Abstract
Cancer is a complex disease, necessitating research on many different levels; at the subcellular level to identify genes, proteins and signaling pathways associated with the disease; at the cellular level to identify, for example, cell-cell adhesion and communication mechanisms; at the tissue level to investigate disruption of homeostasis and interaction with the tissue of origin or settlement of metastasis; and finally at the systems level to explore its global impact, e.g. through the mechanism of cachexia. Mathematical models have been proposed to identify key mechanisms that underlie dynamics and events at every scale of interest, and increasing effort is now being paid to multi-scale models that bridge the different scales. With more biological data becoming available and with increased interdisciplinary efforts, theoretical models are rendering suitable tools to predict the origin and course of the disease. The ultimate aims of cancer models, however, are to enlighten our concept of the carcinogenesis process and to assist in the designing of treatment protocols that can reduce mortality and improve patient quality of life. Conventional treatment of cancer is surgery combined with radiotherapy or chemotherapy for localized tumors or systemic treatment of advanced cancers, respectively. Although radiation is widely used as treatment, most scheduling is based on empirical knowledge and less on the predictions of sophisticated growth dynamical models of treatment response. Part of the failure to translate modeling research to the clinic may stem from language barriers, exacerbated by often esoteric model renderings with inaccessible parameterization. Here we discuss some ideas for combining tractable dynamical tumor growth models with radiation response models using biologically accessible parameters to provide a more intuitive and exploitable framework for understanding the complexity of radiotherapy treatment and failure.







Similar content being viewed by others
References
Araujo RP, McElwain DLS (2004) A history of the study of solid tumour growth: the contribution of mathematical modeling. Bull Math Biol 66:1039–1091
Bao S, Wu Q, McLendon RE (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Barcellos-Hoff MH, Costes SV (2006) A systems biology approach to multicellular and multi-generational radiation responses. Mutat Res 597(1–2):32–38
Basse B, Ubezio P (2007) A generalised age- and phase-structured model of human tumour cell populations both unperturbed and exposed to a range of cancer therapies. Bull Math Biol 69(5):1673–1690
Baumann M, Krause M, Hill R (2008) Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 8:545–554
Brenner DJ, Hlatky LR, Hahnfeldt PJ, Hall EJ, Sachs RK (1995) A convenient extension of the linear-quadratic model to include redistribution and reoxygenation. Int J Rad Onc Biol Phys 32(2):379–390
Brenner D, Armour E, Corry P, Hall E (1998) Sublethal damage repair times for a late-responding tissue relevant to brachytherapy (and external-beam radiotherapy): implications for new brachytherapy protocols. Int J Radiat Oncol Biol Phys 41:135–138
Burnet NG, Jena R, Jefferies SJ, Stenning SP, Kirkby NF (2006) Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol 18(2):93–103
Dale RG (1996) Dose-rate effects in targeted radiotherapy. Phys Med Biol 41:1871–1884
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284
Dawson A, Hillen T (2006) Derivation of the tumour control probability (TCP) from a cell cycle model. Comp Math Methods Med 7(2-3):121–141
Dionysiou DD, Stamatakos GS, Marias K (2007) Simulating cancer radiotherapy on a multi-level basis: biology, oncology and image processing. Digital Human Modeling 4561:569–575
Enderling H, Anderson ARA, Chaplain MAJ, Munro AJ, Vaidya JS (2006) Mathematical modelling of radiotherapy strategies for early breast cancer. J Theor Biol 241(1):158–171
Enderling H, Chaplain MAJ, Anderson ARA, Vaidya JS (2007a) A mathematical model of breast cancer development, local treatment and recurrence. J Theor Biol 246(2):245–259
Enderling H, Anderson ARA, Chaplain MAJ (2007b) A model of breast carcinogenesis and recurrence after radiotherapy. Proc Appl Math Mech 7(1):1121701–1121702
Enderling H, Vaidya JS (2008) Mathematical modelling of breast carcinogenesis, treatment with surgery and radiotherapy, and local recurrence. In: Bellomo N, Chaplain M, DeAngelis E (eds) Selected topics on cancer modelling genesis-evolution-immune competition-therapy. Birkhauser, Boston, pp 337–361
Enderling H, Park D, Hlatky L, Hahnfeldt P (2009a) The importance of spatial distribution of stemness and proliferation state in determining tumor radioresponse. Math Model Nat Phenom 4(3):117–133
Enderling H, Hlatky L, Hahnfeldt P (2009b) Migration rules: tumours are conglomerates of self-metastases. Br J Cancer 100(12):1917–1925
Enderling H, Anderson ARA, Chaplain MAJ, Beheshti A, Hlatky L, Hahnfeldt P (2009c) Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res 69(22):8814–8821
Ergun A, Camphausen K, Wein LM (2003) Optimal scheduling of radiotherapy and angiogenic inhibitors. Bull Math Biol 65(3):407–424
Frieboes HB, Edgerton ME, Fruehauf JP et al. (2009) Prediction of drug response in breast cancer using integrative experimental/computational modeling. Cancer Res 69(10):4484–4492
Gonzalez SJ, Carando DG (2008) A general tumour control probability model for non-uniform dose distributions. Math Med Biol 25(2):171–184
Gordon KE, van Leeuwen IMM, Lain S, Chaplain MAJ (2009) Spatio-temporal modelling of the p53-mdm2 oscillatory system. Math Model Nat Phenom 4(3):97–116
Guerrero M, Allen Li X (2003) Analysis of a large number of clinical studies for breast cancer radiotherapy: estimation of radiobiological parameters for treatment planning. Phys Med Biol 48:3007–3326
Hall EJ (2003) The bystander effect. Health Phys 85(1):31–35
Harpold HL, Alvord EC Jr, Swanson KR (2007) The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol 66:1–9
Hlatky LR, Hahnfeldt P, Sachs RK (1994) Influence of time dependent stochastic heterogeneity on the radiation response of a cell population. Math Biosci 122:201–220
Jones B, Dale RG (2007) Further radiobiologic modeling of palliative radiotherapy: use of virtual trials. Int J Rad Onc Biol Phys 69(1):221–229
Kim JJ, Tannock IF (2005) Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer 5(7):516–525
Kirkby NF, Jefferies SJ, Jena R, Burnet NG (2007) A mathematical model of the treatment and survival of patients with high-grade brain tumours. J Theor Biol 245(1):112–124
Li X, Lewis MT, Huang J, Gutierrez C (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100(9):672–679
Little MP (2007) A multi-compartment cell repopulation model allowing for inter-compartmental migration following radiation exposure, applied to leukaemia. J Theor Biol 245(1):83–97
Liu W, Hillen T, Freedman HI (2007) A mathematical model for M-phase specific chemotherapy including the G0-phase and immunoresponse. Math Biosci Eng 4:239–259
Lowengrub JS, Frieboes HB, Jin F, Chuang Y-L, Li X, Macklin P, Wise SM, Cristini V (2009) Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23:R1–R91
Marcu LG, Bezak E (2009) Radiobiological modeling of interplay between accelerated repopulation and altered fractionation schedules in head and neck cancer. J Med Phys 34(4):206–211
Oldham M (2001) Radiation physics and applications in therapeutic medicine. Phys Educ 36:460–467
O’Rourke SFC, McAneney H, Hillen T (2009) Linear quadratic and tumour control probability modelling in external beam radiotherapy. J Math Biol 58(4–5):799–817
Pawlik TM, Keyomarsi K (2004) Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 59:928–942
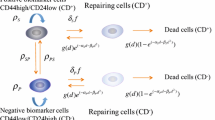
Phillips TM, McBride WH, Pajonk F (2006) The response of CD24(–/low)/CD44+breast cancer-initiating cells to radiation. J Natl Cancer Inst 98:1777–1785
Powathil G, Kohandel M, Sivaloganathan S, Oza A, Milosevic M (2007) Mathematical modeling of brain tumors: effects of radiotherapy and chemotherapy. Phys Med Biol 52(11):3291–3306
Reya R, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Ribba B, Colin T, Schnell S (2006) A multiscale mathematical model of cancer, and its use in analyzing irradiation therapies. Theo Biol Med Model 3:7
Rich JN (2007) Cancer stem cells in radiation resistance. Cancer Res 67(19):8980–8984
Richard M, Kirkby KJ, Webb RP, Kirkby NF (2009) Cellular automaton model of cell response to targeted radiation. Appl Radiat Isot 67:443–446
Roberts SA, Hendry JH (1993) The delay before onset of accelerated tumour cell repopulation during radiotherapy: a direct maximum-likelihood analysis of a collection of worldwide tumour-control data. Radiother Oncol 29(1):69–74
Rockne R, Alvord EC, Szeto M, Gu S, Chakraborty G, Swanson KR (2008) Modeling diffusely invading brain tumors an individualized approach to quantifying glioma evolution and response to therapy. In: Bellomo N, Chaplain M, de Angelis E (eds) Selected topics in cancer modeling: genesis, evolution, immune competition, and therapy. Birkhäuser, Cambridge, pp 207–253
Rockne R, Alvord EC Jr, Rockhill JK, Swanson KR (2009) A mathematical model for brain tumor response to radiation therapy. J Math Biol 58(4–5):561–578
Sachs RK, Shuryak I, Brenner D et al. (2007) Second cancers after fractionated radiotherapy: stochastic population dynamics effects. J Theor Biol 249:518–531
Sancar A, Lindsey-Boltz LA, Ünsal-Kamaz K, Linn S (2004) Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73:39–85
Steel GG, McMillan TJ, Peacock JH (1989) The 5rs in radiobiology. Int J Radiat Biol 56:1045–1048
Swanson KR, Alvord EC Jr, Murray JD (2000) A quantitative model for differential motility of gliomas in grey and white matter. Cell Prolif 33:317–329
Swanson KR, Alvord EC Jr (2002) The concept of gliomas as a traveling wave: the application of a mathematical model to high- and low-grade gliomas. Can J Neurol Sci 29:395
Swanson KR, Rockne R, Rockhill JK, Alvord EC Jr (2007) Combining mathematical modeling with serial MR imaging to quantify and predict response to radiation therapy in individual glioma patient. Neuro-Oncology 9:575
Swanson KR, Rockne R, Rockhill JK, Alvord EC Jr (2007) Mathematical modeling of radiotherapy in individual glioma patients: quantifying and predicting response to radiation therapy. AACR Meeting Abstracts 5059
Swanson KR, Harpold HL, Peacock DL et al. (2008) Velocity of radial expansion of contrast-enhancing gliomas and the effectiveness of radiotherapy in individual patients: a proof of principle. Clin Oncol (R Coll Radiol) 20:301–308
Turesson I, Carlsson J, Brahme A, Glimelius B, Zackrisson B, Stenerlöw B (2003) Biological response to radiation therapy. Acta Oncol 42(2):92–106
Vaidya JS, Baum M, Tobias JS et al. (2001) Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 12:1075–1080
Vaidya JS, Baum M, Tobias JS, Morgan S, D’Souza D (2002) The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol 28:447–454
Withers HR (1975) The four r’s of radiotherapy. Adv Radiat Biol 5:241–247
Withers HR, Taylor JM, Maciejewski B (1988) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27(2):131–146
Acknowledgments
The work was supported by the AACR Centennial Postdoctoral Fellowship in Cancer Research (08-40-02-ENDE, HE) and by a NASA/NSCOR grant (NNJ06HA28G, HE,PH). MAJC acknowledges the support of an ERC Advanced Investigator Grant No. 227619. The authors would also like to thank Afshin Beheshti for kindly providing the glioma radiation response images shown in Fig. 1d, and Russell Rockne and Benjamin Ribba for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enderling, H., Chaplain, M.A.J. & Hahnfeldt, P. Quantitative Modeling of Tumor Dynamics and Radiotherapy. Acta Biotheor 58, 341–353 (2010). https://doi.org/10.1007/s10441-010-9111-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-010-9111-z