Abstract
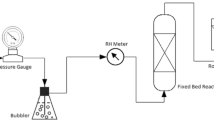
A range of potassium-based alumina sorbents were fabricated by impregnation of alumina with K2CO3 to examine the effects of the structural and textural properties of alumina on the CO2 sorption and regeneration properties. Alumina materials, which were used as supports, were prepared by calcining alumina at various temperatures (300, 600, 950, and 1,200 °C). The CO2 sorption and regeneration properties of these sorbents were examined during multiple tests in a fixed-bed reactor in the presence of 1 vol% CO2 and 9 vol% H2O. The regeneration capacities of the potassium-based alumina sorbents increased with increasing calcination temperature of alumina. The formation of KHCO3 increased with increasing calcination temperature during CO2 sorption, whereas the formation of KAl(CO3)(OH)2, which is an inactive material, decreased. These results is due to the fact that the structure of alumina by the calcination temperature is related directly to the formation of the by-product [KAl(CO3)(OH)2]. The structure of alumina plays an important role in enhancing the regeneration capacity of the potassium-based alumina sorbent. Based on these results, a new potassium-based sorbent using δ-Al2O3 as a support was developed for post-combustion CO2 capture. This sorbent maintained a high CO2 capture capacity of 88 mg CO2/g sorbent after two cycles. In particular, it showed a faster sorption rate than the other potassium-based alumina sorbents examined.








Similar content being viewed by others
References
Abanades, J.C.: The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3. Chem. Eng. J. 90, 303–306 (2002)
Arias, B., Grasa, G., Alonso, M., Abanades, J.C.: Post-combustion calcium looping process with a high stable sorbent activity by recarbonation. Energy Environ. Sci. 5(6), 7353–7359 (2012)
Chen, C., Yang, S.T., Ahn, W.S.: Calcium oxide as high temperature CO2 sorbent: effect of textural properties. Mater. Lett. 75, 140–142 (2012)
Gupta, H., Fan, L.S.: Carbonation–calcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas. Ind. Eng. Chem. Res. 41, 4035–4042 (2002)
Hagewiesche, D.P., Ashour, S.S., Al-Ghawas, H.A., Sandall, O.C.: Absorption of carbon dioxide into aqueous blends of monoethanolamine and N-methyldiethanolamine. Chem. Eng. Sci. 50(7), 1071–1079 (1995)
Hayashi, H., Taniuchi, J., Furuyashiki, N., Sugiyama, S., Hirano, S., Shigemoto, N., Nonaka, T.: Efficient recovery of carbon dioxide from flue gases of coal-fired power plants by cyclic fixed-bed operations over K2CO3-on-carbon. Ind. Eng. Chem. Res. 37, 185–191 (1998)
Hirano, S., Shigemoto, N., Yamaha, S., Hayashi, H.: Cyclic fixed-bed operations over K2CO3-on- carbon for the recovery of carbon dioxide under moist conditions. Bull. Chem. Soc. Jpn. 68, 1030–1035 (1995)
Intergovernmental Panel on Climate Change (IPCC): IPCC special report on carbon dioxide capture and storage. Cambridge University Press, Cambridge (2005)
Kim, D.S., Han, S.J., Kwak, S.Y.: Synthesis and photocatalytic activity of mesoporous TiO2 with the surface area, crystallite size, and pore size. J. Colloid Interface Sci. 316, 85–91 (2007)
Kwon, S.C., Fan, M., Dacosta, H.F.M., Russell, A.G., Tsouris, C.: Reaction kinetics of CO2 carbonation with Mg-rich minerals. J. Phys. Chem. A 115(26), 7638–7644 (2011)
Lee, J.B., Ryu, C.K., Baek, J.I., Lee, J.H., Eom, T.H., Kim, S.H.: Sodium-based dry regenerable sorbent for carbon dioxide capture from power plant flue gas. Ind. Eng. Chem. Res. 47, 4465–4472 (2008a)
Lee, S.C., Choi, B.Y., Lee, T.J., Ryu, C.K., Ahn, Y.S., Kim, J.C.: CO2 absorption and regeneration of alkali metal-based solid sorbents. Catal. Today 111, 385–390 (2006)
Lee, S.C., Kim, J.C.: Dry potassium-based sorbents for CO2 capture. Catal. Surv. Asia 11(4), 171–185 (2007)
Lee, S.C., Chae, H.J., Lee, S.J., Choi, B.Y., Yi, C.K., Lee, J.B., Ryu, C.K., Kim, J.C.: Development of regenerable MgO-based sorbent promoted with K2CO3 for CO2 capture at low temperatures. Environ. Sci. Technol. 42(8), 2736–2741 (2008b)
Lee, S.C., Chae, H.J., Park, Y.H., Ryu, C.K., Yi, C.K., Kim, J.C.: Novel regenerable potassium-based dry sorbents for CO2 capture at low temperatures. J. Mol. Catal. B Enzym. 56(2–3), 179–184 (2009)
Lee, S.C., Kwon, Y.M., Ryu, C.Y., Chae, H.J., Ragupathy, D., Jung, S.Y., Lee, J.B., Ryu, C.K., Kim, J.C.: Development of new alumina-modified sorbents for CO2 sorption and regeneration at temperatures below 200 °C. Fuel 90, 1465–1470 (2011)
Lee, S.C., Kwon, Y.M., Chae, H.J., Jung, S.Y., Lee, J.B., Ryu, C.K., Yi, C.K., Kim, J.C.: Improving regeneration properties of potassium-based alumina sorbents for carbon dioxide capture from flue gas. Fuel 104, 882–885 (2013)
Li, L., Wen, X., Fu, X., Wang, F., Zhao, N., Xiao, F., Wei, W., Sun, Y.: MgO/Al2O3 sorbent for CO2 capture. Energy Fuels 24, 5773–5780 (2010)
Li, L., Li, Y., Wen, X., Wang, F., Zhao, N., Xiao, F., Wei, W., Sun, Y.: CO2 capture over K2CO3/MgO/Al2O3 dry sorbent in a fluidized bed. Energy Fuels 25, 3835–3842 (2011)
Liang, Y., Harrison, D.P., Gupta, R.P., Green, D.A., McMichael, W.J.: Carbon dioxide capture using dry sodium-based sorbents. Energy Fuels 18, 569–575 (2004)
Mavroudi, M., Kaldis, S.P., Sakellaropoulos, G.P.: Reduction of CO2 emissions by a membrane contacting process. Fuel 82, 2153–2159 (2003)
Okunev, A.G., Sharonov, V.E., Aistov, Y.I., Parmon, V.N.: Sorption of carbon dioxide from wet gases by K2CO3-in-porous matrix: influence of the matrix nature. React. Kinet. Catal. Lett. 71(2), 355–362 (2000)
Okunev, A.G., Sharonov, V.E., Gubar, A.V., Danilova, I.G., Paukshtis, E.A., Moroz, E.M., Kriger, T.A., Malakhov, V.V., Aistov, Y.I.: Sorption of carbon dioxide by the composite sorbent potassium carbonate in porous matrix. Russ. Chem. Bull., Int Ed. 52(2), 359–363 (2003)
Salvador, C., Lu, D., Anthony, E.J., Abanades, J.C.: Enhancement of CaO for CO2 capture in an FBC environment. Chem. Eng. J. 96, 187–195 (2003)
Seo, Y.W., Jo, S.H., Ryu, C.K., Yi, C.K.: Effects of water vapor pretreatment time and reaction temperature on CO2 capture characteristics of a sodium-based solid sorbent in a bubbling fluidized-bed reactor. Chemosphere 69, 712–718 (2007)
Shigemoto, N., Yanagihara, T., Sugiyama, S., Hayashi, H.: Material balance and energy consumption for CO2 recovery from moist flue gas employing K2CO3-on-activated carbon and its evaluation for practical adaptation. Energy Fuels 20(2), 721–726 (2006)
Siriwardane, R.V., Shen, M.S., Fisher, E.P., Poston, J.A.: Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 15, 279–284 (2001)
Stichert, W., Schuth, F.: Influence of crystallite size on the properties of zirconia. Chem. Mater. 10, 2020–2026 (1998)
Takamura, Y., Narita, S., Aoki, J., Hironaka, S., Uchida, S.: Evaluation of dual-bed pressure swing adsorption for CO2 recovery from boiler exhaust gas. Sep. Purif. Technol. 24, 519–528 (2001)
Wilson, M., Tontiwachwuthikul, P., Chakma, A., Idem, R., Veawab, A., Aroonwilas, A., Gelowitz, D., Barrie, J., Mariz, C.: Test results from a CO2 extraction pilot plant at boundary dam coal-fired power station. Energy 29, 1259–1267 (2004)
Yi, C.K., Jo, S.H., Seo, Y.W., Lee, J.B., Ryu, C.K.: Continuous operation of the potassium-based dry sorbent CO2 capture process with two fluidized-bed reactors. Int. J. Green Gas Cont. 1(1), 31–36 (2007)
Yi, C.K., Jo, S.H., Seo, Y.W.: The effect of voidage on the CO2 sorption capacity of K-based sorbent in a dual circulating fluidized bed process. J. Chem. Eng. Jpn. 41(7), 691–694 (2008)
Zhang, B.T., Fan, M., Bland, A.E.: CO2 separation by a new solid K–Fe sorbent. Energy Fuels 25, 1919–1925 (2011)
Zhao, C.W., Chen, X.P., Zhao, C.S., Liu, Y.K.: Carbonation and hydration characteristics of dry potassium-based sorbents for CO2 capture. Energy Fuels 23(3), 1766–1769 (2009a)
Zhao, C.W., Chen, X.P., Zhao, C.S.: CO2 absorption using dry potassium-based sorbents with different supports. Energy Fuels 23(9), 4683–4687 (2009b)
Zhao, C.W., Chen, X.P., Zhao, C.S.: Effect of crystal structure on CO2 capture characteristics of dry potassium-based sorbents. Chemosphere 75, 1401–1404 (2009c)
Zhao, C.W., Chen, X.P., Zhao, C.S.: K2CO3/Al2O3 for capturing CO2 in flue gas from power plants. Part 4: abrasion characteristics of the K2CO3/Al2O3 sorbent. Energy Fuels 26(2), 1395–1400 (2012)
Acknowledgments
This work was supported by R&D program under Agency for Defense Development, Republic of Korea. We acknowledge the financial support by grants from Korea CCS R&D Center, funded by the Ministry of Education, Science and Technology of Korean government. This work was supported by the Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy (2010201020007A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.C., Cho, M.S., Jung, S.Y. et al. Effects of alumina phases on CO2 sorption and regeneration properties of potassium-based alumina sorbents. Adsorption 20, 331–339 (2014). https://doi.org/10.1007/s10450-013-9596-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9596-2