Abstract
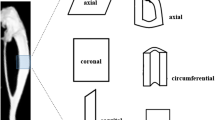
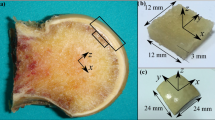
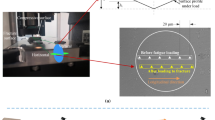
Bone tissue is subjected to increased mechanical stress during high-intensity work. Inadequate bone remodeling reparability can result in the continuous accumulation of microdamage, leading to stress fractures. The aim of this work was to investigate the characteristics and repair mechanisms of tibial microdamage under several degrees of overload. Also, we aimed at better understanding the effects of overload on the multi-scale structure and mechanical properties of bone. Sixty 5-month female rats were divided into three groups with different time points. Micro-CT was used to evaluate the three-dimensional microstructure, and three-point bending, quasi-static fracture toughness and creep mechanical test were carried out to evaluate the mechanical properties. SEM was used to observe the morphological characteristics of fracture surfaces. Section staining was used to count the microdamage parameters and numbers of osteoblasts and osteoclasts. The microarchitectures of cancellous and cortical bones in the three overload groups showed different degrees of damage. Overload led to a messy crystal structure of cortical bone, with slender microcracks mixed in, and a large number of broken fibers of cancellous bone. The properties associated with the elastic plasticity, fracture toughness, and viscoelasticity of cortical bone reduced in three groups, with that corresponding to day 30 presenting the highest damage. The accumulation of microdamage mainly occurred in the first 14 days, that is, the crack density peaked on day 14. Peak-targeted bone remodeling of cortical and cancellous bones occurred mainly between days 14 and 30. The influence of overload mechanical environment on bone quality at different time points was deeply investigated, which is of great significance for the etiology and treatment of stress fractures.







Similar content being viewed by others
References
Chapurlat, R. D., and P. D. Delmas. Bone microdamage: a clinical perspective. Osteoporos. Int. 20(8):1299–1308, 2009. https://doi.org/10.1007/s00198-009-0899-9.
Lee, T. C., S. Mohsin, D. Taylor, R. Parkesh, T. Gunnlaugsson, F. J. O’Brien, M. Giehl, and W. Gowin. Detecting microdamage in bone. J. Anat. 203(2):161–172, 2003. https://doi.org/10.1046/j.1469-7580.2003.00211.x.
Diab, T., and D. Vashishth. Effects of damage morphology on cortical bone fragility. Bone. 37(1):96–102, 2005. https://doi.org/10.1016/j.bone.2005.03.014.
Burr, D. B., M. R. Forwood, D. P. Fyhrie, R. B. Martin, M. B. Schaffler, and C. H. Turner. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J. Bone Miner. Res. 12(1):6–15, 1997. https://doi.org/10.1359/jbmr.1997.12.1.6.
Hoenig, T., K. E. Ackerman, B. R. Beck, M. L. Bouxsein, D. B. Burr, K. Hollander, K. L. Popp, T. Rolvien, A. S. Tenforde, and S. J. Warden. Bone stress injuries. Nat. Rev. Dis. Primers. 8(1):26, 2022. https://doi.org/10.1038/s41572-022-00352-y.
Yan, C. X., H. Song, J. Pfister, T. L. Andersen, S. J. Warden, R. Bhargave, and M. E. Kersh. Effect of fatigue loading and rest on impact strength of rat ulna. J. Biomech. 123:110449, 2021. https://doi.org/10.1016/j.jbiomech.2021.110449.
Sturznickel, J., N. Hinz, M. M. Delsmann, T. Hoenig, and T. Rolvien. Impaired bone microarchitecture distal radial and tibial reference locations is not related to injury site in athletes with bone stress injury. Am. J. Sport Med. 50(12):3381–3389, 2022. https://doi.org/10.1177/03635465221120385.
Nuti, R., M. L. Brandi, G. Checchia, O. Di Munno, L. Dominguez, P. Falaschi, C. E. Fiore, G. Iolascon, S. Maggi, M. Rossini, G. Sessa, U. Tarantino, A. Toselli, and G. C. Isaia. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 14(1):85–102, 2019. https://doi.org/10.1007/s11739-018-1874-2.
Liu, Z. H., J. Z. Gao, and H. Gong. The adaptive response of rat tibia to different levels of peak strain and durations of experiment. Med. Eng. Phys.102:103785, 2022. https://doi.org/10.1016/j.medengphy.2022.103785.
Li, J. W., and H. Gong. Fatigue behavior of cortical bone: a review. Acta Mech. Sin. 37(3):516–526b, 2020. https://doi.org/10.1007/s10409-020-01012-8.
Hao, L., L. Rui-Xin, H. Biao, Z. Bin, H. Bao-Hui, L. Ying-Jie, and Z. Xi-Zhang. Effect of athletic fatigue damage and the associated bone targeted remodeling in the rat ulna. BioMed. Eng. Online. 16:99, 2017. https://doi.org/10.1186/s12938-017-0384-1.
Liu, X. Y., C. Tang, X. H. Zhang, J. Cai, Z. D. Yan, K. N. Xie, Z. P. Yang, J. Wang, X. E. Guo, E. P. Luo, and D. Jing. Spatiotemporal distribution of linear microcracks and diffuse microdamage following daily bouts of fatigue loading of rat lunae. J. Orthop. Res. 37(10):2112–2121, 2019. https://doi.org/10.1002/jor.24391.
Webster, D. J., P. Schneider, S. L. Dallas, and R. Muller. Studying osteocytes within their environment. Bone. 54(2):285–295, 2013. https://doi.org/10.1016/j.bone.2013.01.004.
Sims, N. A., and T. J. Martin. Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu. Rev. Physiol. 82:507–529, 2020. https://doi.org/10.1146/annurev-physiol-021119-034425.
Jarvinen, T. L. N., P. Kannus, I. Pajamaki, T. Vuohelainen, J. Tuukkanen, A. Jarvinen, and H. Sievanen. Estrogen deposits extra mineral into bones of female rats in puberty, but simultaneously seems to suppress the responsiveness of female skeleton to mechanical loading. Bone. 32(6):642–651, 2003. https://doi.org/10.1016/S8756-3282(03)00100-5.
Takano-Yamamoto, T., and S. Nomura. Molecular events caused by mechanical stress in bone. Matrix Biol. 19(2):91–96, 2000. https://doi.org/10.1016/S0945-053X(00)00050-0.
Meng, X. J., C. A. Y. Qu, D. H. Fu, and C. A. Qu. Effects of fatigue damage on the microscopic modulus of cortical bone using nanoindentation. Materials. 14(12):3252–3263, 2021. https://doi.org/10.3390/ma14123252.
Unal, M., S. Uppuganti, S. Timur, A. Mahadevan-Jansen, O. Akkus, and J. S. Nyman. Assessing matrix quality by Raman spectroscopy helps predict fracture toughness of human cortical bone. Sci. Rep. 9:7195, 1981. https://doi.org/10.1038/s41598-019-43542-7.
Galley, S. A., D. J. Michalek, and S. W. Donahue. A fatigue microcrack alters fluid velocities in a computational model of interstitial fluid flow in cortical bone. J. Biomech. 39(11):2026–2033, 2006. https://doi.org/10.1016/j.jbiomech.2005.06.008.
Karali, A., E. D. Ara, J. Zekonyte, A. P. Kao, G. Blun, and G. Tozzi. Effect of radiation-induced damage of trabecular bone tissue evaluated using indentation and digital volume correlation. J. Mech. Behav. Biomed.138:105636, 2023. https://doi.org/10.1016/j.jmbbm.2022.105636.
Burr, D. B., C. H. Turner, P. Naick, M. R. Forwood, W. Ambrosius, M. S. Hasan, and R. Pidaparti. Dose microdamage accumulation affect the mechanical properties of bone. J. Biomech. 31(4):337–345, 1998. https://doi.org/10.1016/S0021-9290(98)00016-5.
Patel, T. K., M. D. Brodt, and M. J. Silva. Experimental and finite element analysis of strain induced by axial tibial compression in young-adult and old female C57Bl/6 mice. J. Biomech. 47(2):451–457, 2014. https://doi.org/10.1016/j.jbiomech.2013.10.052.
Willett, T. L., S. Sutty, A. Gaspar, N. Avery, and M. Grynpas. In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone. Bone. 52(2):611–622, 2013. https://doi.org/10.1016/j.bone.2012.11.014.
Viguet-Carrin, S., D. Farlay, Y. Bala, F. Munoz, M. L. Bouxsein, and P. D. Delmas. An in vitro model to test the contribution of advanced glycation end products to bone biomechanical properties. Bone. 42(1):139–149, 2008. https://doi.org/10.1016/j.bone.2007.08.046.
Tang, S. Y., U. Zeenath, and D. Vashishth. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 40(4):1144–1151, 2007. https://doi.org/10.1016/j.bone.2006.12.056.
Dorrington, K. L. The theory of viscoelasticity in biomaterials. Symp. Soc. Exp. Biol. 34:289–314, 1980.
Ritchiie, R. O., K. J. Koester, S. Ionova, W. Yao, N. E. Lane, and J. W. Ager. Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone. 43(5):798–812, 2008. https://doi.org/10.1016/j.bone.2008.04.027.
Takahashi, Y. Evaluation of leak-before-break assessment methodology for pipes with a circumferential through-wall crack. Part Ι: stress intensity factor and limit load solutions. Int. J. Press. Vessels Pip. 79(6):385–392, 2002. https://doi.org/10.1016/S0308-0161(02)00036-4.
Standard test method for measurement of fracture toughness. ASTM, E1820-17.
Hansen, L. J., S. L. Bloch, T. Frisch, and M. S. Sorensen. Distribution of microcrack surface density in the human otic capsule. Acta Oto-Laryngol. 141(6):567–571, 2021. https://doi.org/10.1080/00016489.2021.1905875.
O’Brien, F. J., D. Taylor, and T. C. Lee. Microcrack accumulation at different intervals during fatigue testing of compact bone. J. Biomech. 36(7):973–980, 2003. https://doi.org/10.1016/S0021-9290(03)00066-6.
Chapurlat, R. D., M. Arlot, B. Burt-Pichat, P. Chavassieux, J. P. Roux, N. Portero-Muzy, and P. D. Delmas. Microcrack frequency and bone remodeling in postmenopausal osteoporotic woman on long-term bisphosphonates: a bone biopsy study. J. Bone Miner. Res. 22(10):1502–1509, 2007. https://doi.org/10.1359/JBMR.070609.
Zhang, C. G., J. X. Zhu, J. L. Jia, Z. Y. Guan, T. T. Sun, W. Zhang, W. Q. Yuan, H. Wang, H. J. Leng, and C. L. Song. Effect of single versus multiple fractures on systemic bone loss in mice. J. Bone Miner. Res. 36(3):567–578, 2020. https://doi.org/10.1002/jbmr.4211.
Zhou, B. N., Q. Zhang, X. Y. Lin, J. Hu, D. C. Zhao, Y. Jiang, X. P. Xing, and M. Li. The roles of sclerostin and irisin on bone and muscle of orchiectomized rats. BMC Musculoskel. Dis. 23(1):1049, 2023. https://doi.org/10.1186/s12891-022-05982-7.
Chalhoub, D., P. M. Cawthon, K. E. Ensrud, M. L. Stefanick, D. M. Kado, R. Boudreau, S. Greenspan, A. B. Newman, J. Zmuda, E. S. Orwoll, and J. A. Cauley. Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J. Am. Geriatr. Soc. 63(9):1733–1740, 2015. https://doi.org/10.1111/jgs.13605.
Wright, C. S., E. R. Hill, P. C. R. Fernandez, W. R. Thompson, M. A. Gallant, W. W. Campbell, and R. P. Main. Effects of dietary protein source and quantity on bone morphology and body composition following a high-protein weight-loss diet in a rat model for postmenopausal obesity. Nutrients. 14(11):2262, 2022. https://doi.org/10.3390/nu14112262.
Wang, X., D. Kim, K. L. Tucker, M. G. Weisskopf, D. Sparrow, H. Hu, and S. K. Park. Effect of dietary sodium and potassium intake on the mobilization of bone lead among middle-aged and older men: the veterans affairs normative aging study. Nutrients. 11(11):2750, 2019. https://doi.org/10.3390/nu11112750.
Hildebrand, T. O. R., and P. Ruegsegger. Quantification of bone microarchitecture with the structure model index. Comput. Methods Biomech. Biomed. Eng. 1(1):15–23, 1997. https://doi.org/10.1080/01495739708936692.
Yu, W., L. L. Zhong, and L. T. Yao. Bone marrow adapogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J. Clin. Investig. 131(2):e140214, 2021. https://doi.org/10.1172/JCI140214.
Wu, C., and T. S. Kato. Dynamics of bone turnover markers in patients with heart failure and following haemodynamic improvement through ventricular assist device implantation. Eur. J. Heart Fail. 14(12):1356–1365, 2012. https://doi.org/10.1093/eurjhf/hfs138.
Napoli, N., M. Chandran, D. D. Pierroz, B. Abrahamsen, A. V. Schwartz, and S. L. Ferrari. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 13(4):208–219, 2017. https://doi.org/10.1038/nrendo.2016.153.
Vashishth, D., G. J. Gibson, J. I. Khoury, M. B. Schaffler, J. Kimura, and D. P. Fyhrie. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 28(2):195–201, 2001. https://doi.org/10.1016/S8756-3282(00)00434-8.
Vashishth, D. Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J. Biomech. 37(6):943–946, 2004. https://doi.org/10.1016/j.jbiomech.2003.11.003.
Zimmermann, E. A., B. Gludovatz, E. Schaible, B. Busse, and R. O. Ritchie. Fracture resistance of human cortical bone across multiple length-scales at physiological strain rates. Biomaterials. 35(21):5472–5481, 2014. https://doi.org/10.1016/j.biomaterials.2014.03.066.
Morais, G. P., A. da Rocha, A. P. Pinto, L. D. Oliveria, L. G. de Vicente, G. N. Ferreira, E. C. de Freitas, and A. S. R. da Silva. Uphill running excessive training increases gastrocnemius glycogen content in C57BL/6 mice. Physiol. Res. 67(1):107–115, 2018. https://doi.org/10.33549/physiolres.933614.
Yahyazadehfar, M., and D. Arola. The role of organic proteins on the crack growth resistance of human enamel. Acta Biomater. 19:33–35, 2015. https://doi.org/10.1016/j.actbio.2015.03.011.
Anton, S. D., M. G. Perri, J. Riley, W. F. Kanasky, J. R. Rodrigue, S. F. Sears, and A. D. Martin. Differential predictors of adherence in exercise programs with moderate versus higher levels of intensity and frequency. J. Sport Exerc. Psychol. 27(2):171–187, 2005. https://doi.org/10.1123/jsep.27.2.171.
Bentolila, V., T. M. Boyce, D. P. Fyhrie, R. Drumb, T. M. Skerry, and M. B. Schaffler. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 23(3):275–281, 1998. https://doi.org/10.1016/S8756-3282(98)00104-5.
Zhang, X. H., X. Y. Liu, Z. D. Yan, J. Cai, F. Kang, S. Shan, P. Wang, M. M. Zhai, X. E. Guo, E. P. Luo, and D. Jing. Spatiotemporal characterization of microdamage accumulation in rat ulnae in response to uniaxial compressive fatigue loading. Bone. 108:156–164, 2018. https://doi.org/10.1016/j.bone.2018.01.011.
Funding
The work was supported by the National Natural Science Foundation of China (No. 12272029).
Author information
Authors and Affiliations
Contributions
He Gong: conceptualization, resources, supervision, funding acquisition, writing-review & editing. Zhehao Liu: experiment, writing-original draft. Jiazi Gao: supervision, writing-review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Gao, J. & Gong, H. Spatiotemporal Characterization of Microstructure Morphology, Mechanical Properties and Bone Remodeling of Rat Tibia Under Uniaxial Compressive Overload Loading. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03531-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03531-y