Abstract
The kinetic constants for free and immobilized cells were determined by measuring reaction rates at different metal concentrations at the optimum reaction conditions. (Kmax and Vmax) were calculated from the slope and intercept of the straight lines. The pseudo-second-order rate constants are derived based on the sorption capacity of the solid phase, where K2 is the rate constant for the pseudo-second-order model. Determined experimentally by plotting t/q against t. The mean free vitality of adsorption (E) was figured as 2.62 kJ mol−1 and the extent of E communicated gives data on the adsorption mechanism. An E value ranging from 1 to 8 indicates physisorption and 8–16 kJ mol−1 predicts ion exchange. Thus, the evaluated value of 2.62 kJ mol−1 predicts the phenomenon of physisorption, which suggests that metal ions were favorably adsorbed by this biosorbent in a multi-layer fashion. The overall result suggested that 98.2% of U (VI) by biosorption of U in the mechanism of adsorption will include chemisorption mechanistic pathway: Langmuir, Freundlich, equations and the values of Kf 5.791 where KL 3.9 were determined from the linear plot of log qe vs. log Ce at 30 °C, indicating that metal ions were favorably adsorbed by this biosorbent in a multi-layer fashion and instrumentation of beads characterizing novel Binding sites using FTIR & SEM beside change in peaks position which assigned for its groups confirm biosorption of metal.
Similar content being viewed by others
Introduction
This isotherm is a blind equation and is widely used for the description of adsorption, including the adsorption of natural and inorganic mixes on a wide assortment of adsorbents. qe is the amount adsorbed, Kf is the characteristic constant related to the adsorption capacity, Ce is the equilibrium concentration, and n is the specific consistency related to the level of ideality of adsorption. When 1/n = 1, the value of Kf is determined by the units in which qe and ce are expressed1.
Kinetic models have been used to test the experimental data. In addition, information on the kinetics of metal uptake is required to select the optimum conditions for full-scale batch metal removal processes. The solute evacuation rate, which regulates the sorbate's live arrangement time at the solid arrangement interface, is how adsorption energy is conveyed2,3,4,5.
The pseudo-second-order kinetic parameters were determined using both linear and nonlinear techniques. Kinetic analysis is required to optimize various conditions for the biosorption process. Pseudo-first-order and pseudo-second-order models were used to study the kinetics of metal sorption. This kinetic model was developed based on the observation that the change in metal concentration with respect to time is proportional to a power of 1. A model for pseudo-second-order kinetics explains the sorption mechanism over the entire range of contact time in a pseudo-second-order kinetic model6. The model as written has the highest coefficient of determination; constants of the pseudo-second-order rate were determined experimentally by plotting t/q vs. t. This approach suggests that the rate-limiting step in the biosorption of heavy metals is chemisorption, which involves valence forces through the sharing or exchange of electrons between sorbent and sorbate (complexation), and is why the nonlinear method is thought to be a better way to determine the desired parameters, coordination, and/or chelation2,3. Several isotherm models, including the Freundlich and Langmuir models, are used to analyze the equilibrium behavior of single-component adsorption. According to the Freundlich model, adsorption occurs on a heterogeneous surface, which is a non-traditional approach. However, according to the Langmuir model, adsorption takes place at an adsorbent surface with homogeneous active sites. The pollutants interact and compete with one another as a result of the sewage's various components. Since single-component systems cannot be represented by the models used for multicomponent systems, they cannot be used. This is caused by various factors, including the complex mechanics involved in multicomponent adsorption. There has been extensive use of advanced models. As a result, the isotherm has just one component, while multi-component models are modified and explained below6.
Methods
Heavy metal adsorption by immobilized bacteria immobilized by calcium alginate beads was prepared by dispensing a 3% (w/v) sodium alginate solution containing 3 mL of a 100% (v/v) E. coli cell-free extract. E. coli S6 beads were dropped into a continuously stirred (250 rpm) 2% (w/v) CaCl2 solution and left in the CaCl2 solution overnight. Bacterial cells were set up according to Leung et al.7 and were maintained in the conical flask containing 50 ml of samples for incubation, after which the specimens were pulled back for substantial metal examination by utilizing the titration method of uranium conc. The uranium content of the sample, the prepared standard solution, and the treated solution was measured at predetermined intervals in accordance with the method developed by the Nuclear Materials Authority and described by Davies and Gray in 1964. The results were utilized to fit various isothermal kinetic equations of adsorption, such as Langmuir, Freundlich, and isothermal models2, in batch mode with U (VI).
The determination of kinetic parameters of free and immobilized cells was performed for measuring reaction rates and kinetic constants at different metal concentrations, U (mg−1), qe (mg−1), and qe = 0.48 ((mg/g−1)) at the optimum reaction conditions. (Km and Vmax) were calculated from the slope and intercept of the straight lines, respectively.
Determination of uranium
The uranium content of the sample, prepared standard, and treated solution were determined according to the method described by and8,9.
Reagents
-
1.
Orthophosphoric acid (H3PO4, 85%)
-
2.
Conc. (HCl, 32%)
-
3.
10% ammonium ferrous sulphate(10 gm A.F. S + 10 ml H2so4 conc. then up to volume of 100 ml with distilled water).
-
4.
Titanium trichloride (TiCl3)
-
5.
Sodium nitrite (NaNO2)
-
6.
20% urea solution
-
7.
Sodium salt (0.2 g diphenyl amino-4-sulfonic acid sodium salt + 0.2 sodium carbonate, then add drops of dist. H2O with stirring and up to volume 100).
-
8.
Ammonium meta vanadate (NH4VO3).
Procedure
-
In a dry and clean 100 ml Erlenmeyer flask, 5 ml of uranium sample was placed, and the following chemicals were added in the same order.
-
ten ml of double-distilled water
-
10 ml (H3PO4)
-
1 mL conc. (HCl).
-
5 drops of 10% ammonium ferrous sulphate.
-
2–3 drops of Ticl3 were added till the solution changed to a purplish color.
-
The reaction was left for 5 min.
-
3 drops of 15% NaNo2 were added till the brownish color appeared, then disappeared. Immediately, 5 ml of (urea 20%) was added and followed by rapid shaking till the air bubbles were stopped.
-
The reaction was left again for 2 min before adding the indicator.
-
2 drops of indicator sodium salt were added.
-
Titration against 0.1 Ammonium meta-vanadates was performed till the end point of pale violet color appeared.
The uranium concentration was calculated according to the following equation:
where (T) is the titration intensity of NH4VO3 Solution, (V1) is the consumed volume of NH4VO3 Solution, and (v) solution is the volume of the measured sample.
Materials
Ammonium meta-vanadates (NH4VO3) from biomaterials India, indicator sodium salt (0.2 gm diphenyl amino-4-sulfonic acid sodium salt + 0.2 g sodium carbonate from biomaterials India, titanium trichlorid (Ticl3) from biomaterials India, Hidrochloric acid (HCl) from biomaterials, orthophosphoric acid (H3PO4) from biomaterials, Sodium nitrite (NaNO2) from biomaterials, 20% Urea Solution co (NH2)2 from biomaterials, sodium alginate From sigma alderich , NaNo2 sodium Nitrite from biomaterials .
Results and discussion
Kinetic study of isotherm models
The pseudo-second-order rate constants were tentatively resolved by plotting t/q against t, as shown in Figs. 1, 2, and 3. Compared to pseudo-first-arrange energy, the pseudo-second-arrange display as composed has the highest coefficient of assurance; this model is thought to be more appropriate to describe the kinetic data in biosorption systems10. Our findings were put to use to fit a number of isotherm kinetic equations for adsorption, including Langmuir, Freundlich, and isotherm models with U parallel with2 findings.
Bio-sorption and slow phase involved active metabolism-dependent transport of metal into the bacterial cells. Although there may have been some concurrent ion inhibition when the modified alginate beads were used to remediate 10 mg−1 U (VI), bioremediation ultimately prevailed, characterization of the newly synthesized product and proof of the physical nature of the newly synthesized alginate materials that are to be cited here in this article The idea of the coupling sites and their inclusion during biosorption can be roughly examined with the help of FTIR. As a result, we calculated the coupling points using FTIR instruments We used FT-IR spectra to confirm the availability of binding sites, as shown in Tables 4 and 5. Data of FT-IR OF Unloaded E. coli. (S6) Bacterial Isolation as indicated in tables after loading For uranium, we discovered amino acid (O–H) stretching, protein (N–H) stretching, phosphate C–O stretching band, P–H stretching, protein amide I band primarily (C=O) stretching, protein (CH2) and (CH3) bending of methyl, lipid (CH2) bending of methyl, carbohydrate (c-o) of polysaccharides, nucleic acid (other phosphate containing compound), >p=o stretching.
Whereas regarding our study under publication, "Application of the FTIR spectra of U-loaded and unloaded free and immobilized cells and Scanning Electron Microscopy (SEM) The microcapsule system had good mechanical strength, flexibility, and biocompatibility between the E. coli capsule and the microcapsule. In addition, the internal, three-dimensional network structure of the microcapsule provided sufficient spaces for E. coli capsule growth and good encapsulating stability. Scanning electron microscopy of these beads, the synthetic solution in the sample, and the control showed that they were hollow from the inside (having smooth inner walls). In SEM/EDS analysis of the Ca-alginate beads after the experiment, void spaces of the beads were found to be filled with precipitates of heavy metals, showing that Ca-alginate beads can be successfully used as a biosorbent for the removal of uranium, which agreed with11,12,13,14. Analysis revealed that the carboxyl and amino groups were responsible for metal binding Negatively charged and easily accessible carboxyl groups are essential for the binding of metal captions12,13,14,15. It has been observed that potentiometric titrations can be used to gather data on the types and numbers of binding sites. Pseudomonas aeruginosa was titrated by15, who also When E. coli was grown in the presence of U (VI), it was discovered that the unstained whole mount of E. coli interacted with the metal. Since the whole cells were collected, the contrast seen in the micrographs was due to the binding/accumulation of metallic U only16.
At various beginning metal concentrations, straight-line graphs of log (qe − qo) against t were generated hypothetically to determine the rate constants and equilibrium metal uptake17. The experimental value is then contrasted with the qe value obtained using this procedure. The heat of adsorption can be used to determine whether the biosorption process is exothermic or endothermic. The Langmuir constant, KL, and temperatures T, where Ko is the adsorption equilibrium constant, E in (kJ mol−1) is the activation energy of adsorption/heat of adsorption, R is the gas constant (0.0083 kJ/mol−1 K−1), and T is the absolute temperature (K)18.
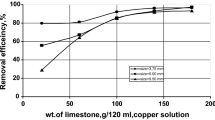
The percentage of remediation of U (VI) was calculated as U (VI) % = (Ci − Cf)/Ci * 100, where Ci = initial concentration and Cf = final concentration. The residual U (VI) in the medium was quantified by titration. At the typical remedial rate (R) of 100 ppm U (VI) m−3 of water per day, it was discovered. The final result was attained after five repetitions when there was no detectable U (VI) in the container. For initial concentrations of 100 of U (VI) (Tables 1 and 2), the values of qe, or the quantity of metal adsorbed (in mg/g) on the bead biomass, were determined using Eq. (1)
The results were fitted with isotherm models of Langmuir and Freundlich. A plot of Ce/qe vs. Ce (Fig. 2).
According to Eq. (1), the square of the regression coefficient, R2, was calculated to be 0.9986, indicating that the Langmuir isotherm could not be the perfect model but completely describe the adsorption, Where from Freundlich isotherm Eq. (2)
the linear plot of log qe vs. log Ce as at 30 °C (Fig. 2), and the values of Kf (5.791), which revealed that metal ions were positively adsorbed by this biosorbent in a multi-layer form. The adsorption data were also plotted as RT ln [1 + 1/Ce] vs. ln qe.
Adsorption capacity is the most important characteristic of an adsorbent. It is defined as the volume of adsorbate that the adsorbent can hold in one unit of mass. As the interaction between sorbent and solute molecules is anticipated to be strong, various mechanisms may be at play. Several characteristics, including specific surface area, cation exchange capacity, and specific volume, affect this value19,20. For example, hydroxyl, carbonyl, carboxyl, sulfhydryl, thioether, sulfonate, amine, imine, amide, imidazole, phosphonate, and phosphodiester groups may be present within the biosorbent structure, and adsorption will not be limited to physical bonding21,22,23.
All of this data points to the functional groups listed in Tables 5 and 6 as being in charge of the uptake of metals in our bacterial biomass. Also supporting the biosorption of metal ions from waste due to ion charge interactions is the change in peak locations ascribed to its groups. When Tables 3, 4, and 5 were compared, we discovered a rise in the number of binding sites, which shows that immobilized bacteria have high efficiency for metal uptake and also alters the peak positions assigned to its groups, confirming the biosorption of metal ions from waste due to ion charge interactions (Table 6).
Energy dispersive X-ray (EDX) and scanning electron microscopy.
Alginate beads (Figs. 4 and 5), predominantly ellipsoidal spheres, with an average diameter of 3–5 mm were used in the packed bed to remediate 10–1000 ppm U (VI) in a synthetic uranium solution. The effectiveness of different dosages of beads was considered, and the optimized ratio of 1:5 (v/v) of beads to water was used in all batch studies of isotherm kinetics. Scanning electron microscopy of these beads, synthetic solution (Figs. 3 and 4), and control (Fig. 2), showed that they were hollow from inside (having smooth inner walls). In SEM/EDS analysis of the Ca-alginate beads after the experiment, void spaces of the beads were found to be filled with precipitates of heavy metals, showing that Ca-alginate beads can be successfully used as a biosorbent for the removal of uranium, which agreed with24 as in Figures 4, 5, 6, and 4, as seen in Figs. 4 and 5. Uranium biosorption has been confirmed in the spot zone.
Isotherm parameters value
It has been stated that the extent of E suggests an adsorption mechanism. E values ranging from 1 to 8 indicate physiosorption and 8–16 kJ mol−1 predict ion-exchange. The mean free vitality of adsorption (E) was stated as 2.62 kJ mol−12,25,26. According to the overall findings, 98.2% of U (VI) was absorbed through the biosorption of the U models used to compute adsorption capacity: Equations show that the same beads might be utilized for biosorption for 15 days, thus the evaluated value of 2.62 kJ mol−1 This suggests physiosorption as the process but also mentions chemisorption, which involves valence forces through the sharing or exchange of electrons between the sorbent and the sorbate, complexation, coordination, and/or chelation2,3,27. The heat of the adsorption of metal ions suggests that a physical mechanism underlies adsorption. It was thought that the diffusion of heavy-metal ions into the porosity of beads was the rate-determining step of the adsorption since the diffusion profile of the bead technique was similar to the profile of active carbon, which physically adsorbs the heavy metal ions23,28,29. According to the researcher cited below, because the pores of the beads were clogged with deposited copper, the researcher here shows that the porous structure of the host polymer has a large number of pores. In the outer part of the beads, clogging or blocking of the pores may occur due to the Cuo deposit, it is probable that the metal ions were adsorbed during the formation of a multi-layer. Therefore, forces of physical attraction might have been at fault. Therefore, the adsorption may have resulted from physical attraction forces. The dense pore structure and rough surface of kaolin can offer the physical space required for the chemical adsorption of heavy metals at high temperatures. A certain surface area, pore size, and pore volume offer a richer physical adsorption space and more active sites for the chemisorption of heavy metals30,31,34 (Fig. 6).
The endpoint was reached after 5 times when there was no amount of U (VI) measured in the present container. The values of qe, or the amount of metal adsorbed (in mg/g) on the bead biomass, were calculated using Eq. U (VI) % = (Ci − Cf)/Ci * 100, where Ci = initial concentration and Cf = final concentration as the interaction between sorbent and solute molecules is expected to be strong, various mechanisms may be involved. Adsorption capacity is the most important characteristic of an adsorbent19,20,35. R is a universal gas constant, and T is the absolute temperature (K). The heat of adsorption can be calculated from the plots of log b versus 1/T 19, 2436,37,38. According to39,40,41 Tables 2, 3, and 4 provide the parameters and the typical percent % biosorption practically and expected theoretically as shown the in tables. The equilibrium isotherm was of a favourable kind, where the Langmuir equation accurately captures the results of our experimental work. The monolayer coverage of the metal ions on the surface of the biomass is shown by the application of the Langmuir isotherm. The overall result suggested that 98.2% of U (VI) by biosorption of U in the mechanism of adsorption will include chemisorption mechanistic pathway42–44.
Conclusion
The kinetic constants for free and immobilized cells were determined by measuring reaction rates at different metal concentrations, U (mg−1), qe (mg g−1), and qe = 0.48 mg g−1 at the optimum reaction conditions. The average free vitality of adsorption (E) was calculated to be 2.62 kJ mol−1. The extent of E communication gives data on the adsorption mechanism. An E value ranging from 1 to 8 indicates that the adsorption mechanism is physiosorption and a range from 8 to 16 kJ mol−1 predicts indicates that the adsorption mechanism is ion-exchange. Thus, the evaluated value of 2.62 kJ mol−1 and this predicts the adsorption mechanism as physiosorption but also includes chemisorption. The overall result suggests that 98.20% of U (VI) by biosorption of U in the mechanism of adsorption will not be restricted to physical bonding, and the kinetic model of pseudo-second-order represents the experimental data, accurately, the Langmuir model of favorable type for the biosorption isotherm indicating that metal ions were favourably adsorbed by this biosorbent in a multi-layer fashion, With characterization of novel binding sites using FTIR and SEM, as well as a change in peak position assigned to its groups.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Ci :
-
Initial concentration
- Cf :
-
Final concentration
- q:
-
Adsorbative capacity per unit mass of adsorbent [mmol/g]
- KL :
-
Langmuir constant
- Kf :
-
Freundlich constant
- qt :
-
Adsorbed amount on the biosorbent at time t [mg/g]
- R2 :
-
Correlation coefficient
- R:
-
Universal gas constant [cal/mol K]
- T:
-
Absolute temperature [K]
References
Calfa, B. A. & Torem, M. L. On the fundamentals of Cr (III) removal from liquid streams by a bacterial strain. Miner. Eng. 21(1), 48–54 (2008).
Baral, S. S., Das, S. N. & Rath, P. Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust. Biochem. Eng. J. 31(3), 216–222 (2006).
Sağ, Y. & Aktay, Y. Kinetic studies on sorption of Cr (VI) and Cu (II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem. Eng. J. 12(2), 143–153 (2002).
Fadl, M. & Zenatkamelmohamed, M. A. A. Enhancement of survival and uranium bioleaching and bio-sorption properties of bacteria by immobilization techniques in Egypt. Ann. Romanian Soc. Cell Biol. 25(6), 7732–7757 (2021).
Vijayaraghavan, K. & Yun, Y. S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 26(3), 266–291 (2008).
Edokpayi, J. N., Odiyo, J. O., Msagati, T. A. & Popoola, E. O. A Novel Approach for the removal of lead (II) ion from wastewater using mucilaginous leaves of diceriocaryum eriocarpum plant. Sustainability 7(10), 14026–14041 (2015).
Leung, W. C., Wong, M. F., Chua, H., Lo, W., Yu, P. H. F. & Leung, C. K. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci. Technol. 41(12), 233–240 (2000).
Davies, W. & Gray, U. A rapid and specific titrimetric method for the precise determination of uranium using iron (II) sulphate as reductant. Talanta 11(8), 1203–1211 (1964).
Ho, Y. S. & McKay, G. Application of kinetic models to the sorption of copper (II) on to peat. Adsorpt. Sci. Technol. 20(8), 797–815 (2002).
Ho, Y. S. & Ofomaja, A. E. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem. Eng. J. 30(2), 117–123 (2006).
Mishra, V. & Tadepalli, S. Biosorption of toxic heavy metals on sawdust. Clean: Soil, Air, Water https://doi.org/10.1002/clen.20130093 (2014).
Yadav, S. et al. Cationic dye removal using novel magnetic/activated charcoal/β-cyclodextrin/alginate polymer nanocomposite. Nanomaterials 10(1), 170 (2020).
Yadav, S. et al. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 409, 124840 (2021).
Yadav, S. et al. Facile preparation of methionine-functionalized graphene oxide/chitosan polymer nanocomposite aerogel for the efficient removal of dyes and metal ions from aqueous solutions. Environ. Nanotechnol. Monit. Manag. 18, 100743 (2022).
Kang, S. Y., Lee, J. U. & Kim, K. W. Biosorption of Cr (III) and Cr (VI) onto the cell surface of Pseudomonas aeruginosa. Biochem. Eng. J. 36(1), 54–58 (2007).
Panda, J. & Sarkar, P. Biosorption of Cr (VI) by calcium alginate-encapsulated Enterobacter aerogenes T2, in a semi-batch plug flow process. Water Air Soil Pollut. 226(1), 1–10 (2015).
Srivastava, V. C., Mall, I. D. & Mishra, I. M. Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash. Chem. Eng. Process. 48(1), 370–379 (2009).
Chandra Srivastava, V., Deo Mall, I. & Mani Mishra, I. Modelling individual and competitive adsorption of cadmium (II) and zinc (II) metal ions from aqueous solution onto bagasse fly ash. Sep. Sci. Technol. 41(12), 2685–2710 (2006).
Dursun, A. Y. A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper (II) and lead (II) ions onto pretreated Aspergillus niger. Biochem. Eng. J. 28(2), 187–195 (2006).
Zafar, M. N., Nadeem, R. & Hanif, M. A. Biosorption of nickel from protonated rice bran. J. Hazard. Mater. 143(1–2), 478–485 (2007).
Agarwal, G. S., Bhuptawat, H. K. & Chaudhari, S. Biosorption of aqueous chromium (VI) by Tamarindus indica seeds. Bioresour. Technol. 97(7), 949–956 (2006).
Abu Al-Rub, F. A. Biosorption of zinc on palm tree leaves: Equilibrium, kinetics, and thermodynamics studies. Sep. Sci. Technol. 41(15), 3499–3515 (2006).
Apiratikul, R. & Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 99(8), 2766–2777 (2008).
Park, S. & Lee, M. Removal of copper and cadmium in acid mine drainage using Caalginate beads as biosorbent. Geosci. J. 21, 373–383 (2017).
Ansari, M. I., Masood, F. & Malik, A. Bacterial biosorption: a technique for remediation of heavy metals. Microbes Microb. Technol. 283–319. (2011).
Junior, O. P. et al. Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: Optimization using RSM and methylene blue adsorption. J. Anal. Appl. Pyrol. 105, 166–176 (2014).
Fadl, M. G. & Kamel, Z. Cholesterol-lowering effects and safety assessment of Lactobacillus spp. in vivo and in vitro testing for human use as probiotic from the dairy product in Egypt. J. Genet. Eng. Biotechnol. 20(1), 1–11 (2022).
McLean, J. S., Beveridge, T. J. & Phipps, D. Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ. Microbiol. 2(6), 611–619 (2000).
Fakhry, H. et al. A treatment of wastewater containing safranin O using immobilized Myriophyllum spicatum L. onto polyacrylonitrile/polyvinylpyrrodlidone biosorbent. J. Inorganic Organomet. Polym. Mater. 1–15. (2022).
Nakajima, A. & Baba, Y. Mechanism of hexavalent chromium adsorption by persimmon tannin gel. Water Res. 38(12), 2859–2864 (2004).
Li, H., Jiao, Y., Xu, M., Shi, Z. & He, B. Thermodynamics aspect of tannin sorption on polymeric adsorbents. Polymer 45(1), 181–188 (2004).
Hassanin, M. A. et al. Sustainable remedy waste to generate SiO2 functionalized on graphene oxide for removal of U (VI) ions. Sustainability 14(5), 2699 (2022).
Godlewska-Żyłkiewicz, B., Sawicka, S. & Karpińska, J. Removal of platinum and palladium from wastewater by means of biosorption on fungi Aspergillus sp. and yeast Saccharomyces sp. Water 11(7), 1522 (2019).
Kociołek-Balawejder, E., Stanisławska, E., Jacukowicz-Sobala, I. & Jasiorski, M. Anomalous effect of Cu2O and CuO deposit on the porosity of a macroreticular anion exchanger. J. Nanopart. Res. 23(6), 1–12 (2021).
Liang, G., Mo, Y. & Zhou, Q. Novel strategies of bioleaching metals from printed circuit boards (PCBs) in mixed cultivation of two acidophiles. Enzyme Microb. Technol. 47(7), 322–326 (2010).
Amini, M. & Younesi, H. Biosorption of Cd (II), Ni (II) and Pb (II) from aqueous solution by dried biomass of Aspergillus niger: Application of response surface methodology to the optimization of process parameters. Clean: Soil, Air, Water 37(10), 776–786 (2009).
Akinhanmi, T. F., Ofudje, E. A., Adeogun, A. I., Aina, P. & Joseph, I. M. Orange peel as low-cost adsorbent in the elimination of Cd (II) ion: Kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 7(1), 1–16 (2020).
Sağ, Y., Kaya, A. R. Z. U. & Kutsal, T. Ü. L. I. N. The simultaneous biosorption of Cu (II) and Zn on Rhizopus arrhizus: Application of the adsorption models. Hydrometallurgy 50(3), 297–314 (1998).
Özer, A. & Özer, D. Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ions onto S. cerevisiae: Determination of biosorption heats. J. Hazard. Mater. 100(1–3), 219–229 (2003).
Kim, T. Y. et al. Adsorption of heavy metals by brewery biomass. Korean J. Chem. Eng. 22(1), 91–98 (2005).
Djeribi, R. & Hamdaoui, O. Sorption of copper (II) from aqueous solutions by cedar sawdust and crushed brick. Desalination 225(1–3), 95–112 (2008).
Low, K. S., Lee, C. K. & Liew, S. C. Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem. 36(1–2), 59–64 (2000).
Fadl, M. G., Farahat, M. G. & Mohamed, Z. K. Optimum biosorption and resistance of uranium by metal-resistant bacteria isolated from rock ore. Geomicrobiol. J. 1–8. (2022).
Fadl, M. G. Characterization of genetic resources of microorganism as microorganism response of climate change. J. Gene Eng. Bio. Res. 4(2), 165–172 (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.G.F. wrote the main manuscript text and prepared figures.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fadl, M.G. Prediction of heavy metal biosorption mechanism through studying isotherm kinetic equations. Sci Rep 13, 1576 (2023). https://doi.org/10.1038/s41598-023-28655-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28655-4
- Springer Nature Limited
This article is cited by
-
On the performance of Sargassum-derived calcium alginate ion exchange resins for Pb2+ adsorption: batch and packed bed applications
Environmental Science and Pollution Research (2024)
-
Experimental and Theoretical Investigation of the Effect of Cellulose Nanowhiskers on the Pb(II) Adsorption by Superabsorbent Hydrogel Nanocomposites
Water, Air, & Soil Pollution (2024)