Abstract
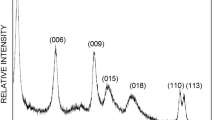
Low cost adsorption technology offers high potential to clean up laundry rinsing water. From an earlier selection of adsorbents (Schouten et al. 2007), layered double hydroxide (LDH) proved to be an interesting material for the removal of anionic surfactant, linear alkyl benzene sulfonate (LAS) which is the main contaminant in rinsing water. The main research question was to identify the effect of process parameters of the LDH synthesis on the stability of the LDH structure and the adsorption capacity of LAS. LDH was synthesized with the co-precipitation method of Reichle (1986); a solution of M2+(NO3)2 and M3+(NO3)3 and a second solution of NaOH and Na2CO3 were pumped in a beaker and mixed. The precipitate that was formed was allowed to age and was subsequently washed, dried and calcined. The process parameters that were investigated are the concentration of the initial solutions, M2+/M3+ ratio and type of cations. The crystallinity can be improved by decreasing the concentration of the initial solutions; this also decreases the leaching of M3+ from the brucite-like structure into the water. The highest adsorption capacity is obtained for Mg2+/Al3+ with a ratio 1 and 2 because of the higher charge density compared to ratio 3. Storing the LDH samples in water resulted in a reduction of adsorption capacity and a decrease in surface area and pore volume. Therefore, LDH is not applicable in a small device for long term use in aqueous surroundings. The adsorption capacity can be maintained during storage in a dry N2 atmosphere.
Similar content being viewed by others
References
Adak, A., Bandyopadhyay, M., Pal, A.: Removal of anionic surfactant from wastewater by alumina: a case study. Colloid Surf. A Physicochem. Eng. Aspects 254(13), 165–171 (2005)
Anbarasan, R., Lee, W.D., Im, S.S.: Adsorption and intercalation of anionic surfactants onto LDH, a XRD study. Bull. Mater. Sci. 28(2), 145–149 (2005)
Cavani, F., Trifiro, F., Vaccari, A.: Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 11, 173–301 (1991)
Frost, R.L., Martens, W.N., Erickson, K.L.: Thermal decomposition of the hydrotalcite: Thermogravimetric analysis and hot stage Raman spectroscopic study. J. Therm. Anal. Calorim. 82(3), 603 (2005)
Holmberg, K., Jonsson, B., Kronberg, B., Lindman, B.: Surfactants and Polymers in Aqueous Solution, pp. 357–387. Wiley, Chichester (2003)
Kloprogge, J.T., Hickey, L., Frost, R.L.: The effects of synthesis pH and hydrothermal treatment on the formation of zinc aluminum hydrotalcites. J. Solid State Chem. 177(11), 4047–4057 (2004)
Kopka, H., Beneke, K., Lagaly, G.: Anionic surfactants between double metal hydroxide layers. J. Colloid Interface Sci. 123(2), 427–436 (1988)
Lazaridis, N.K., Asouhidou, D.D.: Kinetics of sorptive removal of chromium(VI) from aqueous solution by calcined MgAlCO3 hydrotalcite. Water Res. 37, 2875–2882 (2003)
Lazaridis, N.K., Matis, K.A., Webb, M.: Flotation of metal-loaded clay anion exchangers. Part I: the case of chromates. Chemosphere 47, 373–378 (2001)
Lazaridis, N.K., Hourzemanoglou, A., Matis, K.A.: Flotation of metal-loaded clay anion exchangers. Part II: the case of arsenates. Chemosphere 47, 319–324 (2002)
Pavan, P.C., Gomes, G.A., Valim, J.B.: Adsorption of sodium dodecyl sulphate on layered double hydroxides. Microporous Mesoporous Mater. 21, 659–665 (1998)
Pavan, P.C., Crepaldi, E.L., Gomes, G.D., Valim, J.B.: Adsorption of sodium dodecylsulfate on a hydrotalcite-like compound. Effect of temperature, pH and ionic strength. Colloid Surf. A Physicochem. Eng. Aspects 154, 399–410 (1999)
Pavan, P.C., Crepaldi, E.L., Valim, J.B.: Sorption of anionic surfactants on LDH. J. Colloid Interface Sci. 229, 346–352 (2000)
Pavlovic, I., Barriga, C., Ulibarri, M.A., Hermosin, M.C., Cornejo, J.: Adsorption of acidic pesticides 2,4-D, Clopyralid and Picloram on calcined hydrotalcite. Appl. Clay Sci. 30(2), 125–133 (2005)
Pringadi, P.: Sud Chemie Germany, Ostenrieder Str. 15, 85368 Moosburg, Germany. Personal communication, July 2005
Ramakrishnan, V.: Unilever Research India, 64 Main Road, Whitefield P.O. Bangalore 560066, India. Personal communication, December 2004
Reichle, W.T.: Synthesis of anionic clay minerals. Solid State Ionics 22, 135–141 (1986)
Reis, M.J., Silverio, F., Tronto, J., Valim, J.B.: Effect of pH, temperature, and ionic strength on adsorption of sodium dodecylbenzenesulfonate into Mg-Al-CO3 layered double hydroxides. J. Phys. Chem. Solids 65, 487–492 (2004)
Schouten, N., van der Ham, A.G.J., Euverink, G.J., de Haan, A.B.: Selection and evaluation of adsorbents for the removal of anionic surfactants from laundry rinsing water. Water Res. 41, 4233–4241 (2007)
Shin, H.S., Kim, M.J., Nam, S.Y., Moon, H.C.: Phosphorus removal by hydrotalcite-like compounds. Water Sci. Technol. 34(1–2), 161–168 (1996)
Tao, Q., Zhang, Y., Zhang, X., Yuan, P., He, H.: Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chem. 179(3), 708–715 (2006)
Trujillano, R., Holgado, M.J., Pigazo, F., Rives, V.: Preparation, physicochemical characterisation and magnetic properties of Cu-Al layered double hydroxides with CO32- and anionic surfactants with different alkyl chains in the interlayer. Phys. B 373(2), 267–273 (2006)
Ulibarri, M.A., Pavlovic, I., Hermosin, M.C., Cornejo, J.: Hydrotalcite like compounds as potential sorbents of phenols from water. Appl. Clay Sci. 10, 131–145 (1995)
Xu, Z.P., Stevenson, G.S., Lu, C.Q., Lu, G.Q., Bartlett, P.F., Gray, P.P.: Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 128(1), 36–37 (2005)
Zhao, H., Nagy, K.L.: Dodeyl sulfate-hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. J. Colloid Interface Sci. 274, 613–624 (2004)
Zhu, M.X., Li, Y.P., Xie, M., Xin, H.Z.: Sorption of an anionic dye by uncalcined and calcined LDH. J. Hazard. Mater. 120(1–3), 163–171 (2005)
Zou, K., Zhang, H., Duan, X.: Studies on the formation of 5-aminosalicylate intercalated Zn-Al layered double hydroxides as a function of Zn/Al molar ratios and synthesis routes. Chem. Eng. Sci. 62(7), 2022–2031 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schouten, N., van der Ham, L.G.J., Euverink, GJ.W. et al. Optimization of layered double hydroxide stability and adsorption capacity for anionic surfactants. Adsorption 13, 523–532 (2007). https://doi.org/10.1007/s10450-007-9073-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-007-9073-x