Abstract
Machine learning (ML) has led to significant advances in dentistry, easing the workload of professionals and improving the performance of various medical processes. The fields of periodontology and implantology can profit from these advances for tasks such as determining periodontally compromised teeth, assisting doctors in the implant planning process, determining types of implants, or predicting the occurrence of peri-implantitis. The current paper provides an overview of recent ML techniques applied in periodontology and implantology, aiming to identify popular models for different medical tasks, to assess the impact of the training data on the success of the automatic algorithms and to highlight advantages and disadvantages of various approaches. 48 original research papers, published between 2016 and 2023, were selected and divided into four classes: periodontology, implant planning, implant brands and types, and success of dental implants. These papers were analyzed in terms of aim, technical details, characteristics of training and testing data, results, and medical observations. The purpose of this paper is not to provide an exhaustive survey, but to show representative methods from recent literature that highlight the advantages and disadvantages of various approaches, as well as the potential of applying machine learning in dentistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, artificial intelligence (AI) is used in numerous domains, such as automotive, robotics, education, or medicine. It mimics the behavior of humans and, most of the time, it leads to faster and better results. A subfield of AI, machine learning covers a plethora of algorithms that learn from data to accomplish various prediction tasks. To better understand the possible medical tasks that can profit from the employment of ML techniques and how the performance of these algorithms can be assessed, an introduction to ML is provided in the current chapter, presenting various types of ML models, existing problems in ML and evaluation metrics. Also, since the object of the current study is the use of ML in periodontology and implantology, a brief description of the two medical areas is provided in the same chapter. The second chapter presents recent surveys and reviews of artificial intelligence techniques in various subfields of dentistry. The third chapter describes the methodology for selecting 48 original papers related to the use of ML algorithms in periodontology and implantology. It also extracts important information from each paper with regards to aim, technical details, characteristics of data, evaluation, and medical observations, trying to identify similar approaches, as well as advantages and disadvantages of various ML techniques. The last chapter draws the conclusions from the analysis of the selected ML solutions.
Introduction to Machine Learning
A possible classification, based on the learning process [1], groups the machine learning algorithms into supervised ML algorithms (that make predictions based on previously labeled datasets), unsupervised ML algorithms (that process unlabeled datasets and make inferences without the need for human intervention), semi-supervised ML algorithms (that process both labeled and unlabeled data), and reinforcement learning algorithms (that function based on rewards and punishments). Another classification would group ML algorithms based on the task they perform.
Types of Machine Learning Algorithms
Figure 1 groups existing ML algorithms according to other proposed classifications [1,2,3,4].
While there is a plethora of ML models, as can be observed from Fig. 1, we focus on algorithms applied in dentistry. Many techniques used in periodontology and implantology process radiographic images. Still, there are also several algorithms that process non-clinical data or information that was previously extracted from radiographs (by medical specialists), to deliver a diagnostic or to predict the outcome of a surgical procedure. Most algorithms used in periodontology and implantology belong to the supervised learning category. However, unsupervised learning models can also be applied for segmentation tasks.
K-nearest neighbors (k-NN) [5] is a supervised learning technique that assigns new, unlabeled data, to a category, using training data elements that were previously grouped into certain categories. Naïve Bayes is another supervised learning algorithm, based on the Bayes Probability Theorem [6], useful in classification problems. Support vector machine (SVM) [7] algorithms organize data into different categories by using a separation hyperplane that maximizes the differences between classes. Logistic regression (LR) [8] applies a logistic function on a linear combination of independent variables, to predict the result of an output variable. The logistic regression algorithm tries to minimize a cost function that quantifies the error within the predicted probabilities. Discriminant function analysis (DFA) [9] is similar to logistic regression, in the sense that it predicts a categoric result for an output variable, even if the input variables have continuous values. Linear discriminant analysis (LDA) [10] is one of the most popular variants of DFA. It predicts the probability that a certain element belongs to a class or not, using a density function of all the values of the data belonging to that class. Another supervised learning algorithm is a decision tree (DT) [11], which uses a series of rules to classify a new element. The elements in the dataset are organized in a hierarchical structure, where each decision node corresponds to a test that divides the dataset based on the values of a feature, and each leaf node corresponds to a class. To classify a new element, the algorithm starts from the root (the whole, un-labeled dataset), and advances downwards on the nodes that correspond to the values of the element’s features. A random forest (RF) [12] represents a set of decision trees that are obtained from a randomly chosen sub-set of the training dataset. This algorithm combines the votes from several decision trees to assign the new element to a category. Neural networks (NN) are a subset of machine learning models that are inspired by the human brain. Even though neural networks cannot be grouped into a single category based on the learning process, as can be observed in Fig. 1, in the context of periodontology and implantology they depend on previously labeled data, therefore belonging to the supervised learning category. A neural network is comprised of interconnected neurons, organized in three types of layers: input, hidden and output layers. An artificial neural network (ANN) is a neural network that processes the input information only in the forward direction. A recurrent neural network (RNN) is a variant of ANN that contains cycles, so that the output of some neurons can affect the input of the same neurons, in subsequent iterations. A deep neural network (DNN) is a neural network with multiple hidden layers. Convolutional neural networks (CNN) are neural networks specialized in image processing due to the use of kernels that extract salient characteristics from input data with convolution operations. Unlike other neural networks that process previously extracted features from images, CNNs can be applied on raw data. A comprehensive review of artificial neural networks and their applications to computer vision is provided by Abiodun et al. [13].
K-means [14] is one of the simplest unsupervised machine learning algorithms. It divides the elements in a dataset into k clusters, based on similarities or dissimilarities. Each element is assigned to a cluster based on a distance function minimization. Genetic algorithms [15] represent computational models of biological evolution. They cannot be grouped into a single category of ML algorithms based on the learning process. However, in the context of medical imaging, they can be employed for clustering tasks (in an unsupervised manner), similar to k-means. They perform natural selection of the fittest individuals by solving an optimization problem. The optimization is accomplished by mixing genetic material from the parents.
Tasks in Machine Learning
The classification task refers to assigning a new element into one of several categories, based on the training data which contains previously labeled elements. An example of a classification task is detecting the type/manufacturer of an implant based on a patient’s radiography. This problem can be solved with a multitude of AI techniques, including k-NN, Naïve Bayes, SVM, LR, DFA, decision trees or neural networks.
Unlike the classification task, where the classes are already known, the clustering task refers to identifying similarities between elements in an unlabeled dataset. In computer vision, the clustering problem translates into the segmentation task. An example of segmentation in dentistry is detecting teeth pixels in radiographic images, based on tissue density. A popular algorithm for clustering is k-means. An alternative would be the use of genetic algorithms for energy optimization in active contours-based [16] or in edge-based segmentations [17].
Feature extraction is another task in the ML domain. It is necessary when working with neural networks that cannot process raw data such as full images. Several ML models, such as ResNet [18], or MobileNet [19], can be adapted to extract features (for example, a common feature extraction technique is to use ResNet to process an image and to obtain the output of the network from intermediate layers).
The object detection task assumes the identification of an object’s position and bounding box, as well as its labeling into one of the existing classes. An example of object detection in dentistry would be obtaining a bounding box with a characterization of each tooth (e.g., if it’s healthy or if it has caries) in a radiographic image. Some of the most successful object detection models are you only look once (YOLO) [20], single shot multibox detector (SSD) [21], regions with convolutional neural networks (R-CNN) [22] and region-based fully convolutional networks (R-FCN) [23].
Semantic segmentation is another task in computer vision, where each image pixel is assigned to a class of objects. An example of such task in dentistry is obtaining a mask of pixels for all the implants in a radiography. U-net [24], DeconvNet [25] and SegNet [26] are popular convolutional neural networks, with an encoder-decoder architecture, that perform pixelwise classification.
Instance segmentation is a combination of object detection and semantic segmentation: it performs pixelwise classification, but it also differentiates between distinct objects from the same class (e.g., each mask of pixels belonging to a molar in a radiographic image would have a different label). Some of the most common instance segmentation networks are DeepNet [27] and Mask R-CNN [28].
Evaluation Metrics
There are various metrics used to assess the performance of a machine learning algorithm, depending on the problem it tries to solve.
In the binary classification problem, each prediction can lead to one of four results: true positive (TP—the value was true and it was predicted true), true negative (TN—the value was false and it was predicted false), false positive (FP—the value was false, but it was predicted true) and false negative (FN—the value was true, but it was predicted false). One of the most popular metrics is accuracy (Acc), measuring the number of correct predictions relative to the total number of input samples. It is defined as:
The accuracy is a suitable metric when the target variable classes are relatively balanced (for example, for two classes, the number of items assigned to the first class is approximately the same as the number of items assigned to the second class). Another metric is the misclassification rate, or the error rate (Err), measuring the number of wrong predictions, relative to the total number of input samples:
In case of imbalanced classes, more suitable metrics are the precision and the recall. The precision (P), or the positive predictive value (PPV), refers to the proportion of correct positive predictions. It represents the ratio between true positives and predicted positive values:
The negative predictive value (NPV) refers to the proportion of negative predictions. It represents the ratio between true negatives and predicted negative values:
The recall (R), also known as the sensitivity, or the true positive rate (TPR), aims to identify the proportion of actual positive values that were identified incorrectly. It represents the ratio between the true positives and the total number of positive values, either correctly predicted as positives or incorrectly predicted as negatives:
A high value of the TPR is equivalent to a small number of false negatives, whereas a high precision is equivalent to the minimization of the false positives.
F1 score, also known as F-measure or the Dice coefficient, combines the precision and recall metrics, being also very suitable for imbalanced classes:
Another popular metric is the specificity, or the true negative rate (TNR). It is defined as:
The false positive rate (FPR) is defined as:
The receiver operating characteristic (ROC) curve shows the performance of a classification model at different discrimination thresholds, by plotting the TPR against the FPR. A popular metric, namely the area under the ROC curve (AUC), computes the performance across all the thresholds. A high value of AUC (close to 1) is obtained for correct predictions (close to 100%). The Youden index (J), a common summary measure of the ROC curve, indicates a model’s ability to balance the sensitivity and the specificity:
\(J=TPR+TNR-1\). (9)
A confusion matrix is a tabular representation of prediction outcomes of a classifier (binary or general), illustrating the prediction values and the actual values. The values on the diagonal represent the number of elements for which the predicted label is the same as the actual label (for the binary classification, the diagonal would contain the true positives and the true negatives). The values that are not on the diagonal represent elements that were not correctly labeled by the classifier.
Cross-validation is a technique that evaluates the ability of ML models to predict new data that was not used in estimating it. It divides up the training data into k-folds (for example, k = 10). While each time a fold remains out, the model is trained on the remaining data. The model is then used to predict the answers for the observations in the held-out fold. This technique identifies problems such as overfitting or selection bias.
The previously mentioned metrics could be used for classification tasks (for example, to differentiate between various implant brands and types, based on radiographs), as well as for segmentation (e.g., to segment a radiographic image into pixels that belong to healthy teeth, implants, caries, or other tissue).
A very popular metric, used for segmentation or object detection tasks, is the intersection over union (IoU). In the object detection problem, each object has an associated bounding box. The IoU is a measure of the difference between the predicted bounding box and the ground-truth bounding box. It is defined as the ratio between the area of overlap and the area of union for the two bounding boxes. While in the object detection task this measure is computed at bounding box level, in the segmentation task the IoU is computed pixelwise.
Another ML task in dentistry is the estimation of various characteristics in radiographic images, such as root or implant length. A popular metric to assess the quality of an estimator is the mean squared error (MSE), or the mean squared deviation (MSD). It is defined as:
where \({x}_{i}\) represent actual values and \({\widehat{x}}_{i}\), predicted ones. The root-mean-square error (RMSE), also known as the root-mean-square deviation (RMSD), is defined as the square root of MSE. Low values of MSE and RMSE indicate a good performance of the estimator.
The quality of a classification model or of an estimator can also be accomplished by computing the correlation between the results of an automatic process with those of a manual one. The Pearson correlation measures the strength and direction of the relationship between two sets of data. It is a linear correlation, computed as the ratio between the covariance of two variables and the product of their standard deviations. The Pearson correlation has a value in the \(\left[-\text{1,1}\right]\) interval, the two extremes corresponding to perfect correlations. A value close to 0 indicates no correlation between the two sets of data. Other statistical comparisons between automatic and manual processes can be performed with the one-way ANOVA test [29] or with the Tukey test [30], both comparing the means of two or more independent sets of data to determine whether there is statistical evidence that the means of the sets are significantly different.
Introduction to Periodontology and Implantology
Periodontology is concerned with the study of the tooth’s supportive tissue, with the associated diseases and their treatment. The word “periodontology” has Greek roots. The prefix “peri” means “around,” and “odontos” means tooth. The periodontium refers to the entire area surrounding the tooth. The periodontium is made up of four different types of tissues. On the exterior we have the visible structure, namely gum. Next is the periodontal ligament or desmodontium. This has the role of connecting the tooth to the alveolar bone. The third is radicular cementum, which covers the tooth’s root and fixates the fibers of periodontal ligaments. Finally, we have the alveolar bone which is part of the maxilla bone and contains the root of the tooth, fixating the fibers of periodontal ligaments. The periodontium is of two types: superficial, including gingiva, and deep, including ligaments, cement, and bone [31]. For the periodontium to work within normal parameters and to ensure the health and protection of the tooth, each one of the four tissues must be completely healthy.
When periodontal diseases appear, they tend to first affect the superficial periodontium, i.e., the gingiva. This is called gingivitis. It is characterized by swelling, redness, and bleeding of the gingiva [32]. It is caused by poor oral health and biofilm accumulation [33]. If the biofilm is removed, the gingiva can recover. If the biofilm persists, then gingivitis can lead to affliction of the profound supportive tissues, causing periodontal disease [34, 35]. Untreated periodontal disease leads to irreversible destruction of the profound periodontal tissue and to gingival pockets between the tooth and the supportive bone. When gingival pockets form, clinical signs include dental mobility, secondary migrations of the teeth, gingival recession, bleeding gums, and halitosis. All these factors can lead to loss of teeth [36].
When loss of teeth occurs, due to periodontal disease or other causes, a dental implant is required to accommodate new teeth, so that a patient can use the normal functions of the dental-masticatory complex. Dental implants act as artificial dental roots. With the help of dental prosthetics, dental implants can be a good functional and aesthetic replacement for missing teeth. Dental implants aren’t affected by bacteria itself, but bacteria can affect the structural tissue surrounding the implant, causing peri-implantitis. Peri-implantitis is the equivalent of periodontal disease for natural teeth [37].
When periodontal disease or peri-implantitis appear, there are three types of treatments. The first one is etiologic therapy. It includes patient education for good oral hygiene, removal of biofilm, removal of supra- and subgingival tartar, and treatment of all existing dental problems. Removal of bacteria at home is carried out through a good daily dental brushing, flossing, and waterpik. When needed, the patient is called to the clinic where a more thorough removal of bacteria is carried out by mechanical, airflow, perioflow, laser, sonic or ultrasonic instruments. The second type of treatment is surgical therapy. There are two types of surgical therapy: reducing gingival pockets and correcting the anatomical and morphological defects. Finally, the third type of treatment is maintenance and support of obtained results of the previous treatments, to prevent relapse of the disease [38].
Keeping in mind the importance of detecting and treating dental problems before they can cause further complications, regular check-ups at the dentist are essential. However, detection and treatment can be difficult, even for experienced dental professionals, so any tool that can help the dentist to detect and treat these diseases can have a large positive impact [39]. The onset of AI in the dental sector offers exciting opportunities to assist dental professionals in a variety of ways, ranging from interpreting dental images, correlating risk factors with general health problems, detecting type of implants and many other benefits.
Related Work
In recent years artificial intelligence has led to the advance of many areas in medicine, including dentistry. While several surveys give an overview of the whole field of dentistry [40,41,42,43,44], others focus on selected areas, such as implantology or periodontology [45,46,47,48,49,50,51,52].
General surveys provide overviews of various sub-domains of dentistry. Shan et al. [40] outlined the progress and the potential applications of AI in dentistry, which range from diagnosis and treatment to disease prediction. Katne et al. [41] presented applications of AI in various fields of dentistry, including general dentistry, oral and maxillofacial surgery, oral medicine, dental and maxillofacial radiology, forensic odontology, dental education system, prosthodontics, orthodontics, and periodontics. Grischke et al. [42] provided an overview of existing applications of robotic systems and AI in dentistry. They mentioned several robotic assistants in dental implantology, and machine learning tools to predict periodontal diseases or peri-implant infection. However, they pointed out that the use of AI is still restricted to pilot use cases and narrowly defined research questions. Schwendicke et al. [43] identified opportunities and challenges of AI-based dental diagnostics and treatment planning. Kang et al. [44] provided an analysis of deep learning methods used in dentistry and implantology. They identified applications for image quality enhancement, detecting teeth or dental caries, diagnosing periodontal diseases or cancerous lesions, identifying cephalometric landmarks, and assisting during the manufacturing of prostheses.
More focused reviews analyze the use of ANNs or CNNs in periodontology and implantology. Bernauer et al. [48] aimed to assess the usefulness of using ANNs or CNNs in several dentistry tasks, such as identifying and classifying dental implant systems, assisting in the fabrication of implant-supported monolithic zirconia crowns cemented on customized hybrid abutments, predicting periodontally compromised teeth, or classifying teeth in dental prosthetics workflows. Manerikar et al. [50] identified several applications of CNN in different sub-areas of periodontology and implantology, such as dental plaque detection, identification of gingivitis, detection of periodontal diseases, as well as classification of implant design systems. Revilla-León et al. [51] provided a survey of AI models for detecting dental plaque and for diagnosing gingivitis and periodontal disease. The performance evaluation indicated that AI models for detecting plaque (from 2 studies) obtained an accuracy ranging from 73.6 to 99%, while solutions for diagnosing gingivitis (8 studies) reached an accuracy ranging from 74 to 78.2% for intraoral photographs and from 67.7 to 73.72% for fluorescent intraoral images. Three research papers included in their study, related to the diagnosis of periodontal disease, reported an accuracy between 47 and 81%. Analyzing the performance of AI models for detecting alveolar bone loss on 11 studies, they observed an accuracy ranging from 73.4 to 99%. Mohammad-Rahimi et al. [52] included 47 studies in a review of deep learning solutions in periodontology and oral implantology. Their uses cases included the detection of periodontitis and gingivitis or periodontal bone loss, the classification of dental implant systems and the prediction of treatment outcomes in periodontology and implantology.
Other surveys focus on the use of AI in various tasks of implantology such as implant type recognition, implant success prediction or customization of prostheses. Revilla-León et al. [45] provided an analysis of AI models in implant dentistry for implant type recognition (7 studies), implant success prediction (7 studies) and implant design optimization (3 studies). Even though they reviewed a small number of research papers, their conclusions regarding the performance of AI models (accuracy between 93.8 and 98% for solutions that recognize implant types, and between 62.4 and 80.5% for methods that predict osteointegration success) are very encouraging. Saghiri et al. [46] analyzed 10 research papers published between 2000 and 2020 that are related to technology used in the identification of different implant systems. Another recent study [47] reviewed 4 pre-trained CNNs used for the identification of dental implant systems. Out of the selected articles, they extracted information about implant systems, imaging modality, training sample size, validation method, AI architecture and evaluation metrics. The accuracy of the studied AI models ranged from 51 to 99.5%. Pareek and Kaushik [49] provided another review that focuses on recent AI models in dental prosthetics and their efficacy in diagnosing and building customized prostheses.
Among the limitations identified in various surveys we mention the dependence on datasets and the lack of awareness in follow-up treatment [49]. Common limitations of CNN-based applications in periodontology are related to the input data (sample size, image resolution) and to the use of 2D periapical radiographs, as opposed to computer tomography (CT) or magnetic resonance imaging (MRI) datasets [50]. Schwendicke et al. [43] identified a possible future scenario that would considerably improve the field of dentistry, where the training sample size shifts from several thousands to millions of multi-level connected instances, the focus moves from the detection of structures on imagery, association modelling, to multi-class detection of pathologies, predictive modelling, and decision support. They also foresaw a progress, where the testing mode would change from cross-validation to hold-out test sets and independent datasets and where evaluation metrics would not target only measures of accuracy (e.g., accuracy, sensitivity, specificity, area under the curve, F1-score, etc.), but also measures of value (impact on treatment decision, cost-effectiveness) and trustworthiness of AI (explainable AI).
While several surveys are too general, analyzing the use of AI models for all the sub-areas of dentistry, others focus only on specific AI techniques (for example, only deep learning methods) in periodontology and/or prosthodontics, including a very small number of research works in their studies. The purpose of the current research is to provide an overview of all types of ML techniques applied in periodontology and implantology, to identify common procedures based on use cases, to highlight out strengths and weaknesses of various solutions and to point out interesting medical observations.
Selected Papers
Selection Procedure
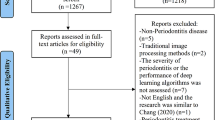
We used several scientific databases to search for recent applications of artificial intelligence in periodontology and implantology (between 2016 and 2023): PubMed, IEEE Xplore, ScienceDirect and Google Scholar. The search keywords included terms from both the AI domain (“artificial intelligence” or “machine learning” or “deep learning”) and from the targeted sub-areas of dentistry (“peri-implantitis” or “periodontology” or “implant planning” or “implantology” or “implant”). The resulting research papers were first screened based on title and abstract and the remaining items were evaluated based on full-text reads, resulting in an initial group of approximately 40 papers. The second selection step led to an additional group of roughly 20 papers, by cross-referencing the initial group. Out of the approximately 60 papers, the surveys and reviews were analyzed separately in the Related work section. We reached a total of 48 original research papers that were included in the current study.
Proposed Classification
The original papers were classified into four categories: periodontology (n = 11), implant planning (n = 9), implant brands and types (n = 14) and success of dental implants (n = 14).
Periodontology
The periodontology category consists of scientific works aimed at predicting teeth in need of extraction or that are periodontally compromised, detecting periodontal bone loss (PBL) or staging periodontitis.
Lee et al. [53] aimed to develop a CNN model for the diagnosis and prediction of periodontally compromised teeth. They applied a deep CNN architecture, using periapical radiographic images. Kim et al. [54] used deep CNNs with transfer learning and clinical prior knowledge, to detect PBL. They trained U-shaped networks to extract regions of interest containing the teeth, as well as a multi-label classification network that predicts the existence of PBL in each tooth. Krois et al. [55] applied a deep feed-forward CNN to detect PBL on panoramic radiographs. Their network was trained and validated using 10-times repeated group shuffling. Also, hyperparameters were systematically tuned using grid search [56]. Comparing the performance of a CNN with that of 6 dentists, the CNN obtained slightly higher accuracy. Thanathornwong and Suebnukarn [57] aimed to adapt the Faster R-CNN [58] model from the natural image domain using a small annotated clinical data, to identify bounding boxes of periodontally compromised teeth. Chang et al. [59] proposed a hybrid method, combining a deep learning architecture with conventional processing for classification, to detect and classify PBL in each individual tooth.
A CNN was employed for the detection of the periodontal bone level, the cementoenamel junction (CEJ) level, and the individual teeth. Next, the percentage rate analysis of the PBL was accomplished based on the tooth long-axis and the periodontal bone and CEJ levels, using the criteria proposed at the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [60]. Lee et al. [61] integrated 3 deep segmentation networks (U-net with ResNet-34/CNN Encoder) for bone area, tooth, and CEJ, and performed measurements of the bone level, assigning the stage of radiographic bone loss (RBL) for each tooth. Kabir et al. [62] applied an end-to-end deep learning network which combines a set of segmentation networks and a classification network to output bone area, tooth and CEJ line masks. The RBL stage assignment follows the periodontitis classification presented by Tonetti et al. [60]. Jiang et al. [63] used a two-stage deep learning architecture based on U-net and YOLO-v4 to localize teeth and key points which led to the calculation of the percentage of bone loss and the staging of the periodontitis. Karacaoglu et al. [64] extracted first-order statistics, shape and size-based features and textual features from periapical images and applied several classifiers (k-NN, SVM, eXtreme Gradient Boosting (XGBoost), RF, LR and DT) on a reduced set of features, to classify periodontal defects.
Unlike other works that handle periodontitis, Deng et al. [65] developed a screening tool for periodontal health status based on non-clinical parameters and salivary biomarkers, using LR and RF on data such as gum disease, rating of gum/teeth health, tooth cleaning, loose teeth, or gingival bleeding on brushing (GBoB). Another research that does not feed radiographic images to the ML models was described by Lakshmi and Dheeba [66]. They applied various classifiers on data containing demographic information, clinical and radiological findings to predict the progression of periodontitis.
Table 1 presents the aim, as well as technical details, characteristics of training/validation/testing data, results of experiments and medical observations of analyzed papers in the periodontology category.
Artificial intelligence fills the need to identify, measure, classify, put a diagnostic and prognosis on periodontal disease more efficiently. The most common imaging modalities for periodontology are bitewing, retroalveolar, and panoramic radiographs. As can be seen from Table 1, the most popular ML algorithms in periodontology are CNNs, which are capable of segmenting teeth and CEJ lines, and predicting PBL lesions, based on radiographic images. Other ML classifiers, such as NB, SVM, RF, LR, k-NN, or DT, process data that was previously extracted from radiographs by medical experts, or non-clinical data (e.g., age, gender, smoking use, etc.).
From the analyzed papers in this category, it can be observed that the training datasets are relatively small (ranging from 100 to 1750 in 7 of the 8 papers, and only one paper with 12179 radiographs). However, data augmentation can compensate for the small sample size. Regarding the evaluation of the proposed solutions, the papers assess the success of different tasks in periodontology: detection of periodontally compromised teeth, prediction of extraction, detection of PBL, staging of periodontitis, segmentation of teeth and CEJ lines. Also, each paper uses different measures: either accuracy, F1-score/F-measure/Dice coefficient, recall/sensitivity, specificity, precision, or AUC. In any case, the evaluation results show that AI is on par or even surpasses the ability of general practitioners to classify periodontal disease. However, automatic solutions are by no means a replacement for medical practitioners, but rather an enhancement to their ability to identify and combat periodontal disease. Some papers hint to the fact that AI models and medical practitioners alike should correlate radiological images with patient dental status.
With the help of AI, dental practitioners can identify periodontal disease faster, thus lowering the chance of tooth loss, cardiovascular disease and other negative health effects associated with periodontal disease.
Implant Planning
The papers in the implant planning category are aimed at detecting regions with missing teeth (and their properties), at determining the implant length and the cervical width, and at predicting the location for the insertion of implants.
Görler and Akkoyun [69] studied the potential of using a layered feed forward ANN to efficiently determine canine implant length and cervical width from panoramic radiographs. Lee et al. [70] applied a CNN with multi-phase training and preprocessing on cone-beam computed tomography (CBCT) images for tooth segmentation (in the context of implant planning), using volumes of different sizes. Roongruangsilp and Khongkhunthian [71] aimed to investigate the learning curve of AI for dental implant treatment planning in the posterior maxillary region. They compared the learning curves of the Faster R-CNN algorithm (using the IBM PowerAI Vision platform) in determining areas with missing teeth and implant size, using four data augmentation procedures: blur, sharpen, color and noise. Bayrakdar et al. [72] applied a CNN to detect bone height and thickness, canals/sinuses/fossae in missing tooth regions. Park et al. [73] aimed to improve the implant planning process by using Mask R-CNN with ResNet-101 for the tooth segmentation task and Faster R-CNN with ResNet-101 for predicting regions of missing teeth. Moufti et al. [74] developed a U-net CNN to segment edentulous alveolar bone (area lacking teeth) in CBCT images in the implant planning phase. Oliveira-Santos et al. [75] proposed a solution that detects the mandibular canal (MC) even in the presence of anatomical variations such as the anterior loop (AL).
Hashem et al. [76] proposed an automatic solution that predicts the exact location of the implant with Guided Local Search with Continuous Time Neural Network (GLCTNN). Liu et al. [77] explored the capability of an AI system in automatically designing implant planning (predicting the implant location). They used Single Shot Detector (SSD) and V2V-PoseNet for edentulous site and related key points detection, for generation of implant axis and computation of implant position.
Table 2 presents the aims, the technical details, information about the data used for training/validation/testing, the results of the evaluation and several medical observations in the implant planning category.
Artificial intelligence in implant planning encompasses key applications like tooth segmentation refinement and automated implant plan design. As can be observed from Table 2, the chosen ML algorithms in implant planning are usually ANNs or CNNs: U-net or Mask R-CNN for pixel-based tooth segmentation, variations of R-CNN (e.g., Faster R-CNN) or SSD for detection of missing teeth’s bounding boxes, ANN to approximate tooth root size, etc. Even if their selected ML model was still a neural network, Hashem et al. [76] also compared the performance of their solution with that of other ML algorithms (W-J48, Naïve Bayes, SVM, K-NN, NNSRM, and GRNN), obtaining the best results in terms of accuracy.
In some scientific works panoramic radiographs are used to detect regions with missing teeth or implant characteristics. However, CBCT images are desired, since they provide a 3D, more exact guidance regarding the position of the implant. The sample sizes in the selected papers range from 42 images to 2500 images, with different proportions for training, validation, and testing. Even if the results hint to the potential of AI, the sample sizes may be too small for a thorough evaluation of the proposed solutions.
Notable achievements include improved tooth segmentation via modified CNNs and successful AI-driven predictions of implant positions, highlighting AI's potential to minimize errors and support dental decision-making, ultimately improving patient evaluation and treatment plan in order to achieve long-term success of the implant.
Implant Brands and Types
The solutions in the implant brands and types category aim to classify brands and models of implants. Most of the selected papers [85,86,87,88,89,90,91,92,93,94] evaluate the efficacy of CNNs in identifying models of implants in radiographic images, either panoramic or intraoral (periapical). Other scientific works [95, 96] compare the performance of clinicians and that of deep learning models in classifying implants. Benakatti et al. [97] analyze the performance of SVM, LR, k-NN and X boost classifiers in identifying implant types based on shape, using Hu and Eigen values.
Table 3 summarizes the technical details, the information about the dataset used for training/validation/testing, the results of the evaluation and several medical observations.
If dental records of the implant are missing, it is challenging for a dental clinical to accurately identify implant brand and type, which can hinder effective prosthetic treatment of the patient. Artificial intelligence has great potential to identify and classify implant brands and types, and to bridge experience gaps in dental professionals' performance.
As observed in the other categories as well, CNNs are the preferred ML models when processing radiographic images. Other classifiers, such as SVM, LR or k-NN, do not reach the accuracies obtained by deep learning models like GoogleNet Inception-v3, ResNet or YOLO.
The selected papers collectively emphasize AI’s transformative potential in enhancing implant type identification accuracy, crucial for sound prosthetic restoration decisions. The utilization of varied imaging data sources, such as panoramic and periapical radiographs, forms the basis for AI model training and evaluation. However, a small sample size can limit the ability of AI to accurately identify implants brands and types, highlighting the codependence of automatic solutions and dental practitioners for best results in this medical field.
Success of Dental Implants
The papers in this category aim to predict implant success probability and risk of periimplantitis. Moayeri et al. [98] applied an ML model which combines results of several classifiers (W-J48, SVM, NN, k-NN) to predict implant success probability, surpassing the accuracy of the best individual classifier. Papantonopoulos et al. [99] used k-means to cluster implants and principal component analysis (PCA) as a variable reduction method for ensemble selection and SVM to predict each implant’s main bone level (IIMBL). Cha et al. [100] and Liu et al. [101] processed various types of radiographic images with convolutional networks in order to detect bone loss around implants. Recent works [102,103,104] also applied convolutional neural networks on radiographic images (periapical or panoramic) to determine bone loss around implants. Lee et al. [105] evaluated various deep learning architectures in the task of identifying and classifying fractured dental implants.
Ha et al. [106] aimed to identify the most significant factors in predicting the success of dental implants. Wang et al. [107] followed the hypothesis that the probability of periimplantitis can be predicted based on the immune system and applied AI to annotate the tissue-resident immune landscape. Other selected works [108,109,110] also aimed to predict the risk of periimplantitis based on other data besides radiographs.
Table 4 contains technical details, information about the data used for training/validation/testing, as well as technical and medical results.
AI demonstrates impressive capabilities in predicting implant success probabilities, phenotypes, and bone levels. It excels in identifying factors influencing implant prognosis, emphasizing precise placement.
From Table 4 it can be observed that a popular strategy among the solutions in the periimplantitis category is to not feed the raw data (radiographic images) directly to the ML algorithms, but to use other types of information: either radiographic measurements (determined by doctors), such as implant length, or data extracted from the patient’s medical record, such as smoking habits, age, or gender. The preferred ML models when processing other data besides radiographic images were W-J48, SVM, k-NN, LR, DT, RF or Naïve Bayes. When processing panoramic or periapical radiographs, convolutional networks, such as YOLO, VGGNet-19, GoogLeNet Inception-v3, automated DCNN ResNet, Faster R-CNN or Mask R-CNN were employed.
As can be observed in the other categories as well, each scientific work has its own evaluation metrics: either accuracy, sensitivity, AUC, precision, recall in classifying the implant success probability, or RMSE for predicting each implants’ main bone level, or average precision, recall, PPV in detecting bone loss around implants. Even if the obtained performances presented in the Results column demonstrate AI’s capabilities of predicting periimplantitis risk, detecting fractured dental implants and successfully identifying bone loss around implants, the small sample sizes hint to the need for further, more thorough training and testing procedures.
Discussions and Conclusion
This paper sets out to identify the most popular ML algorithms applied in the fields of periodontology and implantology, to present technical details and characteristics of data used for training/validation/testing, and to extract interesting medical information.
From the analyzed papers, several observations were drawn:
-
When handling radiographic images (either intraoral, panoramic or CBCT datasets), the convolutional neural networks are preferred, since they are able to process raw data with the help of kernels that extract salient characteristics and do not require additional feature extraction steps.
-
When processing other data besides radiographic images (for example, when predicting implant success probability based on gender, age, smoking habits, implant placement and other parameters), other ML algorithms, such as SVAM, k-NN, LR, DT, RF or Naïve Bayes are employed.
-
For most of the tasks in periodontology and implantology (e.g., predicting periodontally compromised teeth, detecting periodontal bone loss, staging periodontitis, classifying implant brands and types, detecting bone loss around implants), intraoral and panoramic radiographic images provide sufficient information for an accurate result. However, for the task of implant planning (determining the exact location of the implant), CBCT images are preferred.
-
Each paper has its own procedure for evaluation, proposing different measurements, such as accuracy, sensitivity, specificity, AUC, RMSE, etc. As presented in “Evaluation Metrics” section, each metric has its own contribution in evaluating the performance of a certain algorithm. Also, depending on the evaluated task (e.g., classification, segmentation), some metrics are applicable while others cannot be used.
-
Most of the selected papers have their own datasets which are split into training, validation, and testing. In some cases, for already trained algorithms, the authors provide only a small set for testing. Usually, the sample sizes are small (ranging from tens of images to thousands of images—with only one research work that processed over 150000 radiographs [93]), because of the cumbersome process of manually annotating data. As already stated by Schwendicke et al. [43], a desired scenario would be to shift training sample sizes from several thousands to millions of multi-level connected instances. In a survey by Daneshjou et al. [111] it is mentioned that from a total of 70 analyzed research works and 1 065 291 images which were used to develop or test AI algorithms, only 24.2% were publicly available. Sengupta et al. [112] also addressed the scarcity of publicly available image datasets for machine learning research, claiming that from a total of 332 articles/datasets only one met the selection criteria for oral cancer and was available publicly. Possible reasons for this lack of publicly available datasets are intellectual property protection and commercial benefits. Even though there is a clear trend in the direction of open science, a lot of companies still prefer to protect their investments by limiting the access to source code or labeled data, both being obtained with considerable resources. Another possible reason is the lack of interoperability among imaging and labeling solutions. Actions must be taken world-wide, at government level, to encourage large-scale collaborations between hospitals and e-health providers, to ensure interoperability and standardization in imagining and labeling workflows. For now, data augmentation with simple procedures such as blur, sharpen, color, introduction of noise, translation/rotation, or with complex algorithms such as GAN, can compensate for the limitations of the datasets.
Considering the studied papers, several conclusions are highlighted. Firstly, all the analyzed works underline AI’s role in predicting compromised teeth, staging periodontitis, refining implant predictions, aiding dental decisions and guiding implant suitability. However, there is still room for improvement. The small sample sizes represent an important limitation of the presented solutions. There is a need for publicly available, very large datasets for training, validation, and testing. These would improve the performance of the ML algorithms but would also open the door for the creation of public benchmarks that would allow for more objective evaluations of the proposed solutions. These benchmarks should not only use the same datasets, but the same sets of metrics when evaluating different solutions. Lastly, the aim of these automatic solutions should not be the replacement of doctors, but the assistance offered to medical professionals in all the tasks of periodontology and implantology, to increase both the speed and the quality of the medical act.
Change history
21 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10439-024-03565-2
References
Common Machine Learning Algorithms for Beginners. https://www.projectpro.io/article/common-machine-learning-algorithms-for-beginners/202#mcetoc_1g7709sni2l. Accessed 21 Aug 2022.
Sindayigaya, L., and A. Dey. Machine learning algorithms: a review. Info. Syst. J. 11(8):1127–1133, 2022.
Le Duc, T., R. G. Leiva, P. Casari, and P. O. Östberg. Machine learning methods for reliable resource provisioning in edge-cloud computing: a survey. ACM Comput. Surv. 52(5):1–39, 2019.
Qiang, W., and Z. Zhongli. Reinforcement learning model, algorithms and its application. In: 2011 International Conference on Mechatronic Science, Electric Engineering and Computer (MEC), IEEE, 2011, pp. 1143–1146.
Goldstein, M. k_n-nearest neighbor classification. IEEE Trans. Inf. Theory. 18(5):627–630, 1972. https://doi.org/10.1109/TIT.1972.1054888.
Joyce, J. Bayes’ theorem. In: The Stanford Encyclopedia of Philosophy, edited by E. N. Zalta. Stanford: Stanford University, 2003.
Hearst, M. A., S. T. Dumais, E. Osuna, J. Platt, and B. Scholkopf. Support vector machines. IEEE Intell. Syst. their Appl. 13(4):18–28, 1998. https://doi.org/10.1109/5254.708428.
De Stefano J. J. Logistic regression and the Boltzmann machine. In: 1990 IJCNN International Joint Conference on Neural Networks, 1990, pp. 199–204, https://doi.org/10.1109/IJCNN.1990.137845.
Discriminant function analysis. In: Resource Selection by Animals: Statistical Design and Analysis for Field Studies. Springer Netherlands, Dordrecht, 2002, pp. 171–178.
Xanthopoulos, P., P. M. Pardalos, and T. B. Trafalis. Linear discriminant analysis. In: Robust Data Mining, New York: Springer, 2013, pp. 27–33.
Kotsiantis, S. B. Decision trees: a recent overview. Artif. Intell. Rev. 39(4):261–283, 2013. https://doi.org/10.1007/s10462-011-9272-4.
Breiman, L. Random forests. Mach. Learn. 45(1):5–32, 2001. https://doi.org/10.1023/A:1010933404324.
Abiodun, O. I., et al. Comprehensive review of artificial neural network applications to pattern recognition. IEEE Access. 7:158820–158846, 2019. https://doi.org/10.1109/ACCESS.2019.2945545.
Likas, A., N. Vlassis, and J. J. Verbeek. The global k-means clustering algorithm. Pattern Recognit. 36(2):451–461, 2003. https://doi.org/10.1016/S0031-3203(02)00060-2.
Forrest, S. Genetic algorithms. ACM Comput. Surv. 28(1):77–80, 1996. https://doi.org/10.1145/234313.234350.
Paulinas, M., and A. Usinskas. A survey of genetic algorithms applications for image enhancement and segmentation. Inf. Technol. Control. 36:278–284, 2007.
Caponetti, L., N. Abbattista, and G. Carapella. A genetic approach to edge detection. Proc. Int. Conf. Image Process. 1:318–322, 1994. https://doi.org/10.1109/ICIP.1994.413327.
He K., X. Zhang, S. Ren, and J. Sun. Deep residual learning for image recognition. 2016.
Sandler M., A. Howard, M. Zhu, A. Zhmoginov, and L. C. Chen. MobileNetV2: inverted residuals and linear bottlenecks. 2018.
Redmon J., S. Divvala, R. Girshick, and A. Farhadi. You only look once: unified, real-time object detection. 2016.
Liu W. et al., SSD: single shot multibox detector BT-computer vision—ECCV 2016, 2016, pp. 21–37.
Girshick, R., J. Donahue, T. Darrell, and J. Malik. Rich feature hierarchies for accurate object detection and semantic segmentation. IEEE Conf. Comput. Vision Pattern Recognit. 2014:580–587, 2014. https://doi.org/10.1109/CVPR.2014.81.
Dai J., Y. Li, K. He, and J. Sun. R-FCN: object detection via region-based fully convolutional networks. In: Proceedings of the 30th International Conference on Neural Information Processing Systems, 2016, pp. 379–387.
Ronneberger O., P. Fischer, and T. Brox. U-Net: convolutional networks for biomedical image segmentation. In: Medical Image Computing and Computer-Assisted Intervention MICCAI 2015, 2015, pp. 234–241.
Noh H., S. Hong, and B. Han. Learning deconvolution network for semantic segmentation. 2015 IEEE Int. Conf. Comput. Vis. pp. 1520–1528, 2015.
Badrinarayanan, V., A. Kendall, and R. Cipolla. SegNet: a deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 39(12):2481–2495, 2017. https://doi.org/10.1109/TPAMI.2016.2644615.
Pinheiro P. O., R. Collobert, and P. Dollár. Learning to segment object candidates. In Proceedings of the 28th International Conference on Neural Information Processing Systems, 2015, pp. 1990–1998.
He K., G. Gkioxari, P. Dollár, and R. Girshick. Mask R-CNN. In: 2017 IEEE International Conference on Computer Vision (ICCV), 2017, pp. 2980–2988, https://doi.org/10.1109/ICCV.2017.322
Bewick, V., L. Cheek, and J. Ball. Statistics review 9: one-way analysis of variance. Crit. Care. 8(2):130, 2004. https://doi.org/10.1186/cc2836.
Keselman, H. J., and J. C. Rogan. The Tukey multiple comparison test: 1953–1976. Psychol. Bull. 84(5):1050, 1977.
Caton, J. G., G. Armitage, T. Berglundh, I. L. C. Chapple, S. Jepsen, K. S. Kornman, B. L. Mealey, P. N. Papapanou, M. Sanz, and M. S. Tonetti. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J. Clin. Periodontal. 45(Suppl 20):S1–S8, 2018. https://doi.org/10.1111/jcpe.12935.
Pini Prato, G. P., R. Di Gianfilippo, and H. L. Wang. Success in periodontology: an evolutive concept. J. Clin. Periodontal. 46(8):840–845, 2019. https://doi.org/10.1111/jcpe.13150.
Saygun, I., N. Nizam, I. Keskiner, V. Bal, A. Kubar, C. Acıkel, M. Serdar, and J. Slots. Salivary infectious agents and periodontal disease status. J. Periodontal. Res. 46:235–239, 2011.
Slots, J. Periodontology: past, present, perspectives. Periodontology 2000. 62(1):7–19, 2013. https://doi.org/10.1111/prd.12011.
Armitage, G. C. Learned and unlearned concepts in periodontal diagnostics: a 50-year perspective. Periodontology 2000. 2013(62):20–36, 2000.
Dannewitz, B., B. Holtfreter, and P. Eickholz. Parodontitis—Therapie einer Volkskrankheit [Periodontitis-therapy of a widespread disease]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 64(8):931–940, 2021. https://doi.org/10.1007/s00103-021-03373-2.
Darby, I. Risk factors for periodontitis & peri-implantitis. Periodontology 2000. 90(1):9–12, 2022. https://doi.org/10.1111/prd.12447.
Chambrone, L., H. L. Wang, and G. E. Romanos. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: an American Academy of Periodontology best evidence review. J. Periodontal. 89(7):783–803, 2018. https://doi.org/10.1902/jop.2017.170172.
Berglundh, T., S. Jepsen, B. Stadlinger, and H. Terheyden. Peri-implantitis and its prevention. Clin. Oral Implant. Res. 30(2):150–155, 2019. https://doi.org/10.1111/clr.13401.
Shan, T., F. R. Tay, and L. Gu. Application of artificial intelligence in dentistry. J. Dental Res. 100(3):232–244, 2021. https://doi.org/10.1177/0022034520969115.
Katne, T., A. Kanaparthi, S. Goud, S. Muppirala, R. Devaraju, and R. Gantala. Artificial intelligence: demystifying dentistry the future and beyond. Int. J. Contemp. Med. Surg. Radiol. 4:2, 2019. https://doi.org/10.21276/ijcmsr.2019.4.4.2.
Grischke, J., L. Johannsmeier, L. Eich, L. Griga, and S. Haddadin. Dentronics: towards robotics and artificial intelligence in dentistry. Dent. Mater. 36(6):765–778, 2020. https://doi.org/10.1016/j.dental.2020.03.021.
Schwendicke, F., W. Samek, and J. Krois. Artificial intelligence in dentistry: chances and challenges. J. Dent. Res. 99(7):769–774, 2020. https://doi.org/10.1177/0022034520915714.
Kang, D. Y., P. Duong, and J. C. Park. Application of deep learning in dentistry and implantology. Korean Acad. Oral Maxillofac. Implantol. 24:148–181, 2020. https://doi.org/10.32542/implantology.202015.
Revilla-León, M., et al. Artificial intelligence applications in implant dentistry: a systematic review. J. Prosthet. Dent. 2021. https://doi.org/10.1016/j.prosdent.2021.05.008.
Saghiri, M. A., P. Freag, A. Fakhrzadeh, A. Saghiri, and J. Eid. Current technology for identifying dental implants: a narrative review. Bull. Natl. Res. Cent. 2021. https://doi.org/10.1186/s42269-020-00471-0.
Lim, H. K., Y. J. Kwon, and E. S. Lee. Application of artificial intelligence in identification of dental implants system: literature review. J. Dent. Implant Res. 39(4):48–52, 2020.
Bernauer, S. A., N. U. Zitzmann, and T. Joda. The use and performance of artificial intelligence in prosthodontics: a systematic review. Sensors. 2021. https://doi.org/10.3390/s21196628.
Pareek, M., and B. Kaushik. Artificial intelligence in prosthodontics: a scoping review on current applications and future possibilities. Int. J. Adv. Med. 9:367, 2022. https://doi.org/10.18203/2349-3933.ijam20220444.
Col, D., et al. Convolutional neural network in periodontology—innovative technology or new era? A review. Ann. Rom. Soc. Cell Biol. 25:17412–17421, 2021.
Revilla-León, M., et al. Artificial intelligence models for diagnosing gingivitis and periodontal disease: a systematic review. J. Prosthet. Dent. 2022. https://doi.org/10.1016/j.prosdent.2022.01.026.
Mohammad-Rahimi, H., et al. Deep learning in periodontology and oral implantology: a scoping review. J. Periodontal Res. 57(5):942–951, 2022. https://doi.org/10.1111/jre.13037.
Lee, J. H., D. H. Kim, S. N. Jeong, and S. H. Choi. Diagnosis and prediction of periodontally compromised teeth using a deep learning-based convolutional neural network algorithm. J. Periodontal Implant Sci. 48(2):114–123, 2018. https://doi.org/10.5051/jpis.2018.48.2.114.
Kim, J., H. S. Lee, I. S. Song, and K. H. Jung. DeNTNet: deep neural transfer network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 9(1):17615, 2019. https://doi.org/10.1038/s41598-019-53758-2.
Krois, J., et al. Deep learning for the radiographic detection of periodontal bone loss. Sci. Rep. 9(1):8495, 2019. https://doi.org/10.1038/s41598-019-44839-3.
Claesen M., and B. De Moor. Hyperparameter search in machine learning. 2015. https://doi.org/10.48550/ARXIV.1502.02127.
Thanathornwong, B., and S. Suebnukarn. Automatic detection of periodontal compromised teeth in digital panoramic radiographs using faster regional convolutional neural networks. Imaging Sci. Dent. 50(2):169–174, 2020. https://doi.org/10.5624/isd.2020.50.2.169.
Ren S., K. He, R. Girshick, and J. Sun. Faster R-CNN: towards real-time object detection with region proposal networks. In: Proceedings of the 28th International Conference on Neural Information Processing Systems, 2015, pp. 91–99.
Chang, H. J., et al. Deep learning hybrid method to automatically diagnose periodontal bone loss and stage periodontitis. Sci. Rep. 10(1):7531, 2020. https://doi.org/10.1038/s41598-020-64509-z.
Tonetti, M. S., H. Greenwell, and K. S. Kornman. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J. Periodontol. 89(1):S159–S172, 2018. https://doi.org/10.1002/JPER.18-0006.
Lee, C. T., et al. Use of the deep learning approach to measure alveolar bone level. J. Clin. Periodontol. 49(3):260–269, 2021.
Kabir, T., et al. An end-to-end entangled segmentation and classification convolutional neural network for periodontitis stage grading from periapical radiographic images. BIBM. 2021:1370, 2021.
Jiang, L., D. Chen, Z. Cao, F. Wu, H. Zhu, and F. Zhu. A two-stage deep learning architecture for radiographic staging of periodontal bone loss. BMC Oral Health. 22(1):106, 2022. https://doi.org/10.1186/s12903-022-02119-z.
Karacaoglu, F., E. K. Mehmet, B. Nilsun, E. Cengiz, and O. Kaan. Development and validation of intraoral periapical radiography-based machine learning model for periodontal defect diagnosis. Proc. Inst. Mech. Eng. J. Eng. Med. 237(5):607–618, 2023.
Deng, K., Z. Francesco, Y. Huan, P. George, and M. S. Tonetti. Development of a machine learning multiclass screening tool for periodontal health status based on non-clinical parameters and salivary biomarkers. J. Clin. Periodontol. 2023. https://doi.org/10.1111/jcpe.13856.
Lakshmi, T. K., and J. Dheeba. predictive analysis of periodontal disease progression using machine learning: enhancing oral health assessment and treatment planning. Int. J. Intell. Syst. Appl. Eng. 11(10s):660–671, 2023.
Kingma D., and J. Ba. Adam: A method for stochastic optimization. Int. Conf. Learn. Represent, 2014.
Bochkovskiy A., C. Y. Wang, and H. Y. Mark Liao. Yolov4: optimal speed and accuracy of object detection. 2020. https://arxiv.org/abs/2004.10934.
Görler, O., and S. Akkoyun. Artificial neural networks can be used as alternative method to estimate loss tooth root sizes for prediction of dental implants. Cumhur. Sci. J. 38:385, 2017. https://doi.org/10.17776/cumuscij.304902.
Lee, S., S. Woo, J. Yu, J. Seo, J. Lee, and C. Lee. Automated CNN-based tooth segmentation in cone-beam CT for dental implant planning. IEEE Access. 8:50507–50518, 2020. https://doi.org/10.1109/ACCESS.2020.2975826.
Roongruangsilp, P., and P. Khongkhunthian. The learning curve of artificial intelligence for dental implant treatment planning: a descriptive study. Appl. Sci. 2021. https://doi.org/10.3390/app112110159.
Kurt Bayrakdar, S., et al. A deep learning approach for dental implant planning on cone-beam computed tomography images. BMC Med. Imag. 21(1):86, 2021.
Park, J., J. Lee, S. Moon, and K. Lee. Deep learning based detection of missing tooth regions for dental implant planning in panoramic radiographic images. Appl. Sci. 2022. https://doi.org/10.3390/app12031595.
Moufti, M. A., N. Trabulsi, M. Ghousheh, T. Fattal, A. Ashira, and S. Danishvar. Developing an artificial intelligence solution to autosegment the edentulous mandibular bone for implant planning. Eur. J. Dent. 17(04):1330–1337, 2023.
Oliveira-Santos, N., R. Jacobs, F. F. Picoli, P. Lahoud, L. Niclaes, and F. C. Groppo. Automated segmentation of the mandibular canal and its anterior loop by deep learning. Sci Rep. 13(1):10819, 2023.
Hashem, M., M. L. Mohammed, and A. E. Youssef. Improving the efficiency of dental implantation process using guided local search models and continuous time neural networks with robotic assistance. IEEE Access. 8:202755–202764, 2020. https://doi.org/10.1109/ACCESS.2020.3034689.
Liu Y., Z. Chen, C. Chu, and F. L. Deng. Transfer learning via artificial intelligence for guiding implant placement in the posterior mandible: an in vitro study. 2021.
Mijiritsky, E., Z. Mazor, A. Lorean, and L. Levin. Implant diameter and length influence on survival: interim results during the first 2 years of function of implants by a single manufacturer. Implant Dent. 22(4):394–398, 2013. https://doi.org/10.1097/ID.0b013e31829afac0.
Anan, N. S., and V. G. Thri. Performance and classification evaluation of J48 algorithm and Kendall’s based J48 algorithm (KNJ48). Int. J. Comput. Trends Technol. 59:73–80, 2018. https://doi.org/10.14445/22312803/IJCTT-V59P112.
Sadat, R., M. Khalili, and M. Nazari. A hybrid method to predict success of dental implants. Int. J. Adv. Comput. Sci. Appl. 2016. https://doi.org/10.14569/IJACSA.2016.070501.
Oliveira, A. L. I., C. Baldisserotto, and J. Baldisserotto. A comparative study on machine learning techniques for prediction of success of dental implants. Adv. Artif. Intell. 2005:939–948, 2005.
Khan, A., and K. Maity. A comprehensive GRNN model for the prediction of cutting force, surface roughness and tool wear during turning of CP-Ti grade 2. Silicon. 10(5):2181–2191, 2018. https://doi.org/10.1007/s12633-017-9749-0.
Isensee, F., P. Kickingereder, W. Wick, M. Bendszus, and K. H. Maier-Hein. Brain tumor segmentation and radiomics survival prediction: contribution to the BRATS 2017 challenge. In: Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries, Cham: Springer, 2018, pp. 287–297.
MONAI framework. https://monai.io/. Accessed 27 May 2024.
Sukegawa, S., et al. Deep neural networks for dental implant system classification. Biomolecules. 2020. https://doi.org/10.3390/biom10070984.
Saïd, M. H., M. K. L. Roux, J. H. Catherine, and R. Lan. Development of an artificial intelligence model to identify a dental implant from a radiograph. Int. J. Oral Maxillofac. Implants. 36(6):1077–1082, 2020. https://doi.org/10.11607/jomi.8060.
Lee, J. H., and S. N. Jeong. Efficacy of deep convolutional neural network algorithm for the identification and classification of dental implant systems, using panoramic and periapical radiographs: a pilot study. Medicine. 99:e20787, 2020. https://doi.org/10.1097/MD.0000000000020787.
Takahashi, T., K. Nozaki, T. Gonda, T. Mameno, M. Wada, and K. Ikebe. Identification of dental implants using deep learning-pilot study. Int. J. Implant Dent. 6(1):53, 2020. https://doi.org/10.1186/s40729-020-00250-6.
da Mata Santos, R. P., et al. Automated identification of dental implants using artificial intelligence. Int. J. Oral Maxillofac. Implants. 36(5):918–923, 2021. https://doi.org/10.11607/jomi.8684.
Sukegawa, S., et al. Multi-task deep learning model for classification of dental implant brand and treatment stage using dental panoramic radiograph images. Biomolecules. 2021. https://doi.org/10.3390/biom11060815.
Kim, H. S., E. G. Ha, Y. H. Kim, K. J. Jeon, C. Lee, and S. S. Han. Transfer learning in a deep convolutional neural network for implant fixture classification: a pilot study. Imaging Sci. Dent. 52(2):219–224, 2022. https://doi.org/10.5624/isd.20210287.
Kong, H. J., J. Y. Yoo, J. H. Lee, S. H. Eom, and J. H. Kim. Performance evaluation of deep learning models for the classification and identification of dental implants. J. Prosthet Dent. 2023. https://doi.org/10.1016/j.prosdent.2023.07.009.
Park, W., J. K. Huh, and J. H. Lee. Automated deep learning for classification of dental implant radiographs using a large multi-center dataset. Sci Rep. 13(1):4862, 2023.
Kong, H. J. Classification of dental implant systems using cloud-based deep learning algorithm: an experimental study. J. Yeungnam Med. Sci. 40(Suppl):S29, 2023.
Lee, J. H., Y. T. Kim, J. B. Lee, and S. N. Jeong. A performance comparison between automated deep learning and dental professionals in classification of dental implant systems from dental imaging: a multi-center study. Diagnostics. 2020. https://doi.org/10.3390/diagnostics10110910.
Lee, J. H., Y. T. Kim, J. B. Lee, and S. N. Jeong. Deep learning improves implant classification by dental professionals: a multi-center evaluation of accuracy and efficiency. J. Periodontal Implant Sci. 52(3):220–229, 2022. https://doi.org/10.5051/jpis.2104080204.
Benakatti, V. B., R. P. Nayakar, and M. Anandhalli. Machine learning for identification of dental implant systems based on shape—a descriptive study. J. Indian Prosthodont. Soc. 21(4):405–411, 2021. https://doi.org/10.4103/jips.jips_324_21.
Moayeri, R. S., M. Khalili, and M. Nazari. A hybrid method to predict success of dental implants. Int. J. Adv. Comput. Sci. Appl. 2016. https://doi.org/10.14569/IJACSA.2016.070501.
Papantonopoulos, G., C. Gogos, E. Housos, T. Bountis, and B. G. Loos. Prediction of individual implant bone levels and the existence of implant ‘phenotypes.’ Clin. Oral Implants Res. 28(7):823–832, 2017. https://doi.org/10.1111/clr.12887.
Cha, J. Y., H. I. Yoon, I. S. Yeo, K. H. Huh, and J. S. Han. Peri-implant bone loss measurement using a region-based convolutional neural network on dental periapical radiographs. J. Clin. Med. 2021. https://doi.org/10.3390/jcm10051009.
Liu, M., S. Wang, H. Chen, and Y. Liu. A pilot study of a deep learning approach to detect marginal bone loss around implants. BMC Oral Health. 22(1):11, 2022. https://doi.org/10.1186/s12903-021-02035-8.
Chen, Y. C., M. Y. Chen, T. Y. Chen, M. L. Chan, Y. Y. Huang, Y. L. Liu, P. T. Lee, et al. Improving dental implant outcomes: CNN-based system accurately measures degree of peri-implantitis damage on periapical film. Bioengineering. 10(6):640, 2023.
Zhang, C., L. Fan, S. Zhang, J. Zhao, and G. Yingxin. Deep learning based dental implant failure prediction from periapical and panoramic films. Quant. Imag. Med. Surg. 13(2):935, 2023.
Vera, M., M. J. Gómez-Silva, V. Vera, C. I. López-González, I. Aliaga, E. Gascó, V. Vera-González, M. Pedrera-Canal, E. Besada-Portas, and G. Pajares. Artificial intelligence techniques for automatic detection of peri-implant marginal bone remodeling in intraoral radiographs. J. Digit. Imag. 36(5):2259–2277, 2023.
Lee, D. W., S. Y. Kim, S. N. Jeong, and J. H. Lee. Artificial intelligence in fractured dental implant detection and classification: evaluation using dataset from two dental hospitals. Diagnostics. 2021. https://doi.org/10.3390/diagnostics11020233.
Ha, S. R., et al. A pilot study using machine learning methods about factors influencing prognosis of dental implants. J. Adv. Prosthodont. 10(6):395–400, 2018. https://doi.org/10.4047/jap.2018.10.6.395.
Wang, C. W., et al. Machine learning-Assisted immune profiling stratifies peri-implantitis patients with unique microbial colonization and clinical outcomes. Theranostics. 11:6703–6716, 2021. https://doi.org/10.7150/thno.57775.
Mameno, T., et al. Predictive modeling for peri-implantitis by using machine learning techniques. Sci. Rep. 11(1):11090, 2021. https://doi.org/10.1038/s41598-021-90642-4.
Sabzekar, M., M. Namakin, H. A. S. Babaki, A. Deldari, and V. Babaiyan. Dental implants success prediction by classifier ensemble on imbalanced data. Comput. Methods Programs Biomed. Updat.1:100021, 2021. https://doi.org/10.1016/j.cmpbup.2021.100021.
Fan, W., J. Tang, X. Huixia, X. Huang, W. Donglei, and Z. Zhang. Early diagnosis for the onset of peri-implantitis based on artificial neural network. Open Life Sci. 18(1):20220691, 2023.
Daneshjou, R., M. P. Smith, M. D. Sun, V. Rotemberg, and J. Zou. Lack of transparency and potential bias in artificial intelligence data sets and algorithms: a scoping review. JAMA Dermatol. 157(11):1362–1369, 2021.
Sengupta, N., S. C. Sarode, G. S. Sarode, and U. Ghone. Scarcity of publicly available oral cancer image datasets for machine learning research. Oral Oncol. 126:105737, 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Șalgău, C.A., Morar, A., Zgarta, A.D. et al. Applications of Machine Learning in Periodontology and Implantology: A Comprehensive Review. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03559-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03559-0