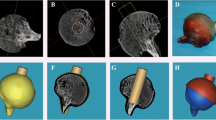
Abstract
This study aimed to investigate the relationship between the micro structural properties of the subchondral trabecular bone (STB) and the macro mechanical properties of the articular cartilage (AC) in patients with osteoporotic (OP) and osteopenic (OPE) fractures. Sixteen femoral head samples (OP;OPE, n = 8 each) were obtained from female patients who underwent hip hemiarthroplasty. STB and AC specimens were harvested from those heads. Bone specimens were scanned using µ-CT to determine the micro structural properties. In-situ nondestructive compressive tests were performed for the cartilages to obtain elastic properties. The finite element technique was implemented on STB models created from µ-CT data to compute apparent elastic modulus. In addition, dynamic cyclic destructive tests were performed on STB and AC specimens to assess failure cycles. The results demonstrated that STB specimens in OPE group have more interconnected structure and higher cyclic dynamic strength than those in OP group. Furthermore, bone mineral density, failure cycle, and trabecular number of STB were positively correlated with the cartilage failure cycle, which indicates that STB alteration may affect the macroscopic mechanical properties of AC. The findings suggest that STB loss correlates with a decrease in cartilage strength and that improving of bone quality may prevent cartilage weakness.







Similar content being viewed by others
References
Arnold, M. P., A. U. Daniels, S. Ronken, et al. Acrylamide polymer double-network hydrogels: candidate cartilage repair materials with cartilage-like dynamic stiffness and attractive surgery-related attachment mechanics. Cartilage. 2(4):374–383, 2011.
Bellido, M., L. Lugo, J. A. Roman-Blas, S. Castañeda, E. Calvo, R. Largo, and G. Herrero-Beaumont. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr. Cartil. 19(10):1228–1236, 2011.
Bellido, M., L. Lugo, J. A. Roman-Blas, et al. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res. Ther. 12(4):R152, 2010.
Blain, H., P. Chavassieux, N. Portero-Muzy, F. Bonnel, F. Canovas, M. Chammas, P. Maury, and P. D. Delmas. Cortical and trabecular bone distribution in the femoral neck in osteoporosis and osteoarthritis. Bone. 43(5):862–868, 2008.
Bobinac, D., M. Marinovic, E. Bazdulj, O. Cvijanovic, T. Celic, I. Maric, J. Spanjol, and T. Cicvaric. Microstructural alterations of femoral head articular cartilage and subchondral bone in osteoarthritis and osteoporosis. Osteoarthr. Cartil. 21(11):1724–1730, 2013.
Bolcos, P. O., M. E. Mononen, A. Mohammadi, M. Ebrahimi, et al. Comparison between kinetic and kinetic-kinematic driven knee joint finite element models. Sci. Rep. 8:17351, 2018.
Calvo, E., I. Palacios, E. Delgado, O. Sánchez-Pernaute, R. Largo, J. Egido, and G. Herrero-Beaumont. Histopathological correlation of cartilage swelling detected by magnetic resonance imaging in early experimental osteoarthritis. Osteoarthr. Cartil. 12(11):878–886, 2004.
Calvo, E., S. Castañeda, R. Largo, M. E. Fernández-Valle, F. Rodríguez-Salvanés, and G. Herrero-Beaumont. Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthr. Cartil. 15(1):69–77, 2007.
Cao, Z., C. Dou, and S. Dong. Scaffolding biomaterials for cartilage regeneration. J. Nanomater. 2014. https://doi.org/10.1155/2014/489128.
Chen, Y., Y. Hu, Y. E. Yu, et al. Subchondral trabecular rod loss and plate thickening in the development of osteoarthritis. J. Bone Miner. Res. 33(2):316–327, 2018.
Chiba, K., M. Uetani, Y. Kido, M. Ito, N. Okazaki, K. Taguchi, et al. Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: a 3-T MRI study. Osteopor. Int. 23(2):589–597, 2012.
Chu, L., Z. He, X. Qu, X. Liu, W. Zhang, S. Zhang, X. Han, M. Yan, Q. Xu, S. Zhang, X. Shang, and Z. Yu. Different subchondral trabecular bone microstructure and biomechanical properties between developmental dysplasia of the hip and primary osteoarthritis. J. Orthop. Transl. 27(22):50–57, 2019.
Ciarelli, T. E., D. P. Fyhrie, M. B. Schaffler, and S. A. Goldstein. Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J. Bone Miner. Res. 15(1):32–40, 2000.
Cutcliffe, H. C., K. M. Davis, C. E. Spritzer, et al. The characteristic recovery time as a novel, noninvasive metric for assessing in vivo cartilage mechanical function. Ann. Biomed. Eng. 48:2901–2910, 2020.
Day, J. S., J. C. Van der Linden, R. A. M. Bank, et al. Adaptation of subchondral bone in osteoarthritis. Biorheology. 41:359–368, 2004.
De Moor, L., E. Beyls, and H. Declercq. Scaffold free microtissue formation for enhanced cartilage repair. Ann. Biomed. Eng. 48:298–311, 2020.
Démarteau, O., L. Pillet, A. Inaebnit, O. Borens, and T. M. Quinn. Biomechanical characterization and in vitro mechanical injury of elderly human femoral head cartilage: comparison to adult bovine humeral head cartilage. Osteoarthr. Cartil. 14(6):589–596, 2006.
Ding, M., C. C. Danielsen, and I. Hvid. The effects of bone remodeling inhibition by alendronate on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif. Tissue Int. 82:77–86, 2008.
Ding, M., C. C. Danielsen, and I. Hvid. Age-related three-dimensional microarchitectural adaptations of subchondral bone tissues in Guinea pig primary osteoarthrosis. Calcif. Tissue Int. 78(2):113–122, 2006.
Ebrahimi, M., M. J. Turunen, M. A. Finnilä, et al. Structure-function relationships of healthy and osteoarthritic human tibial cartilage: experimental and numerical investigation. Ann. Biomed. Eng. 48:2887–2900, 2020.
Ebrahimi, M., S. Ojanen, A. Mohammadi, et al. Elastic, viscoelastic and fibril-reinforced poroelastic material properties of healthy and osteoarthritic human tibial cartilage. Ann. Biomed. Eng. 47:953–966, 2019.
Ferizi, U., S. Honig, and G. Chang. Artificial intelligence, osteoporosis and fragility fractures. Curr. Opin. Rheumatol. 31:368–375, 2019.
Ferretti, J. L., G. R. Cointry, R. F. Capozza, and H. M. Frost. Bone mass, bone strength, muscle-bone interactions, osteopenias and osteoporoses. Mech. Ageing Dev. 124(3):269–279, 2003.
Genant, H. K., K. Engelke, and S. Prevrhal. Advanced CT bone imaging in osteoporosis. Rheumatology. 47:iv9–iv16, 2008.
Glowacki, J., and T. Vokes. Osteoporosis and mechanisms of skeletal aging. In: Advances in Geroscience, edited by F. Sierra, and R. Kohanski. Cham: Springer, 2016, pp. 277–307.
Gül, O., O. S. Atik, D. Erdoğan, G. Göktaş, and C. Elmas. Transmission and scanning electron microscopy confirm that bone microstructure is similar in osteopenic and osteoporotic patients. Eklem Hastalik Cerrahisi. 24(3):126–132, 2013.
He, Z., L. Chu, X. Liu, X. Han, K. Zhang, M. Yan, X. Li, and Z. Yu. Differences in subchondral trabecular bone microstructure and finite element analysis-based biomechanical properties between osteoporosis and osteoarthritis. J. Orthop. Transl. 2(24):39–45, 2020.
Healey, J. H., V. J. Vigorita, and J. M. Lane. The coexistence and characteristics of osteoarthritis and osteoporosis. J. Bone Joint Surg. 67(4):586–592, 1985.
Keaveny, T. M., B. L. Clarke, F. Cosman, E. S. Orwoll, E. S. Siris, S. Khosla, and M. L. Bouxsein. Biomechanical computed tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos. Int. 31(6):1025–1048, 2020.
Keyak, J. H., and Y. Falkinstein. Comparison of in situ and in vitro CT scan-based finite element model predictions of proximal femoral fracture load. Med. Eng. Phys. 25(9):781–787, 2003.
Klein, T. J., J. Malda, R. L. Sah, and D. W. Hutmacher. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. B. 15(2):143–157, 2009.
Korhonen, R. K., M. S. Laasanen, J. Töyräs, J. Rieppo, J. Hirvonen, H. J. Helminen, and J. S. Jurvelin. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 35(7):903–909, 2002.
Li, B., and R. M. Aspden. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J. Bone Miner. Res. 12(4):641–651, 1997.
Li, B., and R. M. Aspden. Material properties of bone from the femoral neck and calcar femorale of patients with osteoporosis or osteoarthritis. Osteoporos. Int. 7:450–456, 1997.
Li, G., J. Yin, J. Gao, T. S. Cheng, N. J. Pavlos, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthr. Res. Ther. 15(6):223, 2013.
Lloyd, A. A., Z. X. Wang, and E. Donnelly. Multiscale contribution of bone tissue material property heterogeneity to trabecular bone mechanical behavior. J. Biomech. Eng. 137:010801, 2015.
Lv, H., L. Zhang, F. Yang, Z. Zhao, Q. Yao, L. Zhang, and P. Tang. Comparison of microstructural and mechanical properties of trabecular in femoral head from osteoporosis patients with and without cartilage lesions: a case-control study. BMC Musculoskelet. Disord. 31(16):72, 2015.
Malgo, F., N. A. Hamdy, S. E. Papapoulos, and N. M. Appelman-Dijkstra. Bone material strength as measured by microindentation in vivo is decreased in patients with fragility fractures independently of bone mineral density. J. Clin. Endocrinol. Metab. 100(5):2039–2045, 2015.
Mohammadi, A., K. A. H. Myller, P. Tanska, et al. Rapid CT-based estimation of articular cartilage biomechanics in the knee joint without cartilage segmentation. Ann. Biomed. Eng. 48:2965–2975, 2020.
Molino, G., A. Dalpozzi, G. Ciapetti, M. Lorusso, et al. Osteoporosis-related variations of trabecular bone properties of proximal human humeral heads at different scale lengths. J. Mech. Behav. Biomed. Mater. 100:103373, 2019.
Mononen, M. E., M. K. Liukkonen, and R. K. Korhonen. Utilizing atlas-based modeling to predict knee joint cartilage degeneration: data from the osteoarthritis initiative. Ann. Biomed. Eng. 47:813–825, 2019.
Nippolainen, E., R. Shaikh, V. Virtanen, et al. Near infrared spectroscopy enables differentiation of mechanically and enzymatically induced cartilage injuries. Ann. Biomed. Eng. 48:2343–2353, 2020.
Ozan, F., M. Pekedis, Ş Koyuncu, T. Altay, H. Yıldız, and C. Kayalı. Micro-computed tomography and mechanical evaluation of trabecular bone structure in osteopenic and osteoporotic fractures. J. Orthop. Surg. 25(1):1–6, 2017.
Pasco, J. A., E. Seeman, M. J. Henry, E. N. Merriman, G. C. Nicholson, and M. A. Kotowicz. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos. Int. 17(9):1404–1409, 2006.
Radin, E. L., H. G. Parker, J. W. Pugh, R. S. Steinberg, I. L. Paul, and R. M. Rose. Response of joints to impact loading-III: relationship between trabecular microfractures and cartilage degeneration. J. Biomech. 6:51–57, 1973.
Shen, Y., Y. H. Zhang, and L. Shen. Postmenopausal women with osteoporosis and osteoarthritis show different microstructural characteristics of trabecular bone in proximal tibia using high-resolution magnetic resonance imaging at 3 tesla. BMC Musculoskelet. Disord. 14(1):136, 2013.
Šimundić, A. M. Measures of diagnostic accuracy: basic definitions. J. Int. Fed. Clin. Chem. Lab. Med. 19(4):203–211, 2009.
Sun, J., and H. Tan. Alginate-based biomaterials for regenerative medicine applications. Materials. 6(4):1285–1309, 2013.
Sun, S. S., H. L. Ma, C. L. Liu, C. H. Huang, C. K. Cheng, and H. W. Wei. Difference in femoral head and neck material properties between osteoarthritis and osteoporosis. Clin. Biomech. 23:39–47, 2008.
Tamimi, I., A. R. G. Cortes, J. M. Sánchez-Siles, J. L. Ackerman, D. González-Quevedo, Á. García, F. Yaghoubi, M. N. Abdallah, H. Eimar, A. Alsheghri, M. Laurenti, A. Al-Subaei, E. Guerado, D. García-de-Quevedo, and F. Tamimi. Composition and characteristics of trabecular bone in osteoporosis and osteoarthritis. Bone. 140:115558, 2020.
Vakiel, P., M. Shekarforoush, C. R. Dennison, et al. Mapping stresses on the tibial plateau cartilage in an ovine model using in-vivo gait kinematics. Ann. Biomed. Eng. 49:1288–1297, 2021.
Vakiel, P., M. Shekarforoush, C. R. Dennison, et al. Stress measurements on the articular cartilage surface using fiber optic technology and in-vivo gait kinematics. Ann. Biomed. Eng. 48:2836–2845, 2020.
von Sarah R. Dynamic stiffness of articular cartilage and potential repair materials, PhD thesis. Universität Basel, 2012.
Acknowledgments
We are grateful to Ege University Planning and Monitoring Coordination of Organizational Development and Directorate of Library and Documentation for their support in editing and proofreading service of this study
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pekedis, M., Ozan, F. & Yildiz, H. Biomechanics of the Femoral Head Cartilage and Subchondral Trabecular Bone in Osteoporotic and Osteopenic Fractures. Ann Biomed Eng 49, 3388–3400 (2021). https://doi.org/10.1007/s10439-021-02861-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02861-5