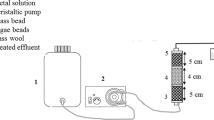
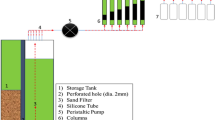
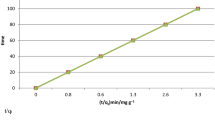
Abstract
Physical and chemical characterization of algae Gelidium particles shows a gel structure, with two major binding groups, carboxylic and hydroxyl groups, with an affinity constant distribution for protons, well described by a Quasi-Gaussian distribution suggested by Sips. A continuous model, considering a heterogeneous distribution of the carboxylic groups, determined by potentiometric titration experiments, was able to predict equilibrium data at different pH. The metal uptake capacity decreases with the solution pH, suggesting that competition exists between hydrogen ions, present in high concentrations for low pH values, and metal ions. For high ionic strengths, adsorption sites will be surrounded by counter ions and partially lose their charge, which weakens the contribution of the electrostatic binding and decreases the overall adsorption. A small influence of the temperature in the adsorption process was observed. Batch kinetic experiments were also performed, at different pH values, and results were well fitted by a mass transfer model, considering the intraparticle diffusion resistance given by the linear driving force model (LDF). Continuous stirred adsorber (CSTA) and packed bed column configurations were also tested for metal adsorption. The biosorbent regeneration was achieved by contacting it with strong acid (0.1 M HNO3). A mass transfer model was applied with success to describe the biosorption/desorption process in CSTA and packed bed column, considering the equilibrium given by the Langmuir equation/mass action law and film and intraparticle diffusion resistances.
Similar content being viewed by others
Abbreviations
- a p :
-
Specific area for thin plates particles
- C b :
-
Metal concentration in the bulk (mg or mmol metal/l fluid)
- \(C_{b_{0}}\) :
-
Initial metal concentration in the bulk (mg or mmol metal/l fluid)
- C E :
-
Feed concentration (mg or mmol metal/l fluid)
- C f :
-
Metal concentration in the film (mg or mmol metal/l fluid)
- C final :
-
Metal concentration in the solution at the end of the saturation or elution process (mg or mmol metal/l fluid)
- C CI :
-
Initial metal concentration in the solution (elution process) (mmol metal/l fluid)
- C H :
-
Equilibrium concentration of proton in the fluid phase (mmol proton/l fluid)
- C M :
-
Equilibrium concentration of metal in the fluid phase (mmol metal/l fluid)
- C T :
-
Total (metal + acid) liquid concentration (mmol/l fluid)
- \(C_{T_{0}}\) :
-
Initial total (metal + acid) liquid concentration (mmol/l fluid)
- \(C_{T_{E}}\) :
-
Total feed (acid) liquid concentration (mmol/l fluid)
- D ax :
-
Axial dispersion coefficient (cm2/s)
- D h :
-
Homogeneous diffusion coefficient (cm2/s)
- IS:
-
Ionic strength (M)
- k f :
-
Film mass transfer coefficient (cm/s)
- k p :
-
Mass transfer coefficient for intraparticle diffusion (cm/s)
- K H :
-
Equilibrium proton constant (l fluid/mmol H)
- K M :
-
Equilibrium metal constant (l fluid/mmol M)
- K H ′:
-
Average value of the affinity constant distribution for the proton (l fluid/mmol H)
- K M ′:
-
Average value of the affinity constant distribution for the metal (l fluid/mmol M)
- K L :
-
Equilibrium constant of Langmuir (l fluid/mg M)
- K M H :
-
Selectivity coefficient between ion M in the particle and ion H in solution
- L :
-
Bed length (cm)
- n :
-
Empirical dimensionless parameter
- n M and n H :
-
Constants that reflect the overall non-ideality of metal and proton
- N d :
-
Number of mass transfer units by intraparticle diffusion
- N f :
-
Number of mass transfer units by film diffusion
- p :
-
Represents the intrinsic heterogeneity of the biosorbent
- Pe :
-
Axial Peclet number based on the bed length
- Pe p :
-
Axial Peclet number based on the particle diameter (spherical) or width (thin plate)
- pH SE :
-
pH of feed solution
- 〈q〉:
-
Average metal concentration in the solid phase (mg or mmol metal/g biomass)
- q E :
-
Solid phase concentration in equilibrium with C E (mg or mmol metal/g biomass)
- q H :
-
Equilibrium concentration of proton in the biomass (mmol metal/g biomass)
- q L e q LF :
-
Maximum amount of metal per g of adsorbent (mg/l)
- q M :
-
Equilibrium concentration of metal in the biomass (mg or mmol metal/g biomass)
- \(q_{M_{0}}\) :
-
Metal concentration in the solid phase in equilibrium with \(C_{b_{o}}\) (mg or mmol metal/g biomass)
- q * :
-
Solid phase concentration in equilibrium with C f (mg or mmol metal/g biomass)
- q t :
-
Concentration of ion species in the sorbent at time t (mg metal g biosorbent−1)
- Q max :
-
Concentration of carboxylic groups or maximum capacity of biomass (mmol/g biomass)
- r :
-
The dimensionless axial coordinate inside the particle
- R :
-
Half of thickness of the thin plate (cm)
- Sh :
-
Sherwood number
- t :
-
Time (s)
- t b :
-
Breakthrough time (s)
- t st :
-
Stoichiometric time (s)
- T :
-
Temperature (°C)
- u i :
-
Interstitial fluid velocity (cm/s)
- V :
-
Metal solution volume (l)
- V r :
-
Volume of the adsorber (CSTA) (cm3)
- x :
-
Axial position normalized by the bed length
- 〈y〉:
-
Dimensionless average concentration in the solid phase
- y b ′ or y b :
-
Dimensionless concentration in the fluid phase
- y f ′:
-
Dimensionless concentration in the fluid phase at the film
- y T :
-
Dimensionless total concentration in the fluid phase
- y* or y M :
-
Dimensionless concentration in the solid phase at the particle surface
- z :
-
The distance to the symmetry plane (cm)
- z′:
-
Bed axial position (cm)
- W :
-
Mass of biosorbent (g)
- ε :
-
Porosity of the bed
- τ :
-
Space time (s)
- τ d :
-
Time constant for intraparticle diffusion
- τ f :
-
Time constant for film diffusion
- θ :
-
Dimensionless time
- ρ ap :
-
Apparent density of particles (g solid/cm3 particle)
- ξ :
-
The batch capacity factor
- ξ′:
-
Adsorber capacity factor for saturation
- ξ″:
-
Adsorber capacity factor for desorption
References
Al-Ashed, S., Duvnjak, Z.: Adsorption of copper and chromium by Aspergillus carbonarius. Biotechnol. Prog. 11, 638–642 (1995)
Atkinson, B., Bux, F., Kasan, H.: Considerations for application of biosorption technology to remediate metal-contaminated industrial effluents. Water SA 24(2), 129–135 (1998)
Babel, S., Kurniawan, T.A.: Low-cost adsorbents for heavy metals uptake from contamined water: a review. J. Hazard. Mater. B97, 219–243 (2003)
Bailey, S.E., Olin, T.J., Bricka, R.M., Adrian, D.D.: A review of potentially low-cost sorbents for heavy metals. Water Res. 33(11), 2469–2479 (1999)
Barba, D., Beolchini, F., Vegliò, F.: A simulation study on biosorption of heavy metals by confined biomass in UF/MF membrane reactors. Hydrometallurgy 59, 89–99 (2001)
Beolchini, F., Pagnanelli, F., Vegliò, F.: Modeling of copper biosorption by Arthrobacter sp. in a UF/MF membrane reactor. Environ. Sci. Technol. 35, 3048–3054 (2001)
Beolchini, F., Pagnanelli, F., Toro, L., Vegliò, F.: Copper biosorption by Sphaerotilus natans confined in UF membrane module: experimental study and kinetic modeling. Hydrometallurgy 72, 21–30 (2004)
Beolchini, F., Pagnanelli, F., Toro, L., Vegliò, F.: Continuous biosorption of copper and lead in single and binary systems using Sphaerotilus natans cells confined by a membrane: experimental validation of dynamic models. Hydrometallurgy 76, 73–85 (2005)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Davis, T.A., Volesky, B., Mucci, A.: A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 37, 4311–4330 (2003)
Dean, J.A.: Lange’s Handbook of Chemistry, 12th edn. McGraw–Hill, New York (1979)
Freundlich, H.: Üeber die Adsorption in Löesungen. Z. Phys. Chem. 57, 385–470 (1907)
Froment, G.F., Bischof, K.B.: Chemical Reactor Analysis and Design. Wiley, New York (1990)
Gavrilescu, M.: Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 4(3), 219–231 (2004)
Glueckauf, E., Coates, J.I.: Theory of chromatography. Part IV. The influence of incomplete equilibrium on the front boundary of chromatograms and on the effectiveness of separation, J. Chem. Soc. pp. 1315–1321 (1947)
Hindmarsh, A.C.: Odepack, a Systematized Collection of ODE Solvers, in Scientific Computing. Scientific Computing, Amsterdam (1983)
Kinniburgh, D.G., Riemsdijk, W.H.V., Koopal, L.K., Borkovec, M., Benedetti, M.F., Avena, M.J.: Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf. A 151, 147–166 (1999)
Kratochvil, D., Volesky, B.: Biosorption of Cu from ferruginous wastewater by algal biomass. Water Res. 32(9), 2760–2768 (1998)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
Madsen, N., Sincovec, R.: PDECOL: General collocation software for partial differential equations. ACM Trans. Math. Soft. 5(3), 326–351 (1979)
Matheickal, J.T., Yu, Q.: Biosorption of lead(II) and copper(II) from aqueous solutions by pre-treated biomass of Australian marine algae. Biores. Technol. 69(3), 223–229 (1999)
Mouradi-Givernaud, A., Hassani, L.A., Givernaud, T., Lemoine, Y., Benharbet, O.: Biology and agar composition of Gelidium sesquipedale harvested along the Atlantic coast of Morocco. In: Hydrobiologia, pp. 391–395 (1999)
Nieboer, E., McBryde, W.A.E.: Free-energy relationship in coordination chemistry, III: a comprehensive index to complex stability. Can. J. Chem. 51, 2512–2524 (1973)
Pagnanelli, F., Beolchini, F., Biase, A.D., Vegliò, F.: Biosorption of binary heavy metal systems onto Sphaerotilus natans cells confined in an UF/MF membrane reactor: dynamic simulations by different Langmuir-type competitive models. Water Res. 38, 1055–1061 (2004)
Pearson, R.G.: Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963)
Radke, C.J., Prausnitz, J.M.: Adsorption of organic solutes from dilute aqueous solution of activated carbon. Ind. Eng. Chem. Fundam. 11(4), 445–451 (1972)
Reddad, Z., Gérente, C., Andrès, Y., Thibault, J.-F., Cloirec, P.L.: Cadmium and lead adsorption by a natural polysaccharide in Mf membrane reactor: experimental analysis and modeling. Water Res. 37, 3983–3991 (2003)
Redlich, O., Peterson, C.: Useful adsorption isotherm. J. Phys. Chem. 63, 1024 (1959)
Reid, R.C., Prausnitz, J.M., Poling, B.E.: The Properties of Gases & Liquids, 4th edn. McGraw–Hill, New York (1987)
Rodrigues, A.E., Beira, E.C.: Staged approach to percolation processes. Part I. Sorption processes in a perfectly mixed reactor: influence of nonlinear equilibrium isotherm and external mass transfer resistance, AIChE J. 25, 416–423 (1979)
Sips, R.: On the structure of a catalyst surface. J. Chem. Phys. 16, 490–495 (1948)
Vignon, M.R., Morgan, E., Rochas, C.: Gelidium sesquipedale (Gelidiales, Rhodophyta), I: soluble polymers. Bot. Mar. 37, 325–329 (1994a)
Vignon, M.R., Rochas, C., Vuong, R., Tekeley, P., Chanzy, H.: Gelidium sesquipedale (Gelidiales, Rhodophyta), II: an untrastructural and morphological study. Bot. Mar. 37, 331–340 (1994b)
Vilar, V.J.P.: Remoção de iões metálicos em solução aquosa por resíduos da indústria de extracção do agar. PhD thesis, Faculty of Engineering University of Porto, Porto (2006)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Proc. Biochem. 40(10), 3267–3275 (2005a)
Vilar, V.J.P., Sebesta, F., Botelho, C.M.S., Boaventura, R.A.R.: Equilibrium and kinetic modeling of Pb2+ biosorption by granulated agar extraction algal waste. Proc. Biochem. 40(10), 3276–3284 (2005b)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Equilibrium and kinetic modeling of Cd(II) biosorption by algae Gelidium and agar extraction algal waste. Water Res. 40(2), 291–302 (2006a)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behavior. J. Hazard. Mater., doi:10.1016/j.jhazmat.2006.1012.1055 (2006b)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Copper desorption from Gelidium algal biomass. Water Res. 41(7), 1569–1579 (2007a)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Copper removal by algae Gelidium, agar extraction algal waste and granulated algal waste: kinetics and equilibrium. Biores. Technol., doi:10.1016/j.biortech.2007.01.042 (2007b)
Vilar, V.J.P., Botelho, C.M.S., Boaventura, R.A.R.: Kinetics and equilibrium modeling of lead uptake by algae Gelidium and algal waste from agar extraction industry. J. Hazard. Mater. 143(1–2), 396–408 (2007c)
Volesky, B.: Biosorption of Heavy Metals. CRC Press, Montreal (1990)
Volesky, B.: Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59, 203–216 (2001)
Volesky, B.: Sorption and Biosorption, 1st edn. BV Sorbex, Quebec (2003)
Wase, J., Forster, C.: Biosorbents for Metal Ions. Taylor & Francis, London (1997)
Yang, J., Volesky, B.: Intraparticle diffusivity of Cd ions in a new biosorbent material. J. Chem. Technol. Biotechnol. 66, 355–364 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilar, V.J.P., Botelho, C.M.S. & Boaventura, R.A.R. Modeling equilibrium and kinetics of metal uptake by algal biomass in continuous stirred and packed bed adsorbers. Adsorption 13, 587–601 (2007). https://doi.org/10.1007/s10450-007-9029-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-007-9029-1