Abstract
The ‘Wieliczka’ Salt Mine with specific stable, microclimatic conditions is a unique place, where pulmonary and allergy-related diseases are treated. The aim of this study was to determine the occurrence of biological particles in the air of the ‘Wieliczka’ Salt Mine in relation to the location and time. Twenty measurements were taken quarterly in 2012–2016, in four salt chambers. The volumetric and impact methods were used to measure microbiological contamination, while the mite and cat allergens were measured using FEIA assay. The statistically significant differences among the study sites and time of measurements, treated as the following seasons, for spores and bacteria were found. Pollen grains were observed in the underground chambers rarely and in significantly lower quantities than outdoors. No statistically significant differences between the content of Der p1 and Fel d1 allergens among the study locations, seasons and patient presence/absence were found. The qualitative content of micro-organisms in the air of salt chambers seems to be related to the biological material carrying in by patients and staff.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Micro-organisms that exist indoors are a mixture of ambient particles that have been infiltrated or carried from outdoors and those originating from known or unidentified indoor sources. Outdoor bioaerosols refer most often to fungal spores and bacteria aerosols, but indoor particles can be also released from other sources, like house dust mites, insects, cockroaches, pets, as well as skin cells from humans and pets (Morawska et al. 2013). Many human activities, like housework, exercises, dealing with pets, etc., can affect bioaerosol.
There are many places, where more or less evident microbiological aerosol is observed. To the most infected places belong: laboratories, air-conditioned rooms, work-specific places with high humidity, crowded, dusty places (metro, drug stories, offices, bakeries), green houses, farms, vineyards (Błaszczyk 2010). In some places, e.g. medical facilities, like theatres, blood stations, clinical pharmacies, because of nosocomial infections, microbiological purity is required.
On the other side, in some places the specific air composition is difficult to estimate. As an example, the underground salt mine chambers are presented in the current paper. They are supposed to be free from any biological particles, because of no indoor sources of their release, a high salt saturation and high-quality ventilation system. The underground inactive mining chambers are commonly used as a place of climatic treatment of persistent respiratory tract diseases (asthma, chronic obstructive pulmonary disease, recurrent infections of upper and lower respiratory tracts) and allergic diseases (allergic rhinitis) (Ponikowska and Ferson 2009; Ponikowska and Kochański 2017).
The treatment is based on the subterraneotherapy method, elaborated by Skulimowski in the ‘Wieliczka’ Salt Mine (Poland) and Spannagel in Ennepetal (Klutterthöhle cave, Germany) in the 1950s (Pąchalska et al. 2002). This innovative method consists of the influence of different physical, chemical, biological stimulative factors occurring in the specific underground environment on the human body. Microclimate changes, caused by patients entering and departing the chambers, influence the effects of treatment strongly. Non-negligible is a stimulogenic effect of daily going down to the depth of 135 m, forcing the body to adapt itself to new environmental conditions twice a day (Obtułowicz et al. 1999; Ponikowska and Ferson 2009; Kalinowska et al. 2013). In the time of the study, the subterraneotherapy has been applied in two Polish salt mines, in Wieliczka and Bochnia (Obtułowicz 2002; Obtułowicz et al. 2013).
The ‘Wieliczka’ Salt Mine chambers are characterized by low temperature (13–14.5 °C in the Health Resort, 14–16 °C in the Tourist Route), high relative humidity (60–75%) and salt aerosol saturation (2.7–8.1 mg/m3), high ionization (1200–4700 aeroions/cm3), almost lack of allergens and particulate matter. The air is free from harmful radiation, external stimulations like electromagnetic, audio waves and toxic agents (Olechnowicz-Bobrowska and Wojkowski 2004; Czajka et al. 2006; Błażejczyk and Wiszniewski 2011; Kostrzon et al. 2015; Wiszniewski 2015). However, the permanent therapy requires microbiological control of chambers, which have been monitored occasionally in the past to estimate the qualitative and quantitative composition of biological components. The first measurement taken in the Bochnia Salt Mine in 2002 (Ważyn Chamber, 250 m below the ground level) and ‘Wieliczka’ Salt Mine (three chambers on levels III (135 m below the ground level) and V (211 m below the ground level) revealed the unique microbiological purity of air (< 5 × 102 bacteria and spores per 1 m3) meeting the standards for treatment rooms. Among bacteria, Micrococcus sp., Bacillus sp. and Corynebacterium sp. dominated, while fungal spores were represented by two genera, Alternaria and Cladosporium. At the time of measurement (June–August), only single pollen grains were recorded (Obtułowicz 2002). The comparative studies in the ‘Wieliczka’ Salt Mine and in the Bochnia Salt Mine were performed by Frączek et al. (2013) in 2008 (January–December) and by Gębarowska et al. (2018), twice in December 2015 (simultaneous measurements in ‘Wieliczka’ Salt Mine and in the ‘Polkowice-Sieroszowice’ Salt Mine).
The aim of the current study was to determine the occurrence of biological particles in the air of the ‘Wieliczka’ Salt Mine in relation to the chamber location, time of the year and the presence or absence of patients. The authors would like to answer three research questions in relation to: fungal spores (separately for each study method), bacteria and allergen occurrence:
-
Does the level of biological particles differ among different salt chambers?
-
Does the level of biological particles measured before and during the patient stay differ in relation to study conditions?
-
Is the level of biological particle variable among the thermal seasons (time of measurements)?
Although the bacteria and fungal spore concentration has been measured before in ‘Wieliczka’ Salt Mine (Obtułowicz et al. 2002) and in Bochnia Salt Mine (Frączek et al. 2013), the novelty of a current study was to analyse the other biological components, existing in the underground, like natural allergens (originated from animals: cuts and house dust mites) and pollen grains. Pollen occurrence in the salt chambers and outdoor has been compared, to estimate how significant is the difference between both environments. Natural allergens and pollen grains have not been detected previously in any underground environments.
2 Materials and methods
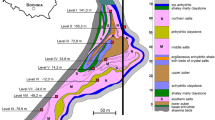
The study was performed in the ‘Wieliczka’ Salt Mine Health Resort, located in Wieliczka, the small city in the south-eastern Poland (Fig. 1). The area of the Health Resort is ventilated by a system of two connected shafts: Regis (inspiratory) and Wilson (exhaust). Two fans working permanently on the surface force the air flow into the underground chambers. The air cleaned by the air conditioning system, off the solid pollution, moves from Regis shaft to the Health Resort on the third level. It is stated that the distance from the surface to the Health Resort is about 700–800 m. On average, 180 m3 of air per minute is transferred through the Health Resort chambers.
The measurements were taken quarterly, in 2012–2016, in six places of four salt chambers of the ‘Wieliczka’ Salt Mine Health Resort. Brief information about these chambers is listed below.
Wessel Lake Chamber is located about 200 m out of the Regis shaft (70 m × 20 m × 10 m in size), including 60 rehabilitation places per one stay. Right in the middle of the chamber, there is a salt lake of a depth equal to 0.6–0.8 m. The measurements were taken in two places within this chamber. The Eastern Maintain Stable Chamber (29 m × 14 m × 3.9 m) is located about 200 m away from the Wessel Lake Chamber. In this chamber, there are two separated rooms: Stable 1 (Graduation Tower) with a device for spraying salt aerosol and Stable 2 (Bedroom), which offers an accommodation for 28 patients, in two-bed ‘stalls’.
The Dragon Chamber (21 m × 9 m × 5 m) is located closely to the Eastern Maintain Stable Chamber and it was exploited in the salt-spelled board. The chamber has the shape of a cuboid and is designed to conduct classes in the field of kinesiotherapy. There is a gymnasium and an inhalatorium located on a separate mezzanine.
The Boczkowski Chamber (21 m × 9 m × 5 m) located near the Dragon Chamber is also leaching chamber exploited in the spiza salt board. This chamber is intended for the rest of the patients and is divided into higher and lower parts. In both parts, there are tables and chairs.
Three groups of biological particles (fungal spores, bacteria and airborne allergens) were measured. Pollen and fungal spore concentrations were collected using a volumetric method and the PARTRAP FA personal sampler (Coppa Biela, Italy) with the air flow of 10 l/min and the air download time 3 h. Identification of pollen grains and fungal spores was carried out microscopically, and the particle concentration was converted to 1 m3 of air/24 h. Fungal spores and pollen grains were identified down to genera, except for Poaceae family.
The fungal spore and bacteria colonies were analysed applying microbiological procedures (impact method), using the Microflow alfa 90 (Aquaria) air sampler, working with 90-mm Petri dishes. A plate was incubated at standard conditions (30 °C, O2) immediately after removing from the impactor. Growth was traced up to 120 h on Tryptic soy agar. Emerging colonies both bacteria and fungi were counted and the final result was converted to CFU/m3. Cultured colonies were isolated on respective selective media, i.e. Columbia agar (suspected Gram-positives), MacConkey agar (suspected Gram-negative rods) and Sabouraud agar (suspected fungi). Micro-organisms were identified using all manual (clumping factor, coagulase detection, Gram-stained preparations), semi-automatic (Nefermtest and Enterotest, Erba-Lachema) and automatic methods (Vitek Systems, Biomerieux). The house dust mite and cat allergens were counted using fluoroimmunoenzymatic assay (FEIA), in dust samples, collected from the ground in salt chambers into the special plastic containers and weighed before analysis. The allergen concentration was given in ng/g of dust using a CITEQ set (Groningen, the Netherlands).
Twenty underground measurement series were arranged. During each measurement, the following samples were collected: fungal spore samples (eight microbiological/impact and six volumetric samples), bacteria (eight impact samples), pollen grains (six volumetric samples) and house dust mites and cat allergens (eight dust samples per allergens source). All measurements were taken before and during the patient stay in salt chambers.
The concentrations of fungal spores and pollen grains obtained using the volumetric method were compared to the particle concentrations in Krakow (15 km distant), measured by the stationary sampler (Lanzoni Ltd., Italy), operating automatically with the airflow similar to the personal samplers. The slides preparation and counting method were the same, according to the International Association for Aerobiology (IAA) requirements (Galan et al. 2014). The obtained concentrations were given in 1 m3 of air/24 h.
The results were analysed according to standard statistical methods. In the first stage, the normality of the distribution was tested using the Shapiro–Wilk test. The Mann–Whitney U test was applied to compare the samples obtained before and during the patient stay. The Kruskal–Wallis ANOVA was used in the case of more than two independent samples (comparison among the chambers and among the seasons). In the detailed analyses, five types of bacteria and three types of fungal spores obtained by both the volumetric and impact methods were discussed (the percentage above 5% of the total amount).
When the statistically significant relations were found among more than two independent samples, the appropriate post hoc tests were applied in the detailed analyses (Dunn’s nonparametric comparison for post hoc KW).
In the case of data analyses among the seasons, the data obtained during eight measurements were grouped into four seasons: winter (1) analyses performed in December–February; spring (2) analyses performed in March–May; summer (3) analyses performed in June–August; and autumn (4) analyses performed in September–November.
The content of pollen grains and Alternaria sp., Cladosporium sp. spores was compared between salt chambers and outdoor air graphically in relation to four seasons and against the background of the daily pollen and fungal spore concentrations in Krakow. For all of these statistical tests, the statistical significance was accepted at the level of α ≤ 0.05. Statistical analysis was performed using the Statistica program version 10.0 (StatSoft, Inc. 1984-2010).
3 Results
3.1 The content of fungal spores and bacteria in the salt mine aerosol
The highest ranges of bioaerosol concentration were found in the case of bacteria. The mean values fluctuated from 143 CFU/m3 in May 2013 to 3787 CFU/m3 in February 2013. The dispersion of spore concentrations determined volumetric method was the lowest (Fig. 2). In the air samples, only ten genera of fungal spores were found, out of which four genera were identified using both methods (Alternaria sp., Cladosporium sp., Aspergillus sp., Penicillium sp.). Cladosporium sp. spores dominated in the volumetric method (almost 70%), while Penicillium sp. in the impact method (almost 45%) (Fig. 3). A wide range of bacteria types was obtained, but only two types Micrococcus sp. and coagulase-negative Staphylococcus prevailed definitely (Fig. 3).
Percentage of particular fungal spores (measured by the volumetric and impact methods) and bacteria (measured by the impact method) in the whole micro-organism spectrum detected in the ‘Wieliczka’ Salt Mine during the study. The taxa with a percentage above 1% were presented, and the other ones were included into ‘other taxa’
The highest spore concentrations, measured by the volumetric method, were obtained in the samples collected in autumn (76.37 spores/m3 on average), measured by the impact method in winter (506.77 CFU/m3 on average), while in the case of bacteria, the highest values were obtained also in winter (1886.87 CFU/m3 on average) (Fig. 4). In the case of fungal spores, the daily highest concentration was achieved in September 2013 (169 spores/m3) using the volumetric method, while in case of the impact method, it was achieved in December 2014 (983 CFU/m3). Spore concentrations measured by the impact method in the study period showed more or less clear ciclicity of higher and lower values (Fig. 4). In the case of other contaminants, their concentration did not show the tendency to higher or lower values during the entire study period.
3.2 The level of biological particles measured in different salt chambers
In the case of some micro-organism types, statistically significant differences among the study sites were found (Table 1). It refers to: Alternaria sp. and Cladosporium sp. in the volumetric method, Penicillium sp. and Geotrichum sp. in the impact method, Micrococcus sp., Bacillus sp. and Corynephorm bacteria types. Post hoc tests indicated the most frequent differences between two studied places in the Wessel Lake Chamber and also between the Wessel Lake Chamber and Eastern Mountains’ Stable 2.
3.3 The level of biological particles before and during the patient stay
The Mann–Whitney U (z) test did not indicate any statistically significant differences between fungal spore and bacteria concentrations measured before and during the patient stay in the underground chambers (Table 2).
3.4 The level of biological particles among the seasons
Statistically significant differences were found among time of measurements, treated as the following seasons, for both fungal spores and bacteria (Table 3). The fungal spore content differed in both methods in the case of Alternaria sp. and Cladosporium sp. between spring and summer, and summer and winter. Moreover, in the impact method the spore concentrations of these taxa differed between summer and autumn, while in the volumetric method they differed between autumn and winter in the case of Cladosporium sp. No statistically significant differences between Aspergillus sp. and Penicillium sp. spore concentrations measured by the volumetric method were found. For almost all analysed bacteria occurring in higher numbers, the differences between the content in spring and autumn were observed, except for Bacillus sp. It was observed that the content of the most frequent bacteria type Microcossus sp. varied significantly also between spring–summer and summer–winter.
3.5 Comparison of fungal spore content between the ‘Wieliczka’ Salt Mine and Krakow
Cladosporium sp. and Alternaria sp. spore concentrations obtained by the volumetric method in the ‘Wieliczka’ Salt Mine in Krakow on the same days differed (among others, but the differences were statistically significant in the case of Cladosporium sp. spores only (p = 0.014). The concentrations of Cladosporium sp. in Wieliczka reached up to 114 spores/m3, while in Krakow they were 1971 spores/m3(data from Department of Clinical and Environmental Allergology, Jagiellonian University Medical College) (Fig. 5). In the case of Alternaria sp., the highest values detected in the ‘Wieliczka’ Salt Mine were 29 spores/m3, whereas in Krakow they were 79 spores/m3.
3.6 Comparison between pollen content in the ‘Wieliczka’ Salt Mine and in Krakow
Pollen grains were observed in the underground chambers rarely and in lower quantities than outdoors (data from Department of Clinical and Environmental Allergology, Jagiellonian University Medical College). In the case of data obtained during the ‘spring’ measurements, the differences were statistically significant (p = 0.000) (Fig. 6).
Among them, pollen of three taxa (Pinus, Poaceae, Urtica) prevailed. A single ‘spring’ observation was arranged in April, during Betula pollen season. In this case, on 12 April 2012 no Betula pollen was found in the ‘Wieliczka’ Salt Mine, in contrast to the high concentration of 567 pollen/m3 outdoors in Krakow (data from Department of Clinical and Environmental Allergology, Jagiellonian University Medical College). Because of a low number of samples, no statistical tests were used. In the case of Pinus, the difference between mean concentrations in both places was the highest (2 pollen/m3 in Wieliczka vs. 316 pollen/m3 in Krakow) (Fig. 6). For most of the taxa, only the single grains were captured (Juglans, Salix, Populus, Ulmus, Fagus, Carpinus, Fraxinus, Juniperus, Picea, Tilia, Quercus, Plantago, Asteraceae, Rosaceae, Artemisia, Chenopodiaceae and Rumex).
Comparing the taxa, of which pollen grains were observed in the ‘Wieliczka’ Salt Mine, two situations were distinguished: (1) in autumn and winter, when the atmospheric air is almost free from pollen grains, they were observed in the air samples in salt chambers; (2) in spring and summer the pollen grains of taxa occurring in the atmospheric air were also observed in the underground air, especially in summer. In samples collected in June–August, pollen of only 33.3% of taxa found in the salt chambers did not normally occur in the air during summer (Fig. 7).
3.7 House dust mite and cat allergens
The occurrence of house dust mite and cat allergens in the air of salt chambers was extremely low, measured in ng/g of dust, and the greater range between the results was found in spring in the case of Fel d1 allergens and in autumn in the case of Der p1 allergens (Fig. 8). No statistically significant differences between the content of Fel d1 and Der p1 allergens in both measurement conditions (before and during patient stay) (N = 32; Z = 0.2215; p = 0.8247), and among study places (N = 16; p = 0.3295) and time of measurements (four seasons) (N = 64; p = 0.1834) were found.
The very low Der p1 concentration in comparison with the threshold value (2 µg/1 g of dust) (Lau et al. 1989; Kuehr et al. 1994), below which is the maximum level for the primary prevention of sensitization in atopic children and young adults, was independent from the study conditions. Moreover, the mean concentration of Der p1 per sample fluctuated from 1.15 ng/1 g of dust up to 1.88 ng/1 g of dust, what makes the concentration about 1000 times lower than the threshold values for indoor air. In the case of Fel d1, the values ranged from 0.7 to 6.9 ng/1 g of dust, up to 100 times lower than 8 μg/1 g of dust, associated with a rapid onset of allergic symptoms in patients (Woodfolk et al. 1993).
4 Discussion
The content of bioaerosols in outdoor and indoor environments is more or less variable. About 1200 species of bacteria and more than 40,000 fungi species exist outdoors. Among them, the filamentous fungi dominate (70% of the total fungal spore content). To the most frequent genera belong: Cladosporium sp., Alternaria sp., Penicillium sp., Aspergillus sp., Botrytis sp., Ulocladium sp., Fusarium sp. (D’Amato et al. 2007). The concentration of bacteria fluctuates from 18 to 20% with the significant share of Micrococcus sp., Achromobacter sp., Bacillus sp.
On the other hand, the composition of indoor aerosols is more stable and originates mainly from the indoor sources, like air conditioning, ventilation systems, human body and other organic matter, less from outdoor air. Saprotrophic microflora dominates, including bacteria (Micrococcus sp., Staphylococcus sp., Bacillus sp. (68–80% of the total content), viruses and fungal spores with dominating Alternaria sp., Rhisopus sp., Aspergillus sp. and Penicilium sp. (D’Amato et al. 2007).
There are only few papers describing the problem of microbiological contamination in salt caves, because of lack of standards for the underground chambers (Bis et al. 2004; Grzyb et al. 2004; Chervinskaya 2007; Frączek et al. 2013. Moreover, the group of experts concluded that there is still no scientific evidence for establishing the limit values or guidelines for particulate matter concentrations in the non-industrial indoor environment (Schneider et al. 2003). In 2007, in Krakow, microbiological analyses in the underground of the Main Market Square were performed. The high concentration of fungal spores and bacteria in the air, on the walls and on the ground was found. In the most of the chambers, the Polish Norms (1989) were exceeded and the places were defined as ‘highly polluted’. Among bacteria, the species of Bacillus, Micrococcus, Sarcina, Streptococcus dominated, while among fungal spores: Aspergillus (incl. A. fumigatus and A. flavus), Penicillium sp., Cladosporium sp., Fusarium sp. and Trichoderma sp. prevailed. The authors concluded that the microbiological flora developed in this specific place after oxygen supply to the mediaeval layers (personal communication).
The fungal spores found in the samples belong to airborne spores, e.g. Alternaria sp., Cladosporium sp., Penicillium sp. and Aspergillus sp., prevailed in the samples. The similar results were published by Frączek et al. (2013), who used the impact method in the Bochnia Salt Mine Health Resort. The authors reported that the concentration of fungal spores indoors was significantly lower than outdoors (88 CFU/m3 vs. 538 CFU/m3). In our study, two genera of fungi measured by the volumetric method were compared. In both studies, the limit of 5000 CFU/m3 was not exceeded (Górny 2004). Among the recognized spores, known as allergic factors, no type was observed in the concentration responsible for clinical symptoms. In Poland, the highest concentrations of Alternaria sp. spores are observed in July and August, reaching the level of several hundred spores in 1 m3 of air. The concentration of Alternaria alternata spores with the value of 80 in 1 m3 of air is considered as threshold concentration responsible for the occurrence of disease symptoms in people allergic to this species in Poland (Samoliński et al. 2010).
The similar genera of spores and bacteria exist in coal mine chambers, as reported by Rdzanek et al. (2015). They sampled at six locations in a coal mine in Poland at about 500–600 m below ground level and found 11 fungal species, with Penicillium meleagrinum and P. notatum as the most common. Similarly to our results, Micrococcus spp. released to be the most common among the collected bacteria. The study in Niedźwiedzia Cave in Poland indicated that in the air samples, 123 and 214 CFU fungi per 1 m3 were noted with domination of Cladosporium herbarum and Rhizopus stolonifer (Ogórek et al. 2014). The contamination of caves differs in micro-organism profile. Example Dupont et al. (2007) reported the colonization of Lascaux Cave by a Fusarium solani species.
The difficulties occurring among study points seem to be related to the specific features of a given cave. Even in one chamber (Wessel Lake) the concentration differed, probably because one study site was located closely to the patient exercise place and the second was far away from the patient group. The observed increase in the fungal spore concentrations in the Dragon Chamber could have been caused by the higher patient activity in this chamber equipped with the wooden floor. The detailed analyses indicated that two genera (Penicillium sp. and Geotrichum sp.) could have caused the increase in fungal spores. Both of them are common in a human environment.
The higher microbiological contamination during the patient presence in the chamber, clearly reported by Frączek et al. (2013), was not confirmed in our study, probably because of the differences in research methods. It is hard to take measurements before the real patient stay, because they are present in salt chambers of the Health Resort permanently.
The highest concentration of fungal spores was observed in summer (July–August), except for Penicillium/Aspergillus sp. spores, which were observed in winter. It is clearly showed that the fungal spore occurrence in the caves coincided with the outdoor exposure. In Krakow, concentration of fungal spores is estimated against the background of current outdoor exposure and previous papers by Myszkowska et al. (2002), Stępalska and Wołek (2005) and Mędrela-Kuder (2008a, b). The results confirm that the spores could be carried by patients and staff during the whole year. On the other side, the occurrence of fungal spores could be also related to the inside sources, like wooden floors, wooden stamps, supports.
Isolated bacteria are typical commensal micro-organisms, for which the surface of the skin and mucous membranes of the airways is a natural reservoir and living environment. They are ubiquitous in the open air as well as inside the utility rooms, especially on numerous solid surfaces of furniture, utensils, floors and wooden elements. The qualitative and quantitative composition of the bred flora indicates its good adaptation to an inanimate environment, very poor in nutrients, rather dry, with the high oxygen content. Above 98% of micro-organisms are distinguished by their ability to grow in the presence of 7.5% NaCl (e.g. Micrococcus, Bacillus, Staphylococcus). Sodium chloride is a known selection agent, particularly strongly inhibiting the growth of Gram-negative flora, including Enterobacteriaceae. It is worth emphasizing that Gram-negative flora in the air of the ‘Wieliczka’ Salt Chambers was not found at all. The presence of enterococci, which is a component of the faecal flora, has been also found, with a relative tolerance of sodium chloride. Generally, bacteria present in caves often survive by modifying their metabolic pathway or other mechanism. During the study, statistically significant differences in bacterial counts were observed depending on the salt chamber where the collection was made. This may be due to differences in architecture and the type of organized classes in a given space. None of the chambers has shown that the number of bacteria depend on the presence of patients. Despite the observed increase in bacterial concentrations, no significant increase in the diversity of bacterial flora in the chambers nor the presence of typical pathogenic organisms for patients that could be re-incubated (noted) in the premises was observed. The presence of Staphylococcus aureus MSSA was observed only once (collected on 16-09-2015, during patient stay). The number of bacteria depends on time of the year in which the test was performed, but there are no standards to which these values can be compared (Górny 2004).
The pollen grains, of a greater size than the fungal spores, are more difficult to transport from the ambient air, so their occurrence is sporadic. These particles more often stand over in the caves. Lee et al. (2006) reported that in spring only a small fraction of pollen penetrated from outdoor to indoor environment in the buildings because of their size. The presence of pollen in air samples in salt chambers indicates that it is delivered to the chambers by patients and staff (clothes, hair) and about biological material, as evidenced by the presence of pollen that does not occur in analogous periods in the outdoor air. The pollen content is so small that it does not pose a threat to allergic people, although Holmquist and Vesterberg (1999) reported that in the studied school rooms, the substantial number of birch and grass pollen allergens penetrated easily from outdoor as particles of smaller than pollen grains diameter.
The system of ventilation responsible for incoming air filtering makes the air breathing by patients more and more clean, what was confirmed by the analyses of particulate matter (PM), in three chambers of the ‘Wieliczka’ Salt Mine in July 2015. PM concentration outdoor did not exceed 30 µg/m3 and average concentration of respirable PM (PM4) was 7 µg/m3 (Rogula-Kozłowska et al. 2016).
Considering this fact, it is not surprising that so tiny number of house dust mites and cat allergens was also found in the quarterly monitoring. Many studies showed the pet allergens level at homes, cars, dwelling (Niesler et al. 2016) in µg/g of dust, regardless of whether the animals stay in the rooms or not. As a result, the occurrence of animal allergens in salt chambers, delivered from outdoors on clothes and hair (Karlsson and Renström 2005), should not be considered as a risk factor for allergic disease. In conclusion, the low threat of disease encourages patients to a stay in the salt mine, in isolation from other environmental factors, like pollution increasing, e.g. asthma symptoms (Erwin et al. 2005). Moreover, the low concentration of mite allergens indicates unfavourable conditions for the development of these arachnids in salt chambers (Calderon et al. 2015).
5 Conclusions
Concentrations of fungal spores determined by the volumetric and impact methods indicated the existence of fungal spores in the salt chambers.
The concentration of all studied contaminants does not clearly depend on the patient stay in salt chambers. The concentration of fungal spores and bacteria differ among the seasons.
The presence of pollen in air samples in salt chambers is definitely lower than on the surface and indicates the presence of biological material, delivered to the chambers by patients and staff, not posing a threat to people.
The indoor bioaerosol content seems to be typical for public facilities and is more stable than in the outdoor air.
Change history
24 April 2019
In the original publication of the article, the authors given and family names were swapped in the author group. The correct author group is given in this correction.
References
Bis, H., Grzyb, J., Barabasz, W., & Frączek, K. (2004). Prevalence of fungi: Micromycetes in the health resort chambers in Bochnia and Wieliczka salt mines. Acta Agraria et Silvestria, Series Agraria,42, 29–39.
Błaszczyk, M. (2010). Mikrobiologia powietrza./Microbiology of air. In: M. Błaszczyk (Ed.), Mikrobiologia środowisk (pp. 357–358). Wydawnictwo Naukowe PWN.
Błażejczyk, K., & Wiszniewski, A. (2011). Operat uzdrowiskowy dla sanatorium uzdrowiskowego w urządzonych podziemnych wyrobiskach górniczych Kopalni Soli W Wieliczce./A health spa resort in decorated underground mining excavations of the Wieliczka Salt Mine. Instytut Geografii i Przestrzennego Zagospodarowania im. Stanisława Leszczyckiego PAN (IGiPZ PAN).
Calderon, M. A., Linneberg, A., Kleine-Tebbe, J., De Blay, F., Fernandez, Hernandez, de Rojas, D., et al. (2015). Respiratory allergy caused by house dust mites: What do we really know? Journal of Allergy and Clinical Immunology,36, 38–48.
Chervinskaya, A. (2007). Halotherapy in controlled salt chamber microclimate for recovering medicine. Balneologia Polska,2, 133–141.
Czajka, K., Sziwa, D., Drobnik, M., & Latour, T. (2006). Porównanie właściwości mikroklimatu i aerozoli w wyrobiskach kopalnianych i naziemnych grotach solnych./Comparison of properties of microclimate and aerosols in mine excavations and above-ground salt caves. Acta Balneologica,3, 176–181.
D’Amato, D., Cecchi, L., Bonini, S., Nunes, C., Annesi-Maesano, I., & Behrendt, H. (2007). Allergenic pollen and pollen allergy in Europe,62(9), 976–990.
Dupont, J., Jacquet, C., Dennetière, B., Lacoste, S., Bousta, F., et al. (2007). Invasion of the French Paleolithic painted cave of Lascaux by members of the Fusarium solani species complex. Mycologia,99, 526–533.
Erwin, E. A., Custis, N., Ronmark, E., Wickens, K., Sporik, R., Woodfolk, J. A., et al. (2005). Asthma and indoor air; contrasts in the dose response to cut and dust-mite. Indoor Air,15(10), 33–39.
Frączek, K., Górny, R., & Ropek, D. (2013). Bioaerosols os subterraneotherapy chambers at salt mine health resort. Aerobiologia,29, 481–493.
Galán, C., Smith, M., Thibaudon, M., Frenguelli, G., Oteros, J., Gehring, R., et al. (2014). Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia,30, 385–395.
Gębarowska, E., Pusz, W., Kucińska, J., & Kita, W. (2018). Comparative analysis of airborne bacteria and fungi in two salt mines in Poland. Aerobiologia. https://doi.org/10.1007/s10453-017-9502-6.
Górny, R. (2004). Biologiczne czynniki szkodliwie: normy zalecenia i propozycje wartości dopuszczalnych./Biological harmful factors: standards, recommendations and proposals for limit values. Podstawy i metody oceny środowiska pracy,3(41), 17–39.
Grzyb, J., Bis, H., Barabasz, W., Frączek, K., & Chmiel, M. J. (2004). Studies upon bacteria occurrence in air of Bochnia and Wieliczka Salt Mine chambers. Acta Agraria et Silvestria, Series Agraria,42, 163–176.
Holmquist, L., & Vesterberg, O. (1999). Quatification of birch and grass pollen allergens in indoor air. Indoor Air,9, 85–91.
Kalinowska, A. K., Mirska, A., & Dmitruk, E. (2013). Subterraneoterapia jako swoista metoda klimatoterapii./Subterraneotherapy as a specific method of climate therapy. Acta Balneologica,1(131), 55–58.
Karlsson, A. S., & Renström, A. (2005). Human hair a is a potential source of cat allergen contamination of ambient air. Allergy,60, 961–964.
Kostrzon, M., Czarnobilski, K., & Badyda, A. (2015). Climate characteristics of salt chambers used for therapeutic purposes in the ‘Wieliczka’ Salt Mine. Acta Balneologica,57(1), 52–58.
Kuehr, J., Frischer, T. R., Meinert, R., Barth, R., Forster, J., & Schraub, S. (1994). Mite allergen exposure is a risk for the incidence of specific sensitization. Journal of Allergy and Clinical Immunology,94, 44–52.
Lau, S., Falkenhorst, G. A., Weber, I., Werthmann, I., Lind, P., Buettner-Goetz, P., et al. (1989). High mite-allergen exposure increases the risk of sensitization in atopic children and young adults. Journal of Allergy and Clinical Immunology,84, 718–725.
Lee, T., Grinshpun, S. A., Martuzevicius, D., Adhikari, A., Crawford, C. M., Luo, J., et al. (2006). Relationship between indoor and outdoor bioaerosols collected with a button inhalable aerosol sampler in urban homes. Indoor Air,16, 37–47.
Mędrela-Kuder, E. (2008a). Fungal Spores—Significant allergens in outdoor and indoor environments. Polish Journal of Environmental Studies,17(4A), 258–262.
Mędrela-Kuder, E. (2008b). Changes in fungal spores concentrations in the atmospheric air of Krakow in 2006–2007 taking into consideration meteorological factors. Polish Journal of Environmental Studies,17(4B), 478–483.
Morawska, L., Afshari, A., Bae, G. N., Buonanno, G., Chao, C. Y. H., & Hänninen, O. (2013). Indoor aerosols: From personal exposure to risk assessment. Indoor Air,23, 62–487.
Myszkowska, D., Stępalska, D., Obtułowicz, K., & Porębski, G. (2002). The relationship between airborne pollen and fungal spore concentrations and seasonal pollen allergy symptoms. Aerobiologia,18, 153–161.
Niesler, A., Ścigała, G., & Łudzeń-Izbiska, B. (2016). Vat (Fel d 1) and dog (Can f 1) allergen levels in cars, dwellings and schools. Aerobiologia,32, 571–580.
Obtułowicz, K. (2002). Badania mikrobiologiczne i palinologiczne./Microbiological and palynological tests. In R. Ney (Ed.), Modelowe Studium Kompleksowego Wykorzystania i Ochrony Surowców Balneologicznych Krakowa i Okolicy (pp. 40–41). Krakow: Sigma Polska Akademia Nauk.
Obtułowicz, K., Myszkowska, D., Dyga, W., Mazur, M., & Czarnobilska, E. (2013). Hypoalergenowa subterraneoterapia w leczeniu alergii dróg oddechowych i skóry w komorach solnych Kopalni w Wieliczce. Znaczenie bioaerosolu./Hypoallergenic subterraneotherapy in the treatment of airway and skin allergies, in salt chambers of the Wieliczka Salt Mine. The importance of bioaerosol. Alergologia. Immunologia,10(2–3), 20–23.
Obtułowicz, K., Składzień, J., Michalak, J., Gawlik, J., & Wróblewska, I. (1999). Skuteczność leczenia subterraneoterapią alergicznego nieżytu nosa w komorach solnych szpitala uzdrowiskowego Kinga w Wieliczce./Effectiveness of subtherraneotherapy of allergic rhinitis in salt chambers of the Kinga spa in Wieliczka. Przegląd Lekarski,56(12), 760–762.
Ogórek, R., Lejman, A., & Matkowski, K. (2014). Influence of the external environment on Airborne fungi isolated from a cave. Polish Journal of Environmental Studies,23(2), 435–440.
Olechnowicz-Bobrowska, B., & Wojkowski, J. (2004). Bioklimat komór sanatoryjnych w Kopalniach Soli Bochni i Wieliczce./Bioclimate of sanatorium chambers in the Bochnia and Wieliczka Salt Mines. Acta Agrophisica,3(2), 343–349.
Pąchalska, M., Pąchalski, A., & Schmidt-Pospuła, M. (2002). Profesor Skulimowski: w poszukiwaniu korzeni krakowskiej rehabilitacji./Professor Skulimowski: in search of the roots of Krakow’s rehabilitation. Ortopedia Traumatologia Rehabilitacja,1, 101–114.
Polish Standards. Air purity protection—Microbiological tests—Determination of the number of bacteria in atmospheric air (imission), when collecting samples by aspiration and sedimentation. PN-89/Z-04111/02.
Polish Standards. Air purity protection—Microbiological tests—Determination of the number of airborne fungi in atmospheric air (imission), when collecting samples by aspiration and sedimentation. PN-89/Z-04111/03.
Ponikowska, I., & Ferson, D. (2009). Nowoczesna medycyna uzdrowiskowa./Modern spa medicine. Krakow: Medi Press.
Ponikowska, I., & Kochański, J. W. (2017). Wielka Księga balneologii, medycyny fizykalnej i uzdrowiskowej./The Great Book of Balneology, Physical and Spa Medicine. Warszawa: Wydawnictwo Aluna.
Rdzanek, M., Pusz, W., Gębarowska, E., & Pląskowska, E. (2015). Airborne bacteria and fungi in a coal mine in Poland. Journal of Cave and Kurst Studies,77(3), 177–182.
Rogula-Kozłowska, W., Kostrzon, M., Rogula-Kopiec, P., & Badyda, A. J. (2016). Particulate matter in the air of the underground chamber complex of the Wieliczka Salt Mine Health Resort. In M. Pokorski (Ed.), Advances in Experimental Medicine and Biology (pp. 9–18). Berlin: Springer International Publishing.
Samoliński, B., Rapiejko, P., Lipiec, A., & Kurzawa, R. (2010). Metody ograniczenia narażenia na alergen./Methods to reduce exposure to allergens. In: J. Kruszewski, M. L. Kowalski (Eds.), Standardy w alergologii. Część I, (pp. 143–149), Krakow: Medycyna praktyczna.
Schneider, T., Sundell, J., Bischof, W., Bohgard, M., Cherrie, J. W., & Clausen, P. A. (2003). ‘EUROPART’. Airborne particles in the indoor environment. A European interdisciplinary review of scientific evidence on associations between exposure to particles in buildings and health effects. Indoor Air,13, 38–48.
Stępalska, D., & Wołek, J. (2005). Variation in fungal spore concentrations of selected taxa associated to weather conditions in Cracow, Poland, in 1997. Aerobiologia,21, 43–52.
Wiszniewski, A. (2015). Environment of air-ins in healing chambers in the “Wieliczka” Salt Mine. Acta Physica Polonica A,127, 1661–1665.
Woodfolk, J. A., Luczynska, C. M., de Blay, F., Chapman, M. D., & Platts-Mills, T. A. (1993). The effect of vacuum cleaners on the concentration and particle size distribution of airborne cat allergen. J Allergy Clin Immunol,91(4), 829–837.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The authors given and family names were swapped. The author group has been correctly updated.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Myszkowska, D., Kostrzon, M., Dyga, W. et al. Bioaerosol of salt chambers in the ‘Wieliczka’ Salt Mine, Poland. Aerobiologia 35, 297–311 (2019). https://doi.org/10.1007/s10453-019-09561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-019-09561-7