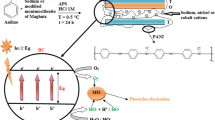
Abstract
Organic azo-dyes, including Congo Red, present a significant environmental concern due to their widespread industrial usage and resistance to biodegradation, leading to severe contamination of effluents. This study explores the efficacy of two basic perovskites (MSnO3, where M = Ca and Sr) in removing Congo Red by adsorption, offering a potential solution for wastewater treatment. The synthesis of the adsorbents was performed by a coprecipitation technique, an effective and no-waste producing method. By adjusting reaction conditions, the physical-chemical characteristics of the perovskites, including crystallinity, morphological features, surface area and porosity, were controlled. Adsorption studies conducted across a range of Congo Red concentrations (10–100 mg L− 1) at pH 10 revealed MSnO3 to possess exceptional adsorption capacity exceeding 100 mg per gram. The results indicate irreversible adsorption and potential adsorbent regeneration by thermal treatment. Slow kinetics also suggest strong binding forces aligned with the fundamentals of pseudo-second-order adsorption kinetic model. Regarding the impact of the synthesis parameters, while the precipitation conditions may not significantly influence adsorption performance, perovskite samples synthesized at higher temperatures are considered more suitable for this application due to their enhanced stability and regenerative capabilities for repeated use. Estimated correlations between sample parameters and adsorption efficiency provide a valuable insight for the practical application of oxide perovskites in addressing dye contamination issues.
Highlights
MSnO3 with tuneable properties can be obtained via MSn(OH)6 as an intermediate.
CaSnO3 consistently demonstrates high adsorption capacities of 98–105 mg g− 1.
SrSnO3 adsorption performance is significantly influenced by experimental conditions.
Desorption experiments indicate irreversibility, suggesting strong binding forces.
Both perovskites exhibit successful regeneration capabilities by thermal treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Congo Red (CR, natrium-3,3′-(4,4′-bifenyldiylbisazo)bis-(4-amino-1-naftaleen-sulfonaat), C32H22N6Na2O6S2) belongs to the azo-dyes class of organic colourants extensively used in chemical industries, textile dyeing, paper, cosmetics, and pharmaceutical due to their excellent stability properties [1, 2]. Azo-dyes exhibit superior stability to light, heat and aggressive chemicals owing to their distinctive structural characteristics, which, on the other hand, makes them hardly degradable [3]. Consequently, widespread application of azo-dyes in different industrial sectors endangers the aquatic ecosystem by the release of heavily polluted effluents with high COD [2, 4]. The azo-dyes themselves are toxic whereas their biodegradation yields aromatic amines known to be mutagenic or even carcinogenic [2, 3]. Moreover, even at low concentration, azo dyes significantly diminish the light intensity of sub-surface water system, which hinders photosynthesis processes and disrupts ecosystem balance [5]. Therefore, development of effective approaches for purifying dye-polluted wastewaters on a commercial-scale is a pressing scientific pursuit.
Regarding the discharge of wastewater from textile industries containing dyes, legislation typically lacks specific restrictions addressing azo dyes content apart from other physical and chemical characteristics of treated wastewater. Treatment methods for such wastewater vary and may include physical, chemical, biological, or hybrid approaches, depending on its properties. Specifically, typical approaches for the removal of organic dues, including Congo Red, include heterogeneous photocatalytic degradation, coagulation-flocculation, electrochemical and advanced oxidation, ion exchange, membrane filtration, reverse osmosis, biological treatment and adsorption [1, 2, 6, 7]. Despite their high efficiency, the applicability of each method is often limited by operational costs and the production of concentrated sludge, toxic metabolites or by-products, which may create new environmental problems [2].
Physical and chemical adsorption stands out as a cost-effective strategy for azo-dye removal, widely adopted on an industrial scale. Adsorption is a combined method with the features of physical and chemical processes. Its advantages, compared to other physic-chemical methods, include economical operation, high efficiency, the potential treatment of heavily polluted effluents, rapid pollutant removal, and the ability to regenerate adsorbents for reuse [1, 2]. The removal of CR by adsorption is a vividly developing topic and over the past two decades, numerous adsorbents have been proposed for this application. Commonly explored adsorbents for CR removal from wastewater include zeolites, hydrogels, silica gels, carbon materials, layered double hydroxides, and some inorganic oxide materials [8,9,10,11,12,13,14,15,16]. The adsorption capacity of typical CR adsorbents rarely exceeds 100 mg L− 1, although a few superior adsorbents with higher capacity have been reported in recent years [5, 17,18,19].
Oxide perovskites are one of the least studied adsorbents of organic pollutants, but are considered highly prospective for this application [20]. Due to their high thermal stability, perovskite oxides can be easily regenerated by heating for repeated application, which makes them economically viable even for developing countries. Dye-adsorbents based on oxide perovskites in previous studies demonstrated a satisfactory CR adsorption capacity ranging from 24 to 43 mg g− 1 [20, 21]. Nevertheless, it is crucial to acknowledge that the studied perovskite compounds often comprise costly rare earth elements (REE) or elements with potential carcinogenic properties, such as Co or Ni, which poses challenges for their widespread applicability within environmental ecosystems.
The present study focuses on two environmentally friendly and affordable basic perovskite oxides of MSnO3 (M = Ca or Sr). These compounds have previously shown effectiveness as catalyst for the photo-degradation of monoazo and diazo-dyes [22, 23]. Considering that typical photodegradation processes often begin with adsorption, MSnO3 may also possess a high adsorption capacity towards azo-dyes. This hypothesis is verified in the current study, which includes extended characterisation of the perovskite compounds prepared under varied conditions, evaluation of their effectiveness as CR adsorbents, modelling of the adsorption kinetics and assessment of the reusability of the adsorbents.
2 Materials and methods
2.1 Synthesis
The formation of pure phase perovskites of Ca and Sr stannates was achieved by thermal decomposition of double hydroxides (MSn(OH)6, M = Ca, Sr), which were obtained by coprecipitation. For synthesis, CaCl2, SrCl2 and K2SnO3 of analytical reagent grade were used as starting materials. The ingredients were mixed in a final ratio M:Sn = 1.5:1 under variable conditions. The concentration of the initial ingredients varied from 0.01 to 0.1 M and the solutions were mixed dropwise in different order. When stannate solution was added to alkaline earth metal chloride solution, the initial pH was either remained neutral or adjusted to 9–10 by addition of ammonia. In the latter case, triethanolamine additive was used to prevent the formation of alkali-earth hydroxides in alkali media. Regardless of the initial conditions, the final pH was around 9–10 in the end of the precipitation. The precipitate was aged during 24 h, filtered, washed to neutral pH, dried at room temperature overnight and then at 60 oC during 24 h in a drying chamber. Calcination was performed in a furnace at the temperature of 400, 500, 600, 700, 800 and 1000 oC during 5 h.
2.2 Methods
Phase composition analysis was performed by powder XRD using a Miniflex 600 benchtop diffractometer (Rigaku, JAP, CuKα radiation, CuKβ Ni filter, 1D detector D/teX Ultra, continuous scanning over the 2 theta range from 10 to 80o with scanning speed of 5-10o 2 theta min− 1, recorded step size 0.02o 2 theta). Phase identification was performed using PDXL2 Rigaku data analysis software by searching for a match in the PDF-2 database.
A scanning electron microscope (Lyra 3, Tescan) was used at an accelerating voltage of 10 kV to study the surface morphology of the composite. SEM images were recorded using SE detector at a working distance of 7 mm.
The thermal transformation of the double hydroxides and the thermal stability of the perovskites was studied using thermal analysis (DSC-TGA, Labsys Evo 1600, Setaram, FR). Measurements were carried out in corundum crucibles in a temperature range of 25-1200 oC with a heating rate of 10 oC min− 1 in inert or oxidising atmospheres (50 ml min− 1 Ar or airflow, respectively).
The surface area and porosity of the samples were analysed using a Quantachrome NovaWin physisorption analyser (Quantachrome Instruments, Anton Paar Quanta Tec Inc., USA) under the following conditions: analysis gas N2 (adsorbate), analysis temperature 77.35 K, pressure tolerance 0.1/0.1 (ads/des), equilibrium time 90/90 s (ads/des), equilibrium timeout 240/240 s (ads/des). Samples were analysed after outgassing for 3 h at 200 oC. The pore size distribution was calculated using the density functional theory, DFT (nonlocal DFT equilibrium model for N2 at 77 K on silica; cylindrical and spherical pore geometry was assumed).
2.3 Adsorption study
Adsorption experiments were carried out in the water phase at room temperature (20 oC) in a set of 50 mL flasks with an adsorbent loading of 50 mg. The study also included an evaluation of the impact of the initial adsorbate concentration (10, 50 and 100 mg L− 1) at the initial pH 10. For the adsorption experiments, 250 mg of the dye was dissolved in 0.5 L of distilled water, which was used to prepare 10, 50 and 100 mg L− 1 dye solutions with pH adjusted to 10 by adding NaOH (0.01 N). Then 50 mg of adsorbent was added to 50 mL of dye solution and kept without shaking in darkness. A small amount of the dye suspension (1–2 mL) was withdrawn at a regular time interval (from 2 to 240 h) and filtered using a syringe filter (PTFE, 0.22 μm, Fisher Scientific). The light absorption of the supernatant solution was analyzed using a photometer in 1-cm quartz cuvettes at the wavelength of 446 nm.
The adsorption efficiency was characterised using two parameters, i.e. the percentage of dye removal (%R, Eq. 1) and the adsorption capacity (Qt, Eq. 2):
%R = [(C0-Ct)/C0]·100% (Eq. 1).
Qt = [(C0-Ct)/m]·V (Eq. 2).
where C0 is the initial concentration of the dye, mg L− 1; Ct is the concentration of the dye at time t, mg L− 1; m is the weight of the adsorbent, g; V is the volume of the solution, L. The adsorption experiments were performed in triplicate and the average results were used for further data treatment. The standard deviation of the triplicate results was within 0.2–20.4% of the average concentration. Desorption experiments were attempted in various solvents (water, diluted HNO3, HCl and NaOH, methanol, ethanol and acetone) by immersing 50 mg of perovskite sample containing adsorbed CR on its surface (products of the adsorption experiments with 100 mg L− 1 of CR) into 50 ml of the solvent for 72 h.
Multiple kinetic models, including the first-order kinetic, pseudo-second-order kinetic and intraparticle diffusion models, have been utilized to fit the experimental adsorption data. A summary of the fitted models with equations and parameters is given in Table 1. Both non-linear fitting and linearised approaches were utilised (NLF and LF, respectively). Non-linear fitting was solved using the generalized reduced gradient nonlinear solving method (Solver analysis tool, Microsoft Excel). Linear fitting was analysed through linear regression analysis (least squares method, LINEST function, Microsoft Excel). The coefficient of determination (R2) was employed to evaluate goodness of fit and fidelity of the models.
\({Q}_{t}\) - the amounts of dye adsorbed per unite mass of the adsorbent (mg g− 1) at time t (min);
\({Q}_{e}\) - the amounts of dye adsorbed per unite mass of the adsorbent (mg g− 1) at equilibrium;
\({k}_{1}\) - the rate constant of adsorption (min− 1);
\({k}_{2}\) - the rate constant of the pseudo-second order equation (g mg− 1 min− 1);
\({k}_{p}\) - intraparticle diffusion rate constant (mg g− 1 min− 0.5).
3 Results and discussion
3.1 Formation and characterisation of ca and sr stannate perovskites
The synthesis of perovskite stannates, MSnO3 (M = Ca or Sr), was carried out through a two-step procedure. At the first step, the coprecipitation method was employed to synthesize double hydroxides, MSn(OH)6 (M = Ca or Sr), under varying conditions. The resulting precipitate consistently exhibited a pure crystalline phase corresponding to MSn(OH)6. The phase composition of the precipitate remained consistent regardless of the sequence of mixing of the initial ingredients, their concentration, the presence of additives, such as triethanolamine, and the initial pH. The insensitivity of the precipitate’s phase composition to the mentioned parameters emphasizes its robust formation mechanism.
For subsequent investigation, two distinct methodologies were employed, primarily distinguished by the initial pH which was defined by the sequence of mixing of the ingredients: either the stannate solution was introduced to alkali-earth metal solutions with an initial pH close to neutral (method a), or the alkali-earth metal solution was added to the stannate solution with an initial basic pH close to 10 (method b). Both approaches yielded highly crystalline pure phase product with identical XRD patterns. Figure 1 illustrates the XRD patterns of the double hydroxides synthesized using method b (dried at 60 oC), juxtaposed with the corresponding reference database patterns (CaSn(OH)6, ICDD# 01-074-1823; SrSn(OH)6, ICDD# 01-084-3532).
SEM images of the double hydroxides are presented in Fig. 2. CaSn(OH)6 exhibited micron-sized flower-like octagons regardless the precipitation conditions (Fig. 2, CaSn(OH)6-a and b). However, literature reports have indicated that the morphological characteristics of CaSn(OH)6 precipitate can vary ranging from a flower-like octagonal morphology to regular cubic shape, depending to experimental conditions, such as hydrothermal treatment, presence of additives or application of ultrasound [22, 24,25,26,27]. In our opinion, the crystal morphology of CaSnO3 could be also influenced by the source of stannate. For instance, the use of alkali metal stannates resulted in a flower-like octagonal morphology (present study and [25, 26]), while utilization of tin chlorides yielded regular cubic crystallites [22, 24].
Regarding SrSn(OH)6, the synthesized samples comprised agglomerated crystallites displaying either irregular rounded or elongated shape, depending on the synthesis method (Fig. 2, SrSn(OH)6-a and b, respectively). The formation of the crystallites of irregular rounded shape for this double hydroxide has been rarely detected before [28], while needle-like morphology has been reported the most often [27, 29,30,31]. Notably, the SrSn(OH)6 needles typically exhibited a more pronounced rod-like shape akin to wires, with the potential to organize into a dumbbell-like structures through hydrothermal or ultrasound treatment [27, 29]. Nevertheless, a detailed literature analysis indicated that the initial pH played a crucial role in determining the morphology of SrSn(OH)6, which aligned with the results of this study. In particular, needle-like crystallites were typically formed when the initial pH of the reaction was basic. This condition was met either when Sr salts were added to basic stannate precursor (as in ‘method b’ of the present study), or when the initial compounds were added simultaneously into the basic solution [27, 29,30,31]. In contrast, the crystallites of irregular rounded shape were formed when the initial pH was neutral. This condition was fulfilled when Sn precursor was added to a neutral Sr salt solution, corresponding to ‘method a’ of the present study and [28].
At the second step, MSn(OH)6 was calcinated to obtain MSnO3. DSC-TGA analysis was utilized to follow the decomposition process, which showed that the thermal behavior of MSn(OH)6 did not depend on the precipitation conditions or the type of atmosphere (inert or oxidizing), but its decomposition path varied according to the nature of M element (Fig. 3). CaSn(OH)6 decomposes in one step between 300 and 400 oC associated with a very strong endothermic effect and a substantial step-like weight loss, which corresponded to the release of 3 molecules of water (theoretical weight loss of 20.7%) and the formation of metastable amorphous CaSnO3 [32]. The amorphous state was partially hydrated and slowly continued to dehydrate with the further temperature increase. The exothermic effect at 770 oC was attributed to the transformation of metastable amorphous phase to thermodynamically stable crystalline CaSnO3, which took place at higher temperature than it was previously reported [32].
Dehydration of SrSn(OH)6 slowly started just above 100 oC and then the process intensified above 200 oC reaching its extremum at 280 oC. Similar to CaSnO3, the metastable amorphous state of SrSnO3 was hydrated. The exothermic effect at 700–720 oC, associated with the crystallization of SrSnO3, was accompanied by a step-like weight loss. It testified in favor of higher degree of hydration of metastable amorphous phase of SrSnO3 in comparison to CaSnO3. The total weight loss of the sample was close to the theoretical value of the dehydration of 3 molecules of water (17.5%). The observed behavior is quite different from the previously reported, which showed a gradual dehydration of SrSn(OH)6 within a broader temperature range [32]. The results point out high sensitivity of the decomposition process of SrSn(OH)6 to the experimental conditions.
In both cases, the thermal decomposition of MSn(OH)6 (M = Ca or Sr) resulted in the formation of pure MSnO3 perovskite phases, which aligns with prior research findings [22, 23, 25, 28, 33, 34]. The crystallinity of the resultant product exhibited gradual enhancement with increasing calcination temperatures within the range of 400 to 800 oC. The temperature, which was required for the formation of well-crystalline product, varied based on the preparation method and chemical composition, as illustrated in Fig. 1. A noticeable distinction was particularly observed between SrSnO3-a and b calcined at 500 oC. Increase of the calcination temperatures to 1000 oC and beyond induced the degradation of CaSnO3, giving rise to an additional phase Ca2SnO4. Conversely, the SrSnO3 phase remained stable up to 1200 oC. Although the decomposition of SrSn(OH)6 at lower temperatures could potentially lead to the formation of additional impurity phases, such as SrCO3 or SnO2 [22, 24, 30, 32, 34], no such occurrences were noted in this study. However, a gradual transformation of perovskite phase into SrCO3 was detected during storage, particularly in the case of semi-amorphous metastable products calcined at lower temperatures.
The phase transformation from double hydroxide to perovskite phase induced a notable change in the morphology of the samples, as illustrated in Fig. 2. The initial flower-like octagons of CaSn(OH)6 underwent a transformation into a mixture of spherical and cubic particles comprising CaSnO3, which morphologic characteristics did not depend on the preparation method or calcination temperature. According to the SEM images (Fig. 2), smaller CaSnO3 particles typically exhibited close-to-cubic symmetry, which, as their size increases, got softened edges ultimately giving rise to a prevalent regular spherical shape. Similar rounding of CaSnO3 morphology was previously observed when a sodium citrate was added into the reaction mixture [35]. Nonetheless, the predominant shape of CaSnO3 perovskite derived from the decomposition of CaSn(OH)6 was regular cubic [22, 24, 25, 32, 35], which is different from the present study.
Controversary, for SrSnO3, the effect of the preparation method on the sample’s morphology was significant (Fig. 2). In general, the morphology of SrSnO3 resembled the morphology of SrSn(OH)6 and did not change with increase of the calcination temperature. In particular, SrSnO3-a was composed of agglomerated particles of rounded shape which size varies between 50 and 200 nm. SrSnO3-b consisted of individual interconnected rode-like crystals reaching 20 μm in size. According to the literature, SrSnO3 typically preserved the morphology of SrSn(OH)6 precursor and exhibited either dumbbell like particles, consisting of nanorods, or individual nanorods [29, 30, 33], although an occurrence of the submicron agglomerated rounded particles has been evidenced as well [28], which agrees with results of this study.
The surface area and porosity characteristics of the synthesized perovskites were assessed using the N2 adsorption technique. A summary of the specific surface area and pore volume are provided in Table 2. Figure 4 shows the adsorption isotherms and pore size distribution histograms. In general, the surface area and porosity of CaSnO3 did not depend on the preparation method but exhibited an increase with higher calcination temperatures. Specifically, samples calcined at 500 oC displayed an H3 hysteresis loop indicative of slit-shaped pores, with the mean pore radius of 2 nm. On contrary, CaSnO3 samples calcined at 800 oC exhibited an H1 hysteresis loop, characteristic of cylindrical pore geometry. Simultaneously, the pore volume significantly increased, primary due to enhanced microporosity. The development of microporosity in CaSnO3 samples at elevated temperatures is closely associated with the dehydration process [35], which persists up to 800 °C (Fig. 3) despite no discernible changes in the visual appearance of the samples (Fig. 2).
The surface characteristics of SrSnO3 primary depended on the synthesis parameters and morphological features of the samples. In the case of SrSnO3-a, a notable reduction in surface area was observed with increase of the calcination temperature (Table 2; Fig. 4). Despite maintaining the H3 hysteresis loop shape, the mean pore radius decreased substantially from 15 to 0.5 nm. Consequently, the thermal treatment in this case lead to a reduction in both pore volume and the dimensions of the porosity channels, which can be associated with sintering process. For the SrSnO3-b sample, its pore dimensions also reduced, but the pore volume and surface area were only insignificantly affected by the temperature increase.
3.2 Adsorption properties of Ca and Sr stannate perovskites towards Congo Red
The percentage removal (%R) of Congo Red (CR) from solutions with varying initial CR concentration (C0(CR) = 10, 50 and 100 mg L− 1) by the synthesized MSnO3 samples is illustrated in Fig. 5. Accordingly, %R after a 24-hour contact was significantly higher for CaSnO3 in comparison to SrSnO3. For CaSnO3, it consistently surpassed the 90% threshold, while in the case of SrSnO3, %R fluctuated between 8.3 and 76.8%. The percentage removal of CaSnO3, after a 24-hour contact, exhibited minimal dependence on both the calcination temperature and the initial concentration of CR. The chosen preparation method similarly showed an insignificant impact. However, an interesting trend emerged for sample CaSnO3-b, where the %R slightly increased with the elevation of the initial CR concentration. The percentage removal of SrSnO3 followed a common trend, where it gradually reduced with an increase in CR concentration. Furthermore, the preparation method significantly influenced the adsorption characteristics of SrSnO3, with a notable decline observed for both methods. While the calcination temperature also impacted the efficiency of SrSnO3, a clear trend was not readily observed.
To achieve equilibrium, the study extended over a period of 240 h. Within this timeframe, 100% removal was achieved for almost all CaSnO3 samples, with initial CR concentration of 10 and 50 mg L− 1 and SrSnO3 samples with an initial CR concentration of 10 mg L− 1. Kinetic modelling was specifically performed for tests with an initial CR concentration of 100 mg L− 1, which reached equilibrium within the studied period. The results of the adsorption data analysis are summarised in Table 3. The nonlinear and linear pseudo-first-order and pseudo-second-order kinetic models were assessed, and the best fits of these models are presented in Fig. 6. The selection of the best linear pseudo-second-order kinetic model was based on the highest R2model values, corresponding to the following: LF I for CaSnO3-a-800 and CaSnO3-b-800, LF IV for SrSnO3-a-800 and SrSnO3-b-800, LF V for SrSnO3-a-500, LF VI for CaSnO3-a-500, CaSnO3-b-500 and SrSnO3-b-500.
Kinetic model fitting for Congo Red adsorption by MSnO3 (A = Ca or Sr, method a and b, CT = 500 and 800 oC): NLF-1 and LF-1 (red curves) correspond to the fit of non-linear and linear pseudo-first-order kinetic models, respectively; NLF-2 and LF-2 (black curves) correspond to the fit of non-linear and linear pseudo-second-order kinetic models, respectively
In general, Ca and Sr perovskites showed quite different adsorption isotherms with good fit to both pseudo-first-order and pseudo-second-order kinetic model. The behaviour of CaSnO3 samples was very consistent reaching equilibrium within 48 h and adsorption capacities between 98 and 105 m g− 1. On opposite, the adsorption behaviour of SrSnO3 samples was significantly influenced by preparation conditions, where both, the method and calcination temperature strongly affected their performance. Despite the extended timeframe, equilibrium was not attained for SrSnO3, and the calculated equilibrium adsorption capacity fluctuated significantly, ranging from 37 to 140 mg g− 1. Notably, kinetic constants for CaSnO3 exceeded those of SrSnO3 by more than ten times in certain cases. Considering that the adsorption capacity of typical CR adsorbents rarely surpassed 100 mg L− 1 [8,9,10,11,12,13,14,15,16], the adsorption capacities of both MSnO3 perovskites are highly competitive. Moreover, when compared to other perovskites characterised by adsorption capacities ranging from 24 to 43 mg g− 1 [20, 21], the results are exceptionally impressive.
In spite of such high adsorption capacity, the adsorption kinetics of CR on both perovskites was very slow. In general, the contact time, required to reach the equilibrium, depended on the type of adsorbent. For mixed oxides including perovskites, the typical contact time was below four hours [12, 20]. Thus, the studied samples can be classified as slow-acting adsorbents, that require a longer contact time to reach saturation and maximize the adsorption potential. The slow kinetics also implies that the rate-limiting step potentially ties back to fundamental processes that underline the adsorption phenomenon. Furthermore, insights derived from desorption experiments (elusion) revealed an irreversibility in the process: even when subjected to strong solvents, desorption remained undetectable. These observations strongly suggest that the adsorption process is likely associated with stronger binding forces that involve chemisorption or strong interactions between the adsorbate and the adsorbent surface (such as Van der Waals forces, hydrogen bonding or coordination bonds). This implies that the pseudo-second-order kinetic model is more suitable for the studied case given its foundation in chemisorption mechanism. On contrary, the assumption that the adsorption might be driven by the concentration gradient – a cornerstone of the pseudo-first-order kinetic model - is challenged by the observed complete adsorption at lower adsorbate concentrations (10 and 50 mg L− 1). This further supports the rational for favouring the pseudo-second-order kinetic model.
In the case of CaSnO3, the intraparticle diffusion model also showed a strong fit. In particular, the intraparticle diffusion plot unveiled two distinct linear segments, signifying that there is a subsequent adsorption stage where the rate is limited by the intraparticle diffusion of the adsorbate molecules into the internal pores of the adsorbent particles, characterized by much slower and more controlled adsorption. Thus, the overall adsorption mechanism of this sample can be also explained by multiple kinetic stages.
The interaction between the adsorbent and adsorbate was studied by XRD analysis, FTIR spectroscopy, and SEM. XRD analysis showed significant phase transformation taking place during the adsorption experiments (Fig. 7). Specifically, CaSnO3 primarily transformed into CaSn(OH)6, whereas SrSnO3 transformed into SrCO3. The extend of this transformation was highly influenced by the calcination temperature used during the treatment of the samples (500 or 800 oC). In particular, samples treated at 800 oC exhibited greater stability of the perovskite phase. This can be connected with the transformation of metastable amorphous phase to thermodynamically stable crystalline phase, which occurred just below 800 oC (Fig. 3). Importantly, the samples, which were initially calcined at 800 oC, could be completely regenerated to the initial perovskite phase through repeated thermal treatment at 800 oC after the adsorption experiments. FTIR spectroscopy confirmed the results of XRD study. Regarding the adsorbed CR, some of its most intensive bands (1590 cm− 1, 1220 cm− 1, 1175 cm− 1, 1060 cm− 1) were hardly noticed on the spectra after its adsorption. Simultaneously, alterations in the sample’s morphology were noticed by SEM observation (Fig. 2, MSnO3-a/CR and MSnO3-b/CR). In particular, SEM images revealed noticable recrystallization of cubic CaSn(OH)6 on the surface of CaSnO3 following CR adsorption. Significant changes were also observed in the case of SrSnO3-a, with minor changes in SrSnO3-b (Fig. 2), despite similar transformation of phase composition for both samples (Fig. 7).
Furthermore, the reusability of selected samples was tested. CaSnO3-a-800 and SrSnO3-a-800 samples, which were used for the adsorption of CR with an initial concentration 100 mg L− 1, were regenerated through thermal treatment at 800 oC. The thermal treatment effectively removed the adsorbed dye and completely restored the perovskite structure of the samples (Fig. 7, samples 800-AA-800). Subsequent adsorption experiments were conducted under similar conditions, with an initial CR concentration of 100 mg L− 1 and a duration of 24 h. The regenerated samples demonstrated comparable efficiency to the original ones across three consecutive adsorption/regeneration cycles (standard deviation 10%). The results highlight the reusable nature of the perovskite adsorbents for of Congo Red removal.
Phase composition of CaSnO3 (a) and SrSnO3 (b) after adsorption of Congo Red and regeneration by thermal treatment at 800 oC (‘500 AA’ = MSnO3-a-500 after adsorption of CR; ‘800 AA’ = MSnO3-a-800 after adsorption of CR; ‘500 AA 500’ = MSnO3-a-500 after adsorption of CR and recovery by calcination at 500 oC; ‘800 AA 800’ = MSnO3-a-800 after adsorption of CR and recovery by calcination at 800 oC)
4 Conclusion
This study has demonstrated a cost-effective universal method for the synthesis of MSnO3 perovskites (M = Ca and Sr) with tunable characteristics. The method, based on the thermal decomposition of MSn(OH)6 precipitate, provided control over surface properties by modulating coprecipitation conditions or adjusting the calcination temperature. The observed adsorption capacity of MSnO3 for Congo Red exceeded 100 mg per gram, a notably higher value compared to reported data for other oxide perovskites (24–37 mg per gram). Importantly, the adsorption characteristics of SrSnO3 were found to significantly depend on the initial concentration of Congo Red and, in some cases, on the preparation method and calcination temperature. In contrast, the impact of these parameters on CaSnO3 performance remained comparatively restrained, consistently yielding superior adsorption characteristics. Although the precipitation conditions might not significantly influence adsorption performance, perovskite samples synthesized at higher temperatures are considered more suitable for this application due to their enhanced stability and regenerative capabilities for repeated use. Both, the pseudo-first-order and pseudo-second-order kinetic models exhibited a good fit, with the latter aligning better with the slow adsorption process likely involving strong interactions between the adsorbate and the adsorbent surface. Furthermore, the minimal reversibility observed in desorption experiments underscored the presence of strong binding forces emphasizing the fundamental processes governing the adsorption phenomenon. Both perovskites exhibited successful regeneration capabilities by thermal treatment in multiple adsorption/regeneration cycles. The findings enhance our understanding of the synthesis and adsorption mechanisms of MSnO3 perovskites, providing valuable insights for their potential applications.
Data availability
No datasets were generated or analysed during the current study.
References
Harja, M., Buema, G., Bucur, D.: Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 12, 6087 (2022). https://doi.org/10.1038/s41598-022-10093-3
Al-Tohamy, R., Ali, S.S., Li, F., Okasha, K.M., Mahmoud, Y.A.G., Elsamahy, T., Jiao, H., Fu, Y., Sun, J.: A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 231, 113160 (2022). https://doi.org/10.1016/J.ECOENV.2021.113160
Lade, H., Govindwar, S., Paul, D., Bhamidiammarri, R., Tota-Maharaj, K.: Mineralization and detoxification of the carcinogenic azo dye Congo Red and real textile effluent by a polyurethane foam immobilized microbial consortium in an upflow column bioreactor. Int. J. Environ. Res. Public. Health. 12, 6894–6918 (2015). https://doi.org/10.3390/IJERPH120606894
Han, G., Du, Y., Huang, Y., Wang, W., Su, S., Liu, B.: Study on the removal of hazardous Congo red from aqueous solutions by chelation flocculation and precipitation flotation process. Chemosphere. 289, 133109 (2022). https://doi.org/10.1016/J.CHEMOSPHERE.2021.133109
Obayomi, K.S., Lau, S.Y., Ibrahim, O., Zhang, J., Meunier, L., Aniobi, M.M., Atunwa, B.T., Pramanik, B.K., Rahman, M.M.: Removal of Congo red dye from aqueous environment by zinc terephthalate metal organic framework decorated on silver nanoparticles-loaded biochar: Mechanistic insights of adsorption. Microporous Mesoporous Mater. 355, 112568 (2023). https://doi.org/10.1016/J.MICROMESO.2023.112568
Zaamouchi, I., Kaci, M.M., Zidane, Y., Belaid, S., Bouacida, S., Benmerad, B.: The impressive photocatalytic performance of Zn-MOF as a novel photocatalyst for the effective purification of dyes under solar exposure. J. Mol. Struct. 1299, 137070 (2024). https://doi.org/10.1016/j.molstruc.2023.137070
Akkari, I., Graba, Z., Pazos, M., Bezzi, N., Atmani, F., Manseri, A., Kaci, M.M.: Recycling waste by manufacturing biomaterial for environmental engineering: Application to dye removal. Biocatal. Agric. Biotechnol. 50, 102709 (2023). https://doi.org/10.1016/j.bcab.2023.102709
Chen, X., Huang, Z., Luo, S.Y., Zong, M.H., Lou, W.Y.: Multi-functional magnetic hydrogels based on Millettia Speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 423, 130198 (2021). https://doi.org/10.1016/J.CEJ.2021.130198
Lorenc-Grabowska, E., Gryglewicz, G.: Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dye Pigment. 74, 34–40 (2007). https://doi.org/10.1016/J.DYEPIG.2006.01.027
Purkait, M.K., Maiti, A., DasGupta, S., De, S.: Removal of Congo red using activated carbon and its regeneration. J. Hazard. Mater. 145, 287–295 (2007). https://doi.org/10.1016/J.JHAZMAT.2006.11.021
Zhang, H., Chen, H., Azat, S., Mansurov, Z.A., Liu, X., Wang, J., Su, X., Wu, R.: Super adsorption capability of rhombic dodecahedral Ca-Al layered double oxides for Congo red removal. J. Alloys Compd. 768, 572–581 (2018). https://doi.org/10.1016/J.JALLCOM.2018.07.241
Hashemian, S., Foroghimoqhadam, A.: Effect of copper doping on CoTiO3 ilmenite type nanoparticles for removal of Congo red from aqueous solution. Chem. Eng. J. 235, 299–306 (2014). https://doi.org/10.1016/J.CEJ.2013.08.089
Afkhami, A., Moosavi, R.: Adsorptive removal of Congo Red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 174, 398–403 (2010). https://doi.org/10.1016/J.JHAZMAT.2009.09.066
Hao, T., Yang, C., Rao, X., Wang, J., Niu, C., Su, X.: Facile additive-free synthesis of iron oxide nanoparticles for efficient adsorptive removal of Congo red and cr(VI). Appl. Surf. Sci. 292, 174–180 (2014). https://doi.org/10.1016/J.APSUSC.2013.11.108
Xia, H., Chen, L., Fang, Y.: Highly efficient removal of Congo red from aastewater by nano-CaO. Sep. Sci. Technol. 48, 2681–2687 (2013). https://doi.org/10.1080/01496395.2013.805340
Extross, A., Waknis, A., Tagad, C., Gedam, V.V., Pathak, P.D.: Adsorption of Congo red using carbon from leaves and stem of water hyacinth: Equilibrium, kinetics, thermodynamic studies. Int. J. Environ. Sci. Technol. 20, 1607–1644 (2023). https://doi.org/10.1007/s13762-022-03938-x
Zheng, Y., Cheng, B., Fan, J., Yu, J., Ho, W.: Review on nickel-based adsorption materials for Congo red. J. Hazard. Mater. 403, 123559 (2021). https://doi.org/10.1016/j.jhazmat.2020.123559
Hua, Z., Pan, Y., Hong, Q.: Adsorption of Congo red dye in water by orange peel biochar modified with CTAB. RSC Adv. 13, 12502–12508 (2023). https://doi.org/10.1039/d3ra01444d
Shen, T., Ji, Y., Mao, S., Han, T., Zhao, Q., Wang, H., Gao, M.: Functional connector strategy on tunable organo-vermiculites: The superb adsorption towards Congo Red. Chemosphere. 339, 139658 (2023). https://doi.org/10.1016/j.chemosphere.2023.139658
Garba, Z.N., Zhou, W., Zhang, M., Yuan, Z.: A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere. 244, 125474 (2020). https://doi.org/10.1016/j.chemosphere.2019.125474
Santos, A.G., Leite, J.O., Gimenez, I.F., Souza, M.J.B., Garrido Pedrosa, A.M.: Effect of the B-site cation from LaBO3 and LaBO3/TiO2 (B = mn or ni) perovskites prepared by mechanosynthesis in adsorption of Congo red dye from aqueous medium. Mater. Res. Express. 6, 105065 (2019). https://doi.org/10.1088/2053-1591/ab3b22
Moshtaghi, S., Gholamrezaei, S., Salavati, M., Niasari: Nano cube of CaSnO3: Facile and green co-precipitation synthesis, characterization and photocatalytic degradation of dye. J. Mol. Struct. 1134, 511–519 (2017). https://doi.org/10.1016/j.molstruc.2016.12.098
Moshtaghi, S., Gholamrezaei, S., Salavati Niasari, M., Mehdizadeh, P.: New controllable procedure for preparation of SrSnO3 nanostructures: Photo-degradation of azo dyes and photovoltaic measurement. J. Mater. Sci. Mater. Electron. 27, 414–424 (2016). https://doi.org/10.1007/s10854-015-3769-6
Loginov, A.V., Aparnev, A.I., Uvarov, N.F.: Nanocomposites prepared via thermal decomposition of calcium hydroxystannate CaSn(OH)6, Inorg. Mater. 58, 814–821 (2022). https://doi.org/10.1134/S0020168522080088
Cheng, H., Lu, Z.: Synthesis and gas-sensing properties of CaSnO3 microcubes. Solid State Sci. 10, 1042–1048 (2008). https://doi.org/10.1016/j.solidstatesciences.2007.11.001
Fan, C., Song, X., Yu, H., Yin, Z., Xu, H., Cao, G., Zheng, D., Sun, S.: Shape-controlled synthesis of CaSnO3 micro crystals via a precursor route. Mater. Lett. 61, 1588–1591 (2007). https://doi.org/10.1016/j.matlet.2006.07.083
Hu, X., Lv, G., Jia, Z., Jiang, J., Xiao, T., Yuan, M., Tang, Y.: A general sonochemical approach to rapid synthesis of 1D single-crystalline MSn(OH)6 (M = ba, ca, Sr) nanostructures. Appl. Surf. Sci. 257, 9008–9013 (2011). https://doi.org/10.1016/j.apsusc.2011.05.088
Muralidharan, M., Selvakumar, S., Sivakumar, K., Sivaji, K.: Effect of Yb doping on structural, optical and induced ferromagnetism in SrSnO3 perovskite nanostructures. Phys. B Condens. Matter. 615, 413039 (2021). https://doi.org/10.1016/j.physb.2021.413039
Chen, D., Ye, J.: SrSnO3 nanostructures: Synthesis, characterization, and photocatalytic properties. Chem. Mater. 4585–4591. (2007)
Bohnemann, J., Libanori, R., Moreira, M.L., Longo, E.: High-efficient microwave synthesis and characterisation of SrSnO3. Chem. Eng. J. 155, 905–909 (2009). https://doi.org/10.1016/j.cej.2009.09.004
Yang, L., Yu, Y., Yang, W., Li, X., Zhang, G., Shen, Y., Dong, F., Sun, Y.: Efficient visible light photocatalytic NO abatement over SrSn(OH)6 nanowires loaded with Ag/Ag2O cocatalyst. Environ. Res. 201, 111521 (2021). https://doi.org/10.1016/j.envres.2021.111521
Loginov, A.V., Aparnev, A.I., Uvarov, N.F.: Study of thermal decomposition of hexahydroxostannates(IV) MSn(OH)6, (M = mg, Sr, ca). Mater. Today Proc. 25, 477–479 (2019). https://doi.org/10.1016/j.matpr.2019.12.242
Wang, S., Lu, M., Zhou, G., Zhou, Y., Zhang, A., Yang, Z.: Systematic investigations into SrSnO3 nanocrystals (I) synthesis by using combustion and coprecipitation methods. J. Alloys Compd. 432, 265–268 (2007). https://doi.org/10.1016/J.JALLCOM.2006.05.110
Gaudon, M., Salek, G., Kande, M., Andron, I., Frayret, C., Durand, E., Penin, N., Duttine, M., Wattiaux, A., Jubera, V.: CaSn(OH)6 hydroxides, CaSnO3 oxides and CaSnF6 fluorides: Synthesis and structural filiation. Cationic environment impact on Pr3+ doped compounds luminescence. J. Solid State Chem. 265, 291–298 (2018). https://doi.org/10.1016/j.jssc.2018.06.017
Chen, X.Y., Ma, C., Bao, S.P., Zhang, H.Y.: Novel porous CaSnO3:Eu3+ and Ca2SnO4:Eu3+ phosphors by co-precipitation synthesis and postannealing approach: A general route to alkaline-earth stannates. J. Alloys Compd. 497, 354–359 (2010). https://doi.org/10.1016/j.jallcom.2010.03.065
Funding
The authors thank for the financial support to Czech Science Foundation (GACR, project No. 22-11397 S).
The authors also thank to Ministry of Education, Youth and Sports of the Czech Republic for the financial support of SEM measurements at CEMNAT, University of Pardubice (project LM 2023037).
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
NR, JL, ZD, and PS conceived and designed research. NR conducted experiments. JL and ZD were involved in synthesis and characterization. NR wrote the manuscript. All authors read, corrected and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reinders, N., Luxová, J., Dohnalová, Ž. et al. Exploring Ca and Sr stannate perovskites as adsorbents for Congo Red removal. Adsorption (2024). https://doi.org/10.1007/s10450-024-00455-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00455-w