Ca2+ cytosolic. The analytical characterization of the material released by the pollen highlighted in the pollen allergen Ole e 3 and in the p-coumaric acid the possible culprits of the Ca2+-antagonist activity of PMR. Furthermore, the sequence of Ole e 3 could provide information for the possible construction of a synthetic peptide to be used in an allergy-targeted Ca2+-antagonist therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ca2+, as a second messenger, plays a key role in various cellular processes such as gene expression, proliferation, differentiation (Prakriya & Lewis, 2015; Hogan et al., 2010), plant growth, development and signal transduction (Thor, 2019; Kudla et al., 2018; Ortiz-Ramìrez et al., 2017). Changes in cytosolic Ca2+ determine the activation of temporal responses that can be short term, such as muscle contraction and hormonal secretion, or long term, such as cell growth, proliferation, apoptosis and differentiation (Berridge, 2006; Carafoli, 2003; Dupont et al., 2011). The maintenance of Ca2+, in the absence of stimuli, at nanomolar concentrations in the cytoplasm of cells is an indispensable condition to avoid the activation of unwanted transduction signals (Berridge, 2006; Dupont et al., 2011; Ordenes et al., 2002; Urbina et al., 2006). Allergens influence cellular signals including those of Ca2+, determining the elevation of Ca2+ cytosolic, which triggers the production of cytokines as a response with an unknown mechanism (Jairaman et al., 2015; Matsuwaki et al., 2012). Many allergens induce increases in cytosolic Ca2+in airway epithelial cells (AEC) through the release of Ca2+ from internal stores or through the activation of specific Ca2+ channels on the plasma membrane (Jairaman et al., 2015; Matsuwaki et al., 2012). AEC are the first line of defences against inhaled allergens, and play a vital role in immune recognition and response (Jairaman et al., 2015). Allergy is a general condition of impaired host reactivity characterized by varying levels of bronchoconstriction, remodeling and infiltration of immune cells leading to chronic airway inflammation (Dhar, 2001; Jairaman et al., 2015). Bronchial musculature contracts in asthmatics when Ca2+ cytosolic levels rise abnormally due to cell membrane depolarization (Dhar, 2001). One of the main causes of allergy in Mediterranean countries and some areas of North America is the inhalation of olive pollen (Bousquet et al., 1985; Wheeler, 1992). Olea europaea L. is one of the most complex allergenic models known to date. Indeed, 20 allergenic bands of a protein nature were detected in olive pollen extracts, of which only twelve were identified (from Ole e 1 to Ole e 12). Ole e 1, 20 kDa; Ole e 2 (profilin), 16 kDa; Ole e 3 (polcalcin), 9.2 kDa; Ole e 4, 32 kDa; Ole e 5.16 kDa; Ole e 6, 5.8 kDa; Ole e 7, 10 kDa; Ole e 8, 18.8 kDa; Ole e 9, 46.4 kDa; Ole e10, 10.78 kDa; Ole e 11, 37.4 kDa; Ole e 12, 34.06 kDa; Ole e 13, 24.72 kDa (Huecas et al., 2001; Esteve et al., 2012; Quiralte et al., 2007). Ole e 1 is the main allergen of O. europaea found inside the cisterns of the rough endoplasmic reticulum (Napoli et al., 2008). Its function is still unknown, although some authors believe it is a constitutive protein, involved in reproductive functions or the control of the osmotic gradient in the hydration process. It is also the most abundant protein in extracts with a presence of 5–20% of the total protein of olive pollen and is the most widespread allergen affecting more than 80% of patients suffering from hypersensitivity to this pollen. Ole e 1 shows multiple isoforms and variants present with a different degree of distribution (Esteve et al., 2012; de Dios Alché et al., 1999). In addition to those described, a glycosylated allergen was isolated and characterized as 1,3-b-glucanase (Ole e 9, 46.4 kDa). 1,3-b-Glucanases are widely distributed in higher plants, but their function is still unclear. They are supposed to be expressed during the physiological development of healthy plants, in events such as germination, senescence, fertilization and in response to environmental changes (Huecas et al., 2001).
Among the protein bands of 9–10 kDa in pollen extracts of Oleaceae, grasses and birch, there is Ole e 3 (Polcalcin). This protein allergen has Ca2+ binding domains called EF-hands type (helix-loop-helix motifs) like those of calmodulin and parvalbumin. Polcalcins constitute the majority of allergenic Calcium-binding proteins (CBPs) and their expression appear to be restricted to pollen (Batanero et al., 1996; Ledesma et al., 1998). The Ole e 8 allergen has also been identified as a Ca2+ binding protein, and together with Ole e 3 with a molecular mass of 20 kDa, it belongs to different subfamilies of CBPs. The allergenic potential of Ole e 3 is different from Ole e 8 as the quantity of the two proteins expressed in the pollen is different (Ole e 3 > Ole e 8) (Esteve et al., 2012). In this study, the material released during the hydration of olive pollen (PMR) is tested for the first time in the cell line PE/CA-PJ15 (PJ15) for the release of the cytosolic Ca2+. The effects of PMR on the Ca2+ signals of PJ15 were also evaluated in the presence of tapsigargin (TG), a known inhibitor of Ca2+ ATPase of the endoplasmic reticulum. The search for PMR active agents in Ca2+ cytosolic was carried out with gel filtration, electrophoresis and proteomic analysis techniques. The analytical techniques used aim to identify and characterize the components of PMR with biological activity at the biomolecular level.
2 Materials and methods
2.1 Materials
Fura 2-AM (Fura-2-pentakis (acetoxymethyl) ester, Triton X-100 (t-octylphenoxypolyethoxyethanol), EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetracetic acid), Trypan blue, and Iscove’s modified Dulbecco medium, HBSS (Hanks’ Balanced Salt solution), PBS, tapsigargin were purchased from Sigma-Aldrich corporation (St. Louis, Missouri, USA). Other reagents (reagent grade) were obtained from common commercial sources.
2.2 Pollen material release (PMR)
Olive pollen (50 mg), collected in the field, has been hydrated for 72 h in 5 mL of PBS. The supernatant of the released pollen material (PMR) was separated from the pollen grains by centrifugation at 1000 × g. PMR was tested on the cytosolic Ca2+ of PJ15 cells, subjected to gel filtration on G-200 and analyzed by HPLC.
2.3 Cell preparations
PE/CA-PJ15 (PJ 15) cells (Franz et al., 2006; Kulasekara et al., 2009) were cultured (37 °C, 5% CO2, 72 h) in Iscove modified Dulbecco medium (IMDM) complemented with 1% L-glutamine, penicillin/streptomycin (100 U/mL each) and 10% inactivated fetal calf serum. The medium was replaced every 72 h and cells rinsed in PBS to remove excess serum. The incubation medium was then discarded and the cells washed with phosphate-buffered saline (PBS). Trypsin (0.05% in 0.02% EDTA, Euroclone) was added to the flasks to detach cells. The action of trypsin was stopped after 3 min by the addition of fetal calf serum (10%). Cells were harvested by centrifugation at 400 g × 5 min and suspended in a volume of medium to obtain a cell concentration of 1 × 106 cells/mL. The proliferation was monitored by counting viable cells using the Trypan blue exclusion method. The suspension aliquots (20 µL) were diluted 1:10 with Trypan blue (2.22 mg/mL of PBS), and the cells were counted using a Burker chamber after standing at room temperature for 5 min. The blue-stained cells were considered non-viable.
2.4 Cytosolic calcium determination
FURA-2 AM (2 µL of a 2 mM solution in DMSO) was added to 1 mL of PJ 15 cells (about 10 × 106 cells) and incubated for 60 min at 37 °C, in the dark. Cells were then harvested by centrifugation at 800 × g × 10 min and finally suspended in HBSS buffer (140 mM NaCl, 5.3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose, 25 mM Hepes, adjusted to pH 7.4) to a final cell concentration of 1 × 106/mL. An aliquot of this suspension (1 mL) was centrifuged at 800 × g × 5 min, cells harvested and suspended in 3 mL of calcium-free HBSS (0.1 mM EGTA). Fluorescence was measured with a Perkin-Elmer LS 50 B spectrophotofluorimeter equipped with a double excitation system (ex. 340 and 380 nm, em. 510 nm). Slit widths were set at 10 nm for excitation and 7.5 nm for emission. Cytosolic calcium concentrations ([Ca2+]c) on PJ15 were calculated as reported by Grynkiewicz (Grynkiewicz et al., 1985). When necessary, PMR, TG, phenolic acids and CaCl2 were tested on the cytosolic calcium of PJ15 cells, as reported in the Sect. 3.
2.5 Gel filtration of PMR
Olive pollen (400 mg) was hydrated in 5 mL PBS for 72 h. The supernatant, obtained by centrifugation at 10,000 g × 10 min, was placed in a column of Sephadex G-200 (30 × 2 cm) eluted in H2O at a flow rate of 0.2 mL/min. The collected 3 mL fractions were analyzed spectrophotometrically for the absorbance at 280 nm, and the Ca2+-chelating activity on PJ15 cells was measured.
2.6 SDS-PAGE of PMR
The fractions, eluted from G-200, with chelating Ca2+-activity, were pooled and precipitated on ice with 20% HClO4. The protein content was determined in the pellet resuspended in 1 mL of PBS. Aliquots of the resuspended pellet (7 and 15 μg proteins) were mixed with sample buffer 2X (0,5 M Tris–HCl pH 6.8, 10% (w/v) SDS, 87% (v/v) glycerol, 0.01% (w/v) bromophenol blue, 5% (v/v) β-mercaptoethanol). Samples were boiled for 5 min and then subjected to Tris–HCl criterion 4–15% (11 cm, Bio-Rad) gel electrophoresis. Electrophoresis was performed at 120 V, and the protein bands were visualized with Coomassie Brilliant Blue G staining (Brunelle & Green, 2014).
2.7 Proteomic analysis
Gel lanes resulting from Coomassie staining of each sample were cut with the multi-blade cutter, and the gel pieces were stored at 4 °C in separate tubes each containing 50μL of 5% acetic acid. Each resulting gel lane from each sample (analyzed in duplicate) was cut into different bands, and the corresponding slices (two for each cell pool) were digested together in the gel (Shevchenko et al., 2007). Extracted peptides were analyzed by LC on an Ultimate 3000 Micro HPLC apparatus (Dionex, Sunnyvale, CA, USA) directly coupled to an LTQ Orbitrap XL hybrid FT mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse-phase chromatography was performed on a Jupiter C18, 5 μm, 150 × 1.0 mm column (Phenomenex, Torrance, CA, USA) and a 95 min run (gradient 1.6–44% acetonitrile in water with 0.1% formic acid over 60 min) at a flow rate of 80 μL/min. Mass spectra were collected by data-dependent scan mode: full scans (MS) at 60,000 of resolution in the Orbitrap analyzer, mass range 300–2000 Da, and data-dependent fragmentation (MS/MS, in the ion trap) of the three most abundant multiple-charged precursor ions, via collision-induced dissociation (CID). Protein identifications were obtained with the embedded ion accounting algorithm (Sequest) of Proteome Discoverer software (version 1.3, Thermo) after searching UniProtKB/Swiss-Prot Protein Knowledgebase (Olea europeaea database). The search parameters were 10 ppm tolerance for precursor ions and 0.8 Da for product ions, one missed cleavage, carbamidomethylation of cysteine as fixed modification, oxidation of methionine and N-terminal asparagine and glutamine deamidation as variable modifications. We filtered data applying a false discovery rate threshold of 0.05; i.e., the expected fraction of incorrect peptide spectrum match in the entire dataset is less than 5%, calculated on a decoy database.
2.8 Phenolic acids (PAs) determination on PMR
Phenolic acids were analyzed using the method developed by Bravi et al. (2021). An UHPLC system consists in a Knauer 3950 autosampler with a 10 μL loop and a quaternary Azura P 6.1 L pump (Knauer, Berlin, Germany) coupled with an Azura MWD 2.1 L height channel UV–VIS detector. The separation was carried out using a Sun Shell C18 column (Chroma Nik Technologies Inc., 50 mm × 2.1 mm ID) at 25 °C and a flow rate of 0.4 mL/min. Mobile phase A was 0.1 M citric acid and 0.2 M sodium hydrogen phosphate (85/15; v/v), and mobile phase B was phase A, methanol and acetonitrile (30/20/50, v/v/v). The pH of mobile phase A was 2.88, and the pH of mobile phase B was adjusted to 3.44 with 85% o-phosphoric acid. The wavelengths of the three channels used for the detection were 254, 278 and 324 nm. The Clarity Chromatography Software for Windows (Data Apex, Prague, Czech Republic) was used for data acquisition and elaboration. The chromatographic separation was achieved using the following elution gradient: mobile phase A 90% (min 0), 100% (min 2), 70% (min 8), 50% (min 10), 20% (min 12), 90% min (12.5). The external standard method was used for the calibration, and the calibration plots were constructed for standard compounds with linearity between 0.5 and 5 μg/mL. A stock solution of 100 ug/mL of a mix of considered phenolic acids in methanol/eluent A (30:70, v:v) was used to prepare working solutions.
2.9 Statistical analysis
Statistical evaluations were performed using the software OriginPro version 2019b, OriginLab Corporation, Northampton, MA, USA. Variance assessments included homogeneity analysis by the Levene’s test and the normality analysis by D’Agostino Pearson test. Significance of differences was assessed by the Fisher’s least significant differences test, after the analysis of variance according to the one-way completely randomized design with four replicates for Figs. 1 and 2, and randomized complete split-plot design with four replicates for the other figures. The results obtained are expressed as mean values ± standard error of the mean (SEM). Differences with p < 0.05 were considered statistically significant.
Ca2+-chelating activity of the material released by olive pollen (PMR) on Ca2+-cytosolic ([Ca2+]c) of PE/CA-PJ15 cells. Data are expressed as means ± SEM from 4 independent tests. Different letters above bars indicate significant differences at P ≤ 0.05, whereas the same letters indicate nonsignificant differences
3 Results
3.1 Ca2+ chelating activity of the material released by olive pollen (PMR)
The material released by olive pollen (PMR) (10 mg/mL in PBS) was tested for the modulation of cytosolic Ca2+ ([Ca2+]c) in PJ15 cells treated with the fluorescent probe FURA-2AM. As a result, the Ca2+-cytosolic was reduced in a dose-dependent manner upon the application of increasing PMR amounts (0.05–1.0 mg). The effects are shown in Fig. 1 and quantified as Δ[Ca2+]c, nM.
3.2 Effects of thapsigargin (TG) and PMR on the cytosolic Ca2+ of PE/CA-PJ15 cells
Tapsigargin (3.4 μM (29), a known inhibitor of Ca2+ ATPase of the endoplasmic reticulum, causes an increase of [Ca2+]c in mammalian cells due to the lack of up-take of the cytosolic ion (Treiman et al., 1998). The subsequent addition of PMR to the incubation (0.05–1.0 mg) medium resulted in a depletion of TG effects of [Ca2+]c in PJ15 cells (Fig. 2).
3.3 PMR purification and characterization
PMR (0.08 mg/mL) loaded on a Sephadex G-200 column, showed absorbance at 280 nm, in the range of fractions 13–17 (Fig. 3A). Ca2+-chelating activity performed in all the eluted fractions was present in the interval 14–18 (Fig. 3B).
3.4 SDS-PAGE and proteomic analysis
3.4.1 SDS-PAGE
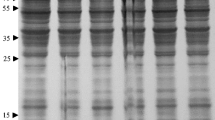
The fractions with Ca2+-chelating activity eluted from sephadex G-200 were pooled, precipitated on ice with HClO4 (20%) and subsequently analyzed by SDS-PAGE. The resulting electrophoretic profile showed a series of protein bands between 5 and 75 KDa (Fig. 4).
3.4.2 Proteomic analysis
The electrophoretic bands comprised between 9 and 37 kDa were cut and submitted to trypsin treatment following the reported protocol (Sect. 2). Proteome Discoverer software analysis performed with the reported parameters (Sect. 2) gave as results the proteins listed in Table 1. All the identified proteins belong to known plant allergens: Major pollen allergen Ole e1(G0YLZ9), Major pollen allergen Ole e1(P19963), Profilin-4 (A4GD58), Profilin-2 (A4GFB7) e Polcalcin Ole e 3 (O81092).
3.5 Determination of phenolic acids in PMR
PMR (25 mg/mL) was analyzed through a UHPLC system. The results obtained underlined eight different phenolic compounds in PMR (five phenolic acids, one alcohol and one flavonoid). The values are reported in Table 2.
The most abundant compounds were tyrosol and quercetin. An interesting amount of p-coumaric acid, sinapic acid and salicylic acid were also found. Other compounds (vanillic and m-coumaric acid) were present only in trace amount (Table 2).
3.6 The activity of phenolic acids in Ca2+ cytosolic of PE/CA-PJ15 cells
Based on the previous investigations (Del Pino et al., 2021), the standard p-coumaric and sinapic acids (5 mg/mL) were chosen, among the hydroxycinnamic acids present in PMR, to verify the chelating effects on Ca2+ cytosolic of PJ-15 cells.
The p-coumaric acid had a Ca2+ chelating activity, while the sinapic acid was a Ca2+ agonist (Fig. 5). Tyrosol and quercetin did not affect cytosolic Ca2+, while salicylic and 2,6-di-hydroxybenzoic acids exhibited a Ca2+ agonist activity (data not shown).
4 Discussion
Olive pollen (Olea europaea L.) is one of the most complex allergenic models known so far (Quiralte et al., 2007). Inhalation of olive pollen (Olea europaea L.) is considered one of the main causes of allergy in Mediterranean countries and some areas of North America (Bousquet et al., 1985; Wheeler, 1992). In some articles, it is reported that allergens influence Ca2+-cytosolic causing an increase by an unknown mechanism (Jairaman et al., 2015; Matsuwaki et al., 2012).
The stimulus for the production of the main inflammatory cytokines: IL-6, IL-8 and TSLP, is attributed to the alteration of Ca2+ cytosolic (Jairaman et al., 2015; Samanta et al., 2014). However, a greater understanding of the molecular mechanisms by which allergens influence Ca2+ signals will allow us to understand their effects on cytokines.
In this work, we tested the biological activity of the material released from olive pollen (PMR) on Ca2+ cytosolic in PE/CA-PJ15 (PJ-15) cells labeled with the FURA 2AM fluorescent probe.
The PJ-15 cell line is obtained from cells of squamous carcinoma of the tongue which represents about 90% of head and neck cancers, and it was used as an experimental model (Franz et al., 2006; Kulasekara et al., 2009). Although the cancer phenotype does not clearly reflect normal physiological events, nevertheless the cells overcome the technical problems associated with the isolation and culture of healthy primary cells and, compared to normal epithelial cells, express similar levels of laminin and cytokeratins (Franz et al., 2006), thus representing, at least in part, the molecular characteristics of the oral cavity, i.e., the most proximal area exposed to pollen.
PMR dose-dependently reduced Ca2+-cytosolic levels in PJ-15 and antagonized the Ca2+-agonist effects of tapsigargin (TG) (Dubois et al., 2013; Treiman et al., 1998). TG is a known inhibitor of endoplasmic reticulum Ca2+ ATPase which caused an increase in the concentration of Ca2+-cytosolic in PJ-15. PMR inhibited the effect of TG probably by blocking the mobilization of Ca2+ from the internal stores.
We believe that the Ca2+-antagonist activity of PMR may play a role in preventing the early activation of pollen. The pollen, in fact, travels considerable distances before reaching the pollination site where germination and pollen tube formation are activated by a Ca2+ gradient in the host's environment.
It is rational to assume that the Ca2+-antagonist effect of PMR can also prevent the allergen-induced increase in Ca2+ cytosolic when olive pollen reaches the upper airways of the oral cavity, thus protecting against the risk of seasonal allergic rhinitis and asthma.
In a subsequent phase of the work, the purification and analytical characterization of the possible agents responsible for the Ca2+-chelating activity in PMR were performed. The size exclusion chromatography (Sephadex G-200), the SDS-PAGE of the active fractions and the proteomic analysis of the single electrophoretic bands highlighted the presence of allergens: major pollen allergen, Ole e1 (G0YLZ9) and (P19963), Profilin-4 (A4GD58), Profilin-2 (A4GFB7) and Polcalcin Ole e 3 (O81092).
HPLC of PMR revealed the presence of eight phenolic acids, including hydroxycinnamic acids (p-coumaric and synapic). Therefore, agents of a protein and non-protein nature can contribute to the marked Ca2+-chelating activity of PMR. Among those of a protein nature, the allergen Ole e 3 (polcalcin) is the one with the Ca2+-antagonist property for the known chelating domains of Ca2+ similar to those of calmodulin and parvalbumin, described by many authors (Quiralte et al., 2007; Batanero et al., 1996; Ledesma et al., 1998; Hauser et al., 2010).
Among the hydroxycinnamic acids identified by HPLC, only p-coumaric, in the unconjugated form, is the one that has Ca2+-antagonist activity, as also emerged in previous works (Del Pino et al., 2021).
The molecules identified in PMR can provide useful information in a therapeutic strategy having as "focus" the Ca2+-antagonist activity.
Whole allergen extracts are used in immunotherapy and have been used for over 100 years, but they are not without side effects34. For this reason, new strategies have led to the use of modified allergens or peptides by exploiting the knowledge on the mechanisms of allergenic responses (Calzada et al., 2018). Protein-based drugs are now designed on the primary structure of the allergen, the length of which is related to the ability to induce tolerance (Calzada et al., 2018). Short peptides derived from the Ole and 1 sequence are used to prevent the proliferative response of olive pollen associated with the production of cytokines IL-10 and IL-35 in allergic patients (Calzada et al., 2019).
The allergen Ole e 3 (polcalcin), identified in PMR, with Ca2+-chelating domains can give indications for the construction of a peptide to be used in olive pollen allergy.
5 Conclusion
The material released by the hydration of olive pollen (PMR) manifests a Ca2+-antagonist activity in the PE/CA-PJ15 (PJ-15) cells and is also capable of inhibiting the effects of tapsigargin.
The Ca2+-antagonist activity of PMR can play a central role in avoiding the activation of pollen grains before pollination, but also in contributing to the homeostasis of Ca2+-cytosolic in the inhalation of allergens.
From the analytical investigations carried out, the Ca2+-antagonist activity of PMR is attributable to the pollen allergen Ole e 3 and to p-coumaric acid. The sequence of Ole e 3 (polcalcin) can provide useful information for the construction of synthetic peptides with Ca2+-antagonistic properties to be used in the treatment of allergen-related diseases.
References
Batanero, E., Villalba, M., Ledesma, A., Puente, X. S., & Rodrıguez, R. (1996). Ole e 3, an olive-tree allergen, belongs to a widespread family of pollen proteins. European Journal of Biochemistry, 241, 772–778. https://doi.org/10.1111/j.1432-1033.1996.00772.x
Berridge, M. J. (2006). Calcium microdomains: Organization and function. Cell Calcium, 40, 405–412. https://doi.org/10.1016/j.ceca.2006.09.002
Bousquet, J., Guerin, B., Hewitt, B., Lim, S., & Michel, B. (1985). Allergy in the Mediterranean area III: Cross reactivity among Oleaceae pollens. Clinical and Experimental Allergy, 13(5), 439–448. https://doi.org/10.1111/j.1365-2222.1985.tb02293.x
Bravi, E., De Francesco, G., Sileoni, V., Perretti, G., Galgano, F., & Marconi, O. (2021). Brewing by-product upcycling potential: Nutritionally valuable compounds and antioxidant activity evaluation. Antioxidants, 10, 65. https://doi.org/10.3390/antiox10020165
Brunelle, J. L., & Green, R. (2014). Chapter thirteen: Coomassie blue staining. Methods Enzymol, 541, 161–167. https://doi.org/10.1016/B978-0-12-420119-4.00013-6
Calzada, D., Baos, S., Cremades, L., & Cardaba, B. (2018). New treatments for allergy: Advances in peptide immunotherapy. Current Medicinal Chemistry, 25(19), 2215–2232. https://doi.org/10.2174/0929867325666171201114353
Calzada, D., Cremades-Jimeno, L., de Pedro, M. A., Baos, S., Rial, M., Sastre, J., Quiralte, J., Florido, F., Lahoz, C., & Cárdaba, B. (2019). Therapeutic potential of peptides from Ole e 1 in olive-pollen allergy. Scientific Reports, 9, 15942. https://doi.org/10.1038/s41598-019-52286-3
Carafoli, E. (2003). The calcium-signalling saga: Tap water and protein crystals. Nature Reviews Molecular Cell Biology, 4(4), 326–332. https://doi.org/10.1038/nrm1073
Del Pino, M. A., Falcinelli, B., D’Amato, R., Businelli, D., Benincasa, P., & Palmerini, C. A. (2021). Extracts of emmer wheatgrass grown with distilled water, salinity or selenium differently affect germination and cytosolic Ca2+ of maize pollen. Agronomy, 11, 633. https://doi.org/10.3390/agronomy11040633
Dhar, H. L. (2001). Role of calcium channel antagonists in allergy and asthma. Indian Journal of Allergy Asthma and Immunology, 15(2), 107–110. https://doi.org/10.1007/BF02713625
Dios, J., de Alché, Castro, A. J., Olmedilla, A., Fernández, M. C., Rodríguez, R., Villalba, M., & Rodríguez-García, M. I. (1999). The major olive pollen allergen (Ole e I) shows both gametophytic and sporophytic expression during anther development, and its synthesis and storage takes place in the RER. Journal of Cell Science, 112(Pt 15), 2501–2509.
Dubois, C., Abeele, F. V., Sehgal, P., Olesen, C., Junker, S., Christensen, S. B., Prevarskaya, N., & Møller, J. V. (2013). Differential effects of thapsigargin analogues on apoptosis of prostate cancer cells Complex regulation by intracellular calcium. FEBS Journal, 280, 5430–5440. https://doi.org/10.1111/febs.12475
Dupont, G., Combettes, L., Bird, G. S., & Putney, J. W. (2011). Calcium oscillations. Cold Spring Harbor Perspectives in Biology, 3, a004226. https://doi.org/10.1101/cshperspect.a004226
Esteve, C., Montealegre, C., Marina, M. L., & García, M. C. (2012). Analysis of olive allergens. Talanta, 92, 1–14. https://doi.org/10.1016/j.talanta.2012.01.01
Franz, M., Hansen, T., Richter, P., Borsi, L., Böhmer, F. D., Hyckel, P., Schleier, P., Katenkamp, D., Zardi, L., Kosmehl, H., & Berndt, A. (2006). Complex formation of the laminin-5 γ2 chain and large unspliced tenascin-C in oral squamous cell carcinoma in vitro and in situ: Implications for sequential modulation of extracellular matrix in the invasive tumor front. Histochemistry and Cell Biology, 126, 125–131. https://doi.org/10.1007/s00418-005-0126-5
Grynkiewicz, G., Poenie, M., & Tsien, R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry, 260, 3440–3450.
Hauser, J., Wallenius, A., Sveshnikova, N., Saarikettu, J., & Grundström, T. (2010). Calmodulin inhibition of E2A stops expression of surrogate light chains of the pre-B-cell receptor and CD19. Molecular Immunology, 47(5), 1031–1038. https://doi.org/10.1016/j.molimm.2009.11.015
Hogan, P. G., Lewis, R. S., & Rao, A. (2010). Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual Review of Immunology, 28, 491–533. https://doi.org/10.1146/annurev.immunol.021908.132550
Huecas, S., Villalba, M., & Rodríguez, R. (2001). Ole e 9, a major olive pollen allergen is a 1,3-beta-glucanase. Isolation, characterization, amino acid sequence, and tissue specificity. Journal of Biological Chemistry, 276(30), 27959–27966. https://doi.org/10.1074/jbc.M103041200
Jairaman, A., Yamashita, M., Schleimer, R. P., & Prakriya, M. (2015). Store-operated Ca2+ release-activated Ca2+ channels regulate PAR2- activated Ca2+ signaling and cytokine production in airway epithelial cells. The Journal of Immunology, 195, 2122–2133. https://doi.org/10.4049/jimmunol.1500396
Kudla, J., Becker, D., Grill, E., Hedrich, R., Hippler, M., Kummer, U., Parniske, M., Romeis, T., & Schumacher, K. (2018). Advances and current challenges in calcium signaling. New Phytologist, 218, 414–431. https://doi.org/10.1111/nph.14966
Kulasekara, K. K., Lukandu, O. M., Neppelberg, E., Vintermyr, O. K., Johannessen, A. C., & Costea, D. E. (2009). Cancer progression is associated with increased expression of basement membrane proteins in three-dimensional in vitro models of human oral cancer. Archives of Oral Biology, 54(10), 924–931. https://doi.org/10.1016/j.archoralbio.2009.07.004
Ledesma, A., Villalba, M., Batanero, E., & Rodìguez, R. (1998). Molecular cloning and expression of active Ole e 3, a major allergen from olive-tree pollen and member of a novel family of Ca2+-binding proteins (polcalcins) involved in allergy. European Journal of Biochemistry, 258, 454–459. https://doi.org/10.1046/j.1432-1327.1998.2580454.x
Matsuwaki, Y., Wada, K., White, T., Moriyama, H., & Kita, H. (2012). Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. International Archives of Allergy and Immunology, 158(1), 19–29. https://doi.org/10.1159/000337756
Napoli, A., Aiello, D., Di Donna, L., Moschidis, P., & Sindona, G. (2008). Vegetable proteomics: The detection of Ole e 1 isoallergens by peptide matching of MALDI MS/MS spectra of underivatized and dansylated glycopeptides. Journal of Proteome Research, 7, 2723–2732. https://doi.org/10.1021/pr700855u
Ordenes, V. R., Reyes, F. C., Wolff, D., & Orellana, A. (2002). A thapsigargin sensitive Ca2+ pump is present in the pea Golgi apparatus membrane. Plant Physiology, 129, 1820–1828. https://doi.org/10.1104/pp.002055
Ortiz-Ramírez, C., Michard, E., Simon, A. A., Damineli, D. S. C., Hernández-Coronado, M., Becker, J. D., & Feijó, J. A. (2017). Glutamate receptor-like channels are essential for chemotaxis and reproduction in mosses. Nature, 549, 91–95. https://doi.org/10.1038/nature23478
Prakriya, M., & Lewis, R. S. (2015). Store-operated calcium channels. Physiological Reviews, 95, 1383–1436. https://doi.org/10.1152/physrev.00020.2014
Quiralte, J., Palacios, L., Rodríguez, R., Cárdaba, B., Arias-de-Saavedra, J. M., Villalba, M., Florido, J. F., & Lahoz, C. (2007). Modelling diseases: The allergens of Olea europaea pollen. The Journal of Investigational Allergology and Clinical Immunology, 17(1), 24–30.
Samanta, K., Bakowski, D., & Parekh, A. B. (2014). Key role for store-operated Ca2+ channels in activating gene expression in human airway bronchial epithelial cells. PLoS ONE, 9, e105586. https://doi.org/10.1371/journal.pone.0105586
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V., & Mann, M. (2007). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols, 1, 2856–2860. https://doi.org/10.1038/nprot.2006.468
Thor, K. (2019). Calcium-nutrient and messenger. Frontiers in Plant Science, 10, 440–446. https://doi.org/10.3389/fpls.2019.00440
Treiman, M., Caspersen, C., & Christensen, S. B. (1998). A tool coming of age: Thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends in Pharmacological Sciences, 19(4), 131–135. https://doi.org/10.1016/s0165-6147(98)01184-5
Urbina, D. C., Herman, S., & Meisel, L. A. (2006). The Ca2+ pump inhibitor, thapsigargin, inhibits root gravitropism in Arabidopsis thaliana. Biological Research, 39, 289–296. https://doi.org/10.4067/S0716-97602006000200011
Wheeler, A. W. (1992). Hypersensitivity to the allergens of the pollen from the olive tree (Olea europaea). Clinical and Experimental Allergy, 22(12), 1052–1057. https://doi.org/10.1111/j.1365-2222.1992.tb00129.x
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vitali, A., Del Pino, A.M., Regni, L. et al. The calcium-antagonist activity of the material released by olive pollen (PMR), tested on Ca2+-cytosolic of PE/CA-PJ15 cells. Aerobiologia 38, 581–589 (2022). https://doi.org/10.1007/s10453-022-09768-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-022-09768-1